Introduction
Agouti-related protein (ARP) is an orexigenic
peptide that modulates appetite and energy balance (1). ARP-expressing neurons also produce
neuropeptide Y (NPY) and the inhibitory neurotransmitter GABA
(2,3). ARP stimulates robust feeding behavior
when injected into the 3rd ventricle or directly into the
paraventricular nucleus (PVN) or dorsomedial hypothalamic nucleus
(DMH) (1,4). Its mRNA level increases in the
arcuate nucleus (ARC) under starvation conditions, as well as in
obese animals deficient in leptin signaling (5–8).
Several groups have devised strategies whereby ARP neurons are
ablated to investigate their involvement in body weight regulation
(9–12). One such strategy has been used in
adult mice and has demonstrated that ARP neuron ablation leads to
the inhibition of feeding behavior (10). In this ablation technique,
diphtheria toxin receptors (DTR) were targeted under the control of
the Agrp promoter to ensure exclusive expression of DTR in
ARP neurons (9,10). Diphtheria toxin (DT) was then
administered to adult mice, leading to the ablation of the neurons
over the next few days. This treatment led to a significant loss of
body weight and the mice were shown to have developed anorexia
(9,10). Studies have suggested that ablation
of ARP neurons removes NPY and GABA-mediated inhibition of
postsynaptic neurons, leading to hyperexcitation of selected
downstream brain regions and the induction of starvation behavior
(13). However, the cellular
mechanism underlying the excitability of postsynaptic neurons in
response to ablation of ARP neurons is poorly understood.
The immediate early gene, early growth response
factor-1 (Egr1), is a transcription factor that regulates
transcriptional responses to cell stimulation in a variety of
neurons and other cells. It is rapidly induced by stimuli, such as
growth factors or cell depolarization (14,15).
Constitutive expression of Egr1 has been identified in
certain types of neurons (16). In
vagal afferent neurons, it has been demonstrated that
cholecystokinin (CCK) stimulates the redistribution of Egr1
to the nucleus, and leptin stimulates Egr1 expression
(17). Egr1 induces the
expression of the gene encoding the satiety neuropeptide, cocaine-
and amphetamine-regulated transcript (CART), in the nodose
ganglion, which leads to the inhibition of food intake (17).
The present study aimed to determine the functional
association between appetite-controlling ARP neurons and
Egr1 in postsynaptic neurons. Egr1 expression levels
were investigated in different brain regions following acute
ablation of ARP neurons. As ARP neurons promote appetite in part
through inhibiting the pro-opiomelanocortin (POMC) pathway
(2,3,18),
it was also investigated whether blockade of melanocortin signaling
affects Egr1 expression in ARP neuron-ablated mice. It was
demonstrated that the death of ARP neurons was associated with
robust activation of Egr1 expression in selected brain
regions. In addition, it was suggested that Egr1 may induce
a novel signaling cascade in postsynaptic neurons that mediates the
feeding response and energy balance.
Materials and methods
Animal maintenance
Mice were housed in a temperature- and
humidity-controlled environment with a 12-h light/dark cycle. All
experimental protocols and animal handling procedures were
performed according to the protocol approved by the Institutional
Animal Care and Use Committee of the Central South University
(Changsha, China). AgrpDTR mice and Ay mice
were obtained from the Jackson Laboratory (Bar Harbor, ME, USA).
The AgrpDTR mice were generated by targeting a human DTR
cDNA to the Agrp locus of mice to allow the selective killing of
ARP neurons in adult mice by administration of DT. Administration
of DT resulted in reliable loss of food consumption and body weight
(9,10). The Ay mice express
agouti protein ectopically in virtually all tissues, including the
brain (19). Agouti protein
antagonizes the action of α-melanocyte stimulating hormone (α-MSH)
binding to melanocortin 4 receptors (MC4Rs) and thus prevents
melanocortin signaling via Gαs-coupled receptors on postsynaptic
cells (18). AgrpDTR
mice and Ay mice were mated together to generate
Ay; AgrpDTR/+ mice. The
Ay; AgrpDTR/+ mice were generated
to determine the consequence of suppressed melanocortin signaling
on Egr1 gene induction. The yellow mice derived from this
cross comprise were the Ay;
AgrpDTR/+ group, whereas the black
littermates served as AgrpDTR/+ controls.
Mice were group-housed with a standard chow diet and water ad
libitum until the beginning of the experiments. To ablate ARP
neurons, systemic injection of DT (two injections of 50 mg/kg;
Sigma Aldrich, St. Louis, MO, USA) was performed in 9-week-old mice
(9,10).
In situ hybridization
Brains were sectioned (coronal, 25 μm thickness) and
used for in situ hybridization with Egr1 probes
according to the manufacturer’s instructions (Roche, Mannheim,
Germany). Materials and detailed procedures concerning the data
generation process (riboprobe production, in situ
hybridization, image capture and processing) have been described
previously (20). Briefly, an
antisense Egr1 oligonucleotide probe was used for in situ
hybridization. Tissue sections were postfixed in 4%
formaldehyde/phosphate-buffered saline (PBS), rinsed in PBS and
acetylated in 0.25% acetic anhydride/0.1 M triethanolamine.
Hybridization in a solution containing a saturating concentration
(~28 kcpm/μl) of radiolabeled probe was conducted at 65°C for 18 h.
Coverslips were removed in 4X standard sodium citrate and
non-specifically bound probe was removed by treatment with RNase
(Sigma-Aldrich) for 30 min. Sections were run through stringency
washes of 1X saline sodium citrate (SSC) buffer and 0.5X SSC at
37°C and 0.1X SSC at 42°C. Sections were then dehydrated, air-dried
and exposed to Kodak BioMax X-ray film (Kodak Inc., Rochester, NY,
USA) for 3 days along with microscale 14C standards (GE Healthcare,
Little Chalfont, UK).
Statistical analysis
Quantification of Egr1-positive cells was
conducted using ImageJ software (National Institutes of Health,
Bethesda, MA, USA). Anatomical correlations of brain sections and
delineation of individual nuclei were determined by comparing
landmarks of Nissl staining images with those provided in the
Paxinos stereotaxic atlas (21).
From the anatomically matched sections, a region of interest of the
same size was further defined. In addition, an optimized threshold
that discerns round Egr1-positive nuclei from partially
stained ones and background noise was preset for all measurements.
The total number of pixels of Egr1-positive cells inside the
defined region was recorded. Unless otherwise stated, data were
analyzed using one-way analysis of variance followed by a post-hoc
Student-Newman-Keuls test. Data are presented as the mean ± SEM.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Ablation of ARP neurons by administration
of DT induces Egr1 in the ARC
A human DTR cDNA was targeted to the Agrp
locus of mice, which allowed the selective killing of ARP neurons
in adult mice by administration of DT. Administration of DT has
been shown to result in reliable loss of food consumption and body
weight (9,10). The cell bodies of all ARP neurons
located in the ARC were shown to be completely ablated by DT
treatment (9,10). In situ hybridization showed
that Egr1 expression was robustly induced in the ARC of
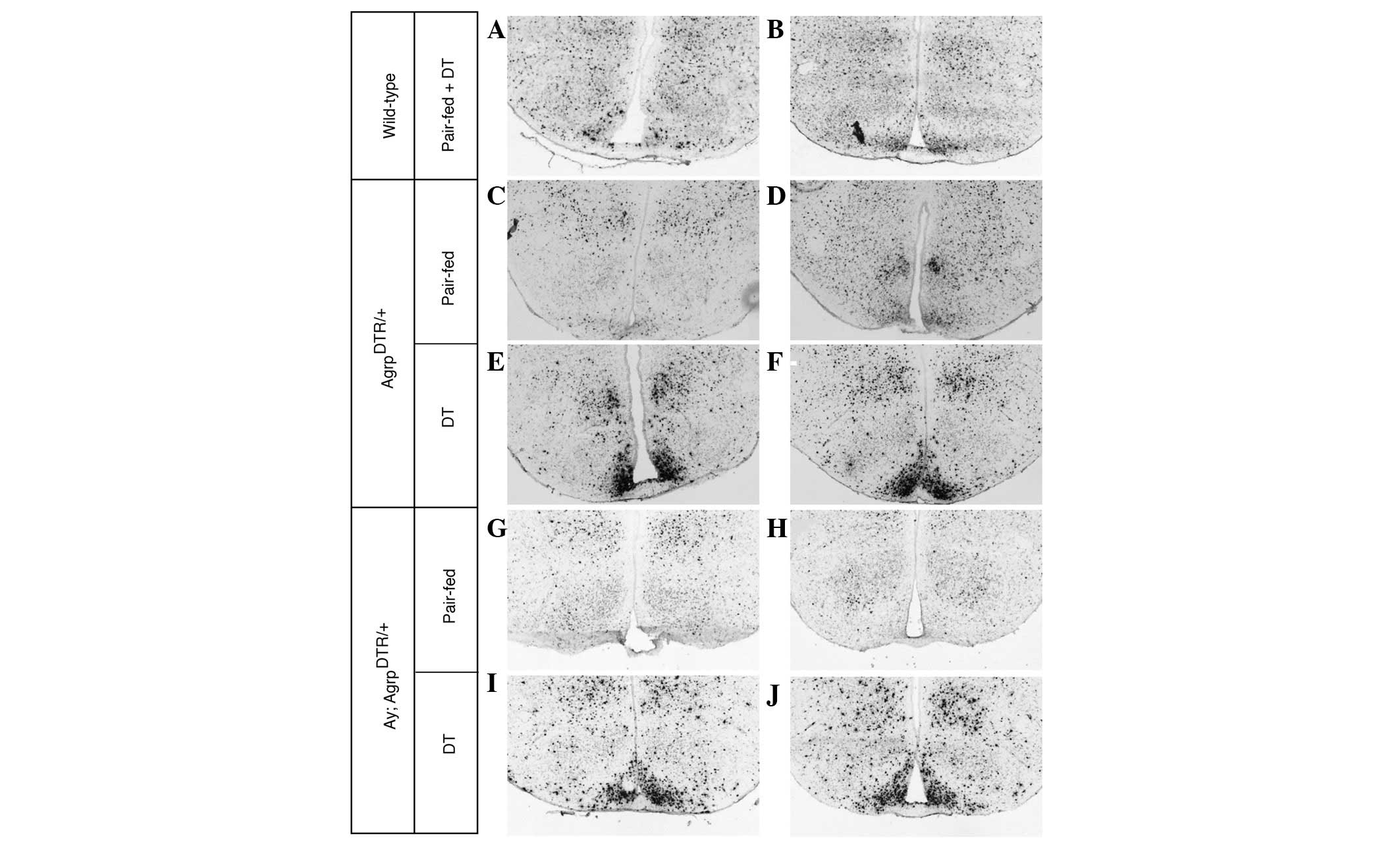
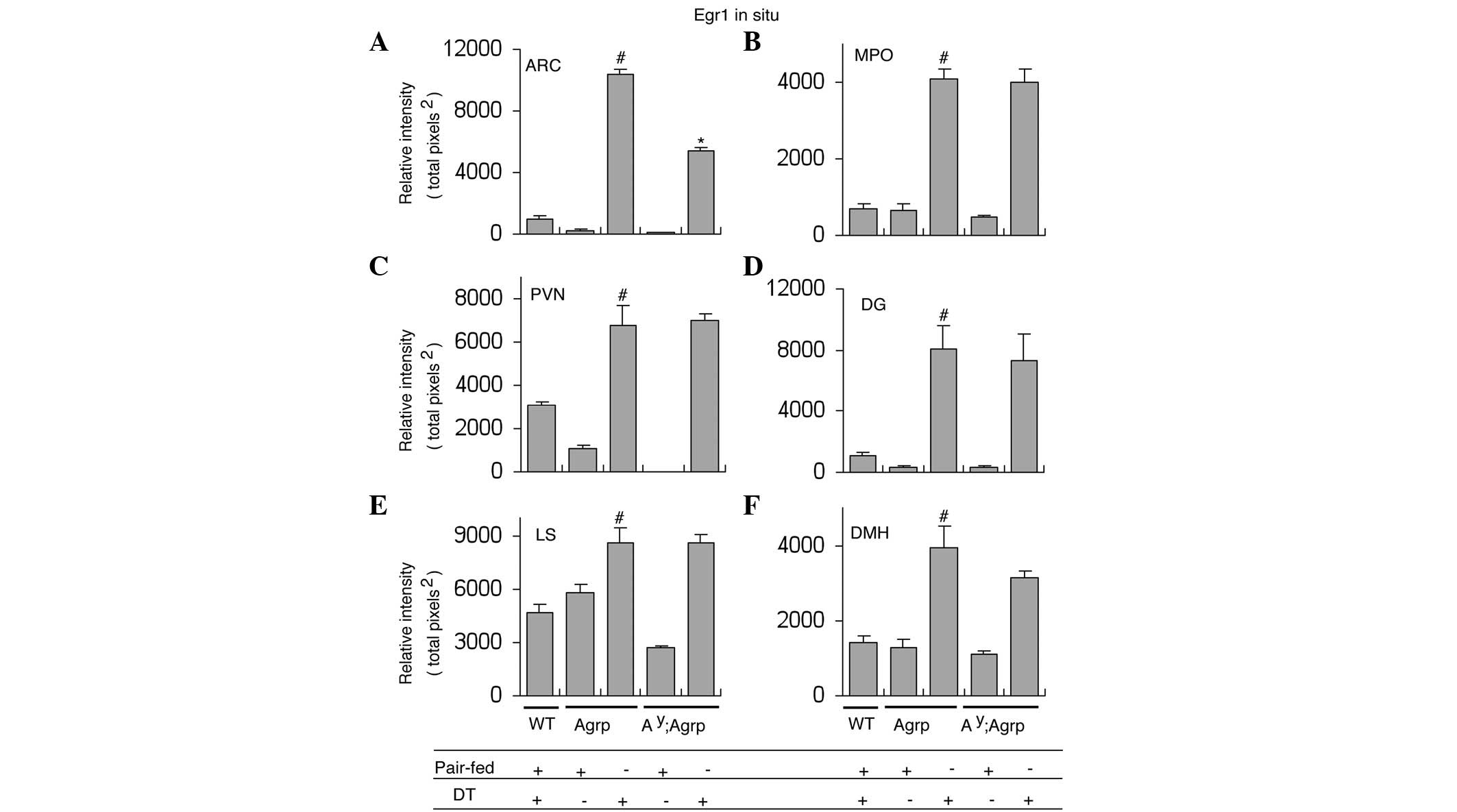
AgrpDTR mice following DT treatment (Fig. 1E and F, and Fig. 2A). In contrast, DT treatment in
wild type (WT) and pair-fed mice exhibited no effect on Egr1
expression (Fig. 1A–D and Fig. 2A).
Induction of Egr1 in the ARC is
attenuated in the absence of melanocortin signaling
The Ay mice express agouti protein
ectopically in virtually all tissues, including the brain (19). Agouti protein antagonizes the
action of α-MSH binding to MC4R, and thus prevents melanocortin
signaling via the Gαs-coupled receptors on postsynaptic cells
(18). AgrpDTR mice and
Ay mice were mated together to generate Ay;
AgrpDTR/+ mice. Ay;
AgrpDTR/+ mice were generated to determine
the consequence of suppressed melanocortin signaling on Egr1
gene induction. In situ hybridization showed that
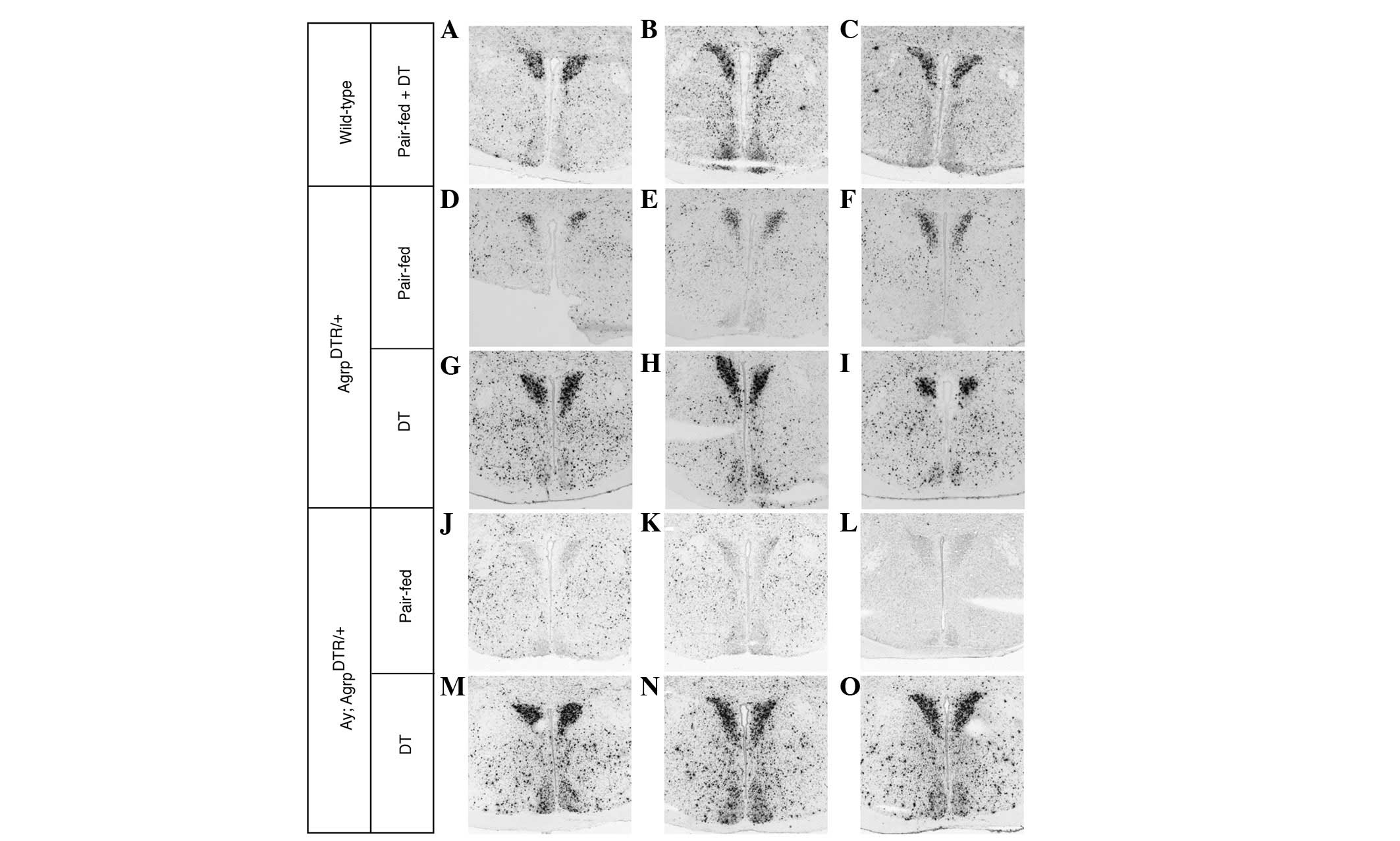
Egr1 expression in the ARC of Ay;
AgrpDTR/+ mice was significantly attenuated
compared with that of AgrpDTR/+ mice
following DT treatment, suggesting that the melancortin signaling
pathway in the ARC modulates Egr1-associated neuronal
hyperexcitability (Fig. 1I and J
and Fig. 2A).
Ablation of ARP neurons induces Egr1 in
other regions of the brain
Egr1 expression was also analyzed in other
brain regions that receive dense projections from ARP neurons.
DT-treated AgrpDTR mice showed significant Egr1
induction in a number of these downstream areas, such as the PVN,
medial preoptic area (MPO), hippocampal dentate gyrus (DG), lateral
septum (LS), and DMH, compared with either WT control or pair-fed
AgrpDTR mice (Fig. 2B–F
and Figs. 3–7). However, no significant difference was
identified in Egr1 induction between
AgrpDTR/+ mice and Ay;
AgrpDTR/+ mice in the PVN, MPO, DG, LS and
DMH nuclei when ARP neurons were ablated (Fig. 2B–F and Figs. 3–7). This suggests that melanocortin
signaling does not contribute to Egr1 induction and
excitability of postsynaptic neurons in these regions.
Disscussion
ARP is an orexigenic peptide that stimulates robust
feeding, following intracerebroventricular administration (1) or direct injection into the PVN or DMH
(4). Ablation of ARP neurons in
adult mice inhibits feeding behavior and results in starvation
within a short period of time (9,10),
indicating that ARP neurons are essential for normal body weight
regulation. Within the central nervous system (CNS), ARP is
expressed exclusively in a small population of neurons located in
the ARC that also co-release NPY and GABA (2). However, the molecular and cellular
mechanisms underlying the orexigenic effect of ARP neurons are
still poorly understood and currently under investigation (22). In the present study, it was
demonstrated that the ablation of ARP neurons markedly induced
Egr1 expression in the majority of known targets of POMC and
ARP neurons, indicating that the loss of ARP neurons leads to
disinhibition of postsynaptic neurons, which eventually results in
anorexia. Egr1 has been observed to stimulate CART expression in
certain types of neurons (17,23).
CART peptides are widely distributed in the CNS and are known to
suppress food intake (24,25) and stimulate Fos expression
in a number of brain areas when administered centrally (26,27).
Leptin-induced Egr1 expression has been shown to increase
the sensitivity of vagal afferent neurons to CCK, thereby
inhibiting food intake (17,27).
Therefore, it was suggested that Egr1-induced CART
expression and hypersensitivity to CCK was responsible, at least
partially, for the anorexia phenotype in ARP neuron-ablated mice,
although functional analysis of the ARP neural circuitry is
required to test this hypothesis.
POMC neurons in the ARC produce α-MSH, an
anorexigenic peptide that activates MC4Rs on postsynaptic cells
(18). Activation of this
melanocortin pathway inhibits feeding and stimulates metabolism.
Studies have suggested that ARP neurons counteract the POMC
signaling pathway by directly inhibiting POMC neurons via GABA
release (28) and antagonizing the
binding of melanocortin to MC4R (18). In the results of the present study,
Egr1 induction in the ARC was attenuated in mice with an
Ay genetic background when ARP neurons were ablated.
This indicated that profound Egr1 expression in the ARC
induced by ablation of ARP neurons is mediated through the
melanocortin pathway. By contrast, other downstream regions that
ARP neurons project to demonstrated no change in Egr1
induction under Ay background, suggesting that other
unknown signaling pathways are responsible for Egr1
induction in these regions.
In conclusion, the results suggested that the
deletion of ARP neurons leads to robust induction of Egr1
expression in the majority of common target regions of ARP and POMC
neurons. The induction of Egr1 is dependent on the
melanocortin signaling pathway in the ARC, but not in other
downstream regions in the brain. It was suggested that Egr1
induction may be involved in the central control of appetite
mediated by ARP neurons. Further analysis of the Egr1 signaling
cascade is required to elucidate the function of postsynaptic
targets of ARP and POMC neurons in the control of energy
metabolism, which may be therapeutically beneficial.
Acknowledgements
This study was supported by the Hunan Science &
Technology Project (grant no. 2011FJ3094).
References
|
1
|
Rossi M, Kim MS, Morgan DG, Small CJ,
Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley
SA, et al: A C-terminal fragment of Agouti-related protein
increases feeding and antagonizes the effect of alpha-melanocyte
stimulating hormone in vivo. Endocrinology. 139:4428–4431. 1998.
View Article : Google Scholar
|
|
2
|
Broberger C, Johansen J, Johansson C,
Schalling M and Hökfelt T: The neuropeptide Y/agouti gene-related
protein (AGRP) brain circuitry in normal, anorectic, and monosodium
glutamate-treated mice. Proc Natl Acad Sci USA. 95:15043–15048.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haskell-Luevano C, Chen P, Li C, Chang K,
Smith MS, Cameron JL and Cone RD: Characterization of the
neuroanatomical distribution of agouti-related protein
immunoreactivity in the rhesus monkey and the rat. Endocrinology.
140:1408–1415. 1999.PubMed/NCBI
|
|
4
|
Kim MS, Rossi M, Abusnana S, Sunter D,
Morgan DG, Small CJ, Edwards CM, Heath MM, Stanley SA, Seal LJ, et
al: Hypothalamic localization of the feeding effect of
agouti-related peptide and alpha-melanocyte-stimulating hormone.
Diabetes. 49:177–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morton GJ, Cummings DE, Baskin DG, Barsh
GS and Schwartz MW: Central nervous system control of food intake
and body weight. Nature. 443:289–295. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barsh GS and Schwartz MW: Genetic
approaches to studying energy balance: perception and integration.
Nat Rev Genet. 3:589–600. 2002.PubMed/NCBI
|
|
7
|
Kalra SP, Dube MG, Pu S, Xu B, Horvath TL
and Kalra PS: Interacting appetite-regulating pathways in the
hypothalamic regulation of body weight. Endocr Rev. 20:68–100.
1999.PubMed/NCBI
|
|
8
|
Ollmann MM, Wilson BD, Yang YK, Kerns JA,
Chen Y, Gantz I and Barsh GS: Antagonism of central melanocortin
receptors in vitro and in vivo by agouti-related protein. Science.
278:135–138. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luquet S, Perez FA, Hnasko TS and Palmiter
RD: NPY/AgRP neurons are essential for feeding in adult mice but
can be ablated in neonates. Science. 310:683–685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gropp E, Shanabrough M, Borok E, Xu AW,
Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et
al: Agouti-related peptide-expressing neurons are mandatory for
feeding. Nat Neurosci. 8:1289–1291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bewick GA, Gardiner JV, Dhillo WS, Kent
AS, White NE, Webster Z, Ghatei MA and Bloom SR: Post-embryonic
ablation of AgRP neurons in mice leads to a lean, hypophagic
phenotype. FASEB J. 19:1680–1682. 2005.PubMed/NCBI
|
|
12
|
Xu AW, Kaelin CB, Morton GJ, Ogimoto K,
Stanhope K, Graham J, Baskin DG, Havel P, Schwartz MW and Barsh GS:
Effects of hypothalamic neurodegeneration on energy balance. PLoS
Biol. 3:e4152005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Q, Boyle MP and Palmiter RD: Loss of
GABAergic signaling by AgRP neurons to the parabrachial nucleus
leads to starvation. Cell. 137:1225–1234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sukhatme VP, Cao XM, Chang LC, Tsai-Morris
CH, Stamenkovich D, Ferreira PC, Cohen DR, Edwards SA, Shows TB,
Curran T, et al: A zinc finger-encoding gene coregulated with c-fos
during growth and differentiation, and after cellular
depolarization. Cell. 53:37–43. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cole AJ, Saffen DW, Baraban JM and Worley
PF: Rapid increase of an immediate early gene messenger RNA in
hippocampal neurons by synaptic NMDA receptor activation. Nature.
340:474–476. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beckmann AM and Wilce PA: Egr
transcription factors in the nervous system. Neurochem Int.
31:477–510; discussion 517–476. 1997. View Article : Google Scholar
|
|
17
|
de Lartigue G, Lur G, Dimaline R, Varro A,
Raybould H and Dockray GJ: EGR1 Is a target for cooperative
interactions between cholecystokinin and leptin, and inhibition by
ghrelin, in vagal afferent neurons. Endocrinology. 151:3589–3599.
2010.PubMed/NCBI
|
|
18
|
Cone RD: Anatomy and regulation of the
central melanocortin system. Nat Neurosci. 8:571–578. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Michaud EJ, Bultman SJ, Stubbs LJ and
Woychik RP: The embryonic lethality of homozygous lethal yellow
mice (Ay/Ay) is associated with the disruption of a novel
RNA-binding protein. Genes Dev. 7:1203–1213. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aher CV, Duwaerts CC, Akama KT and Lucas
LR: Effects of acute diuresis stress on egr-1 (zif268) mRNA levels
in brain regions associated with motivated behavior. Brain Res
Bull. 81:114–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paxinos G and Franklin KBJ: The Mouse
Brain in Stereotaxic Coordinates. 2nd edition. Academic Press;
Waltham, MA: 2001
|
|
22
|
Liu T, Wang Q, Berglund ED and Tong Q:
Action of neurotransmitter: A key to unlock the AgRP neuron feeding
circuit. Front Neurosci. 6:2002013.PubMed/NCBI
|
|
23
|
Dockray GJ and Burdyga G: Plasticity in
vagal afferent neurones during feeding and fasting: mechanisms and
significance. Acta Physiol (Oxf). 201:313–321. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rogge G, Jones D, Hubert GW, Lin Y and
Kuhar MJ: CART peptides: regulators of body weight, reward and
other functions. Nat Rev Neurosci. 9:747–758. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koylu EO, Couceyro PR, Lambert PD, Ling
NC, DeSouza EB and Kuhar MJ: Immunohistochemical localization of
novel CART peptides in rat hypothalamus, pituitary and adrenal
gland. J Neuroendocrinol. 9:823–833. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stanley SA, Small CJ, Murphy KG, Rayes E,
Abbott CR, Seal LJ, Morgan DG, Sunter D, Dakin CL, Kim MS, Hunter
R, Kuhar M, Ghatei MA and Bloom SR: Actions of cocaine- and
amphetamine-regulated transcript (CART) peptide on regulation of
appetite and hypothalamo-pituitary axes in vitro and in vivo in
male rats. Brain Res. 893:186–194. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Lartigue G, Barbier de la Serre C,
Espero E, Lee J and Raybould HE: Leptin resistance in vagal
afferent neurons inhibits cholecystokinin signaling and satiation
in diet induced obese rats. PLoS One. 7:e329672012.PubMed/NCBI
|
|
28
|
Cowley MA, Smart JL, Rubinstein M, Cerdán
MG, Diano S, Horvath TL, Cone RD and Low MJ: Leptin activates
anorexigenic POMC neurons through a neural network in the arcuate
nucleus. Nature. 411:480–484. 2001. View
Article : Google Scholar : PubMed/NCBI
|