Introduction
The formation of new blood vessels is an important
process required for healing wounds and for restoring blood flow to
tissue following injury or insult (1). A number of stem/progenitor cells,
including bone marrow-derived endothelial progenitor cells (EPCs)
and mononuclear cell, have been identified for their pro-angiogenic
potential to improve perfusion as an experimental or clinical
option (2,3). As precursors of mature endothelial
cells (ECs), EPCs are capable of increasing the neovascularization
of ischemic tissue and delaying the initiation and progression of
coronary artery disease (4,5).
Circulating EPCs were found to exhibit similar features of ECs,
possess the ability to home to sites of ischemia and contribute to
the formation of new blood vessels (6). Thus, EPCs have been used as seeding
cells in tissue engineering and stem cell therapy.
Increasing evidence has demonstrated that the Notch
signaling pathway is one of the most important mechanisms involved
in the regulation of neovascularization. In mammals, the Notch
signaling pathway includes five transmembrane ligands, i.e.,
Delta-like (Dll)-1, Dll-3, Dll-4, Jagged (Jag)-1 and Jag-2 and four
transmembrane receptors, i.e., Notch-1, -2, -3 and -4. The
interaction between the Notch receptor and ligand leads to cleavage
of the Notch intracellular domain (NICD) and translocation to the
nucleus, thereby activating downstream target genes, including
basic helix-loop-helix (bHLH) proteins which include hairy/enhancer
of split (Hes) and Hes-related protein (Hey) (7,8). The
Notch ligand Dll-4 has been identified as a promising new target
for angiogenesis in preclinical studies. For example, the balance
between sprout and tube formation is established as being important
for the generation of a new functional vessel, which is
hypothesized to be modulated by Dll-4 from tip ECs to neighboring
stalk ECs in order to restrict the emergence of excessive sprout
through the repression of vascular endothelial growth factor (VEGF)
receptor 2 (VEGFR2) transcription and consequently the reduction of
responsiveness to VEGF (9,10). By contrast, the inhibition of
Dll-4-mediated signaling is hypothesized to retard tumor growth,
despite an increase of tumor vasculature density since the
established vascular network is functionally inefficient (11–13),
suggesting that Dll-4 attenuates the formation of ineffective
vascular branch and promotes the remainder to form functional
vessels. Thus, Dll-4-overexpressing EPCs were hypothesized to be
superior to untreated EPCs in the formation of productive blood
vessels in ischemic myocardium.
Materials and methods
Animal ethics
All procedures were performed in compliance with the
guidelines for the Care and Use of Laboratory Animals published by
the National Institutes of Health (NIH Publication No. 85-23,
revised 1996) and approved by the Animal Care and Use Committee of
the First People’s Hospital of Nanning, China.
Isolation, cultivation and identification
of EPCs
Two 6-week-old C57BL/6 mice were sacrificed by
cervical dislocation. Following removal of the tips of the hind
legs and vertebrae, bone marrow was collected by flushing out the
content of femurs and tibias with PBS. Mononuclear cells were
collected from the bone marrow by density gradient centrifugation
using Histopaque 1077 (Sigma-Aldich, St. Louis, MO, USA) according
to the manufacturer’s instructions. The isolated cells were
cultivated in dishes coated with fibronectin (R&D Systems,
Minneapolis, MN, USA) and induced by EBM-2 Single Quots (Lonza,
Basel, Switzerland) with supplements at 37°C with 5% CO2
in humidified air at a density of 5×106
cells/cm2. Following 3 days in culture, non-adherent
cells were removed by washing with PBS, new medium was applied and
the cultivation was maintained for 7 days. Immunofluorescence
staining and flow cytometry were used to identify EPCs, which was
performed as previously described by Liu et al(14).
Recombinant lentiviral vector
construction and cell infection
The procedures for recombinant lentiviral vector
construction, cell infection and selection of the stable cell line
were performed as previously described by Chen and Zhou (15). In brief, to produce Dll-4
recombinant lentiviral vectors, the plasmids pAJ-Ubi-eGFP-3Flag,
psPAX2 (gag/pol element) and pMD2.G (VSVG element) (Auragene
Bioscience Inc., Changsha, China) were used according to the
manufacturer’s instructions. Following lentiviral vector infection,
the stable cell line overexpressing Dll-4 was cultured in a 5%
CO2-humidified incubator at 37°C. EPCs infected with
Dll-4 recombinant lentiviral vectors were labeled
EPCDll-4+ and EPCs infected with mock vectors were
labeled EPCnull.
To determine whether transplantation of
Dll-4-inhibited EPCs had an opposed effect on neovascularization,
EPCs were infected with lentiviral constructs encoding short
hairpin RNA (shRNA) against Dll-4. The plasmids
pAJ-U6-shRNA-CMV-Puro/eGFP, psPAX2 and pMD2.G (Auragene Bioscience)
were transfected into 293T cells according to the instructions for
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA). Following a 48-h transduction, infected EPCs were selected to
generate a stable Dll-4-shRNA line. EPCs transfected with
recombinant lentiviral vectors encoding Dll-4-shRNA were labled
EPCDll-4−.
Quantitative PCR (qPCR) was used to
identify the efficiency of gene transfection
Total RNA was extracted from each sample with TRIzol
reagent (Invitrogen Life Technologies) and reversed transcribed
into first-strand cDNA by RevertAid™ First Strand cDNA Synthesis
kit (Fermentas, Vilnius, Lithuania) according to the manufacturer’s
instructions. The synthesized cDNA was used for qPCR analysis of
Dll-4 mRNA expression with SYBR® Premix Ex Taq™ (Takara
Bio, Inc., Shiga, Japan). The sense sequence of Dll-4 primers was
5′-CGAGGGAACAGAGTTGAGGAGT-3′ and the antisense sequence was
5′-AATACAGATGCCCACAGGAGC-3′. Fluorescence qPCR was performed on the
ABI PRISM 7300 SDS apparatus (Applied Biosystems, Foster City, CA,
USA).
Western blot analysis
Each sample was lysed in 0.2 ml lysis buffer
(Calbiochem, La Jolla, CA, USA). The protein concentrations were
determined by the Bradford method (Bio-Rad, Hercules, CA, USA).
Total protein (20 μg) was separated on 10% SDS-PAGE gels and
transferred to PVDF membranes (Millipore, Billerica, MA, USA) using
the semi-dry transfer method. Membranes were blocked for 1 h in
Tris-buffered saline containing 0.01% Tween-20 with 10% non-fat
dried milk and incubated overnight at 4°C with the relevant
antibodies: Dll-4 and Hey-1 antibody (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), mammalian target of rapamycin (mTOR)
and phospho-mTOR (Ser2448) antibody (Abcam, Cambridge, UK), p70S6
kinase (p70S6K) and phospho-p70S6K (Thr421/Ser424) antibody (Cell
Signaling Technology, Inc., Danvers, MA, USA). Membranes were
rinsed and incubated for 1 h with the corresponding
peroxidase-conjugated secondary antibodies. Chemiluminescent
detection was performed using the ECL kit (Pierce Biotechnology,
Inc., Rockford, IL, USA). All bands were analysed using Image J
software (version 1.6 NIH).
In vitro angiogenesis assay
Tubulogenesis was induced using an in vitro
Angiogenesis Assay kit (#ECM625, Millipore) following the
manufacturer’s instructions (16).
Briefly, ECMatrix™ solution was thawed on ice overnight, mixed with
10X ECMatrix™ diluents and placed in a 96-well tissue culture plate
at 37°C for 1 h to allow the matrix solution to solidify. EPCs
(1×104 cells/well) in 50 μl of medium were cultured on
the top of the solidified matrix solution. Following 18 h of
incubation at 37°C, the tubule formation was inspected under an
inverted light microscope at ×200 magnification. Tubule formation
was defined as a structure exhibiting a length four times its
width. Five random microscopic fields were assessed for each well
and the average number and the total length of tubules/200× field
was determined and compared with the control cells.
Assessment of cell death induced by
H2O2
The viability of EPCs was determined by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(Sigma-Aldrich) assay. A total of 1×104 cells were
equally seeded into each well in 96-well microplates. Following
incubation with H2O2 for 1 h, the medium was
replaced with MTT solution (0.5 mg/ml in PBS). Incubation was
continued for 4 h and then the supernatant was gently removed.
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich) was added and the
absorbance was read at 490 nm on a spectrophotometer (Bio-Rad) and
the percentage of cell viability was obtained.
Creation of myocardial infarction (MI)
model and cell transplantation
Eight-week-old C57BL/6 mice (weighing 20±2 g)
underwent ligation of the left coronary artery to produce MI.
Following anesthetization, the animals were orally intubated with a
1.0-mm OD intubation cannula and connected to a small animal
volume-control ventilator (HES-HA MiniVent 845, Harvard Apparatus,
Holliston, MA, USA). The left anterior descending artery was
ligated 2–3 mm from its origin between the pulmonary artery conus
and the left atrium using 8-0 sutures. The ligated animals were
monitered by electrocardiograph using the RM6240BD system (Chengdu
Instrument Company, Chengdu, China) to determine MI models. One
week following coronary ligation, the surviving mice were randomly
subdivided into 5 groups (PBS, EPCs, EPCnull,
EPCDll-4+ and EPCDll-4−; 20 animals in each)
and administered with intravenous injection of PBS, EPCs,
EPCnull, EPCDll-4− and EPCDll-4+
in the tail vein. Each animal received an injection of
5×106 cells/100 μl in PBS or PBS alone with a total
volume of 50 μl.
Assessment of cardiac function by
echocardiography
A transthoracic echocardiographic study was
performed by an experienced blinded cardiologist at 14 days
post-transplantation using an echocardiographic system (SONOS 5500,
Hewlett-Packard, Andover, MA, USA) equipped with a 15.0 MHz
transducer. For analysis of left ventricular (LV) function, left
ventricular internal dimensions (LVID) were measured at diastole
(LVIDD) and systole (LVIDS). After two-dimensional images were
obtained, the M-mode cursor was positioned to the parasternal long
axis view at the papillary muscle level. LV ejection fraction (EF)
and fractional shortening (FS) were calculated as follows: EF (%) =
(LVIDD3 - LVIDS3)/LVIDD3 × 100%
and FS (%) = [(LVIDD - LVIDS)/LVIDD] × 100%, respectively. Each
parameter was measured from a minimum of 3 consecutive beat cycles
in each image.
Myocardial blood flow
Measurement of blood flow to the peri-infarcted and
infarcted area of mouse hearts was performed as previously
described (17).
Capillary density
To identify mature capillary density, the tissue
sections (5 μm) of the infarcted zone were stained with anti-VIII
factor antibody (Santa Cruz Biotechnology, Inc.).
Immunohistochemical staining was performed as previously described
(17).
Statistical analysis
Data are presented as the mean ± SD. A method of
ANOVA (analysis of variance) with Scheffe’s post-hoc test was used
to identify differences among the groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Efficiency of gene transfection and
alteration of relative signals
qPCR data indicated that Dll-4 gene expression in
EPCDll-4+ was 17-fold higher compared with the
expression in EPCs. The Dll-4 mRNA level was ~65.4% lower in
EPCDll-4− compared with EPCs. Western blot analysis
showed that the expression of Dll-4, Hey-1, phospho-mTOR and
phospho-p70S6K were markedly increased in EPCDll-4+ and
markedly decreased in EPCDll-4− (Fig. 1). Furthermore, a similar alteration
was also detected in the EPCs-, EPCnull-,
EPCDll-4+- and EPCDll-4−-treated animals when
the peri-infarcted and infarcted heart tissue was extracted for
western blot analysis. These observations suggested that the
overexpression of Dll-4 results in the increase of its downstream
target molecule, Hey-1 and thus, the activation of mTOR signaling
pathway.
 | Figure 1Western blot analysis of Dll-4
expression and alteration of the downstream signaling pathways
in vitro. (A–E) Data show that the expression of Dll-4,
Hey-1, one of the downstream of Notch signaling pathways and the
phosphorylated status of mTOR and p70S6K, was significantly
increased in EPCDll-4+ and markedly decreased in
EPCDll-4−. EPCs, endothelial progenitor cells; Dll-4,
Delta-like-4; Hey-1, Hes-related protein 1; mTOR, mammalian target
of rapamycin, p70S6K, p70S6 kinase; EPCnull, EPCs
infected with mock vectors; EPCDll-4+,
Dll-4-overexpressing EPCs; EPCDll-4−, Dll-4-inhibited
EPCs. |
Protective effect of Dll-4 on the
H2O2-injured EPCs
To determine the effect of Dll-4 on the viability of
H2O2-treated EPCs, the cells of each group
were exposed to increasing concentrations of
H2O2 from 0 to 1,000 μmol/l (data from the
pilot study were not shown). The previous study showed that 150
μmol/l H2O2 is the optimal concentration.
Dll-4-overexpressing EPCs were more resistant to
H2O2 compared with EPCs or EPCnull
following incubation with the apoptotic stimulus (P<0.01). By
contrast, Dll-4-inhibited EPCs were more readily damaged by
H2O2 (Fig.
2).
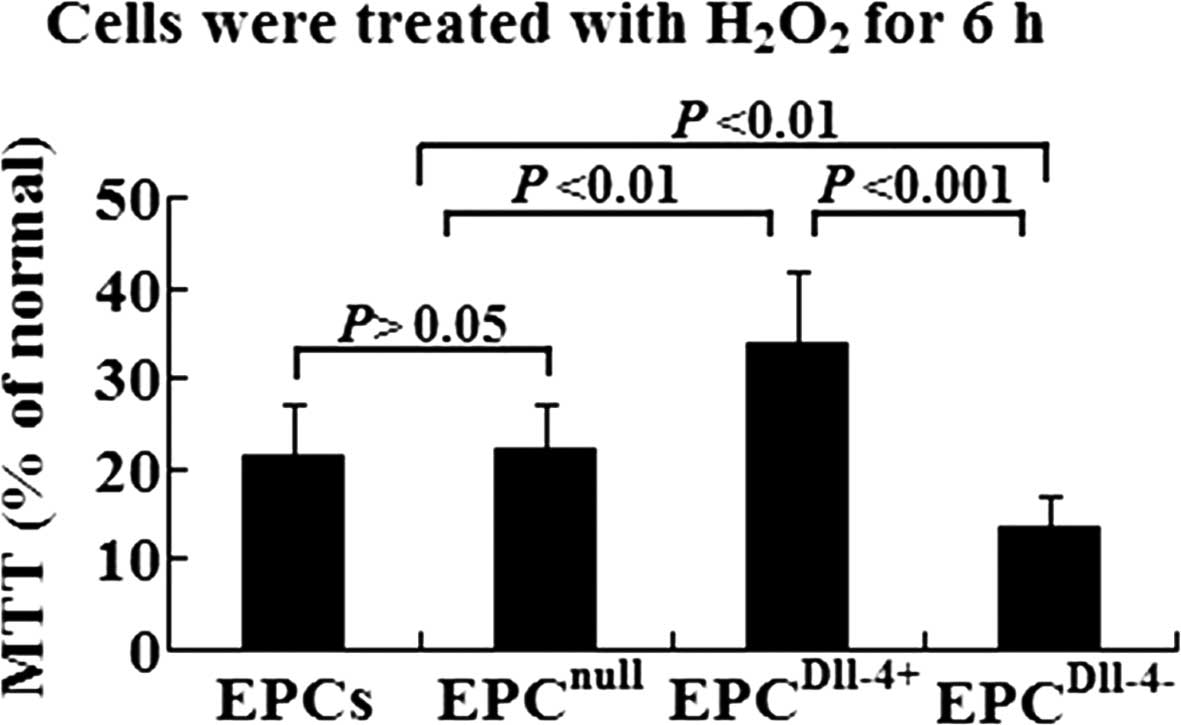 | Figure 2Analysis of cell viability using MTT
assay in EPCs, EPCnull, EPCDll-4+ and
EPCDll-4− following incubation with
H2O2 for 6 h. EPCDll-4+ showed a
higher resistance against hypoxia when compared with the control
cells. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide; Dll-4, Delta-like-4; EPCs, endothelial progenitor cells;
EPCnull, EPCs infected with mock vectors;
EPCDll-4+, Dll-4-overexpressing EPCs;
EPCDll-4−, Dll-4-inhibited EPCs. |
Capillary-like tube formation
EPCs were seeded on a solidified matrix and
incubated with collected medium for 18 h. Quantification of branch
points/200× microscopic field demonstrated that the number of
tubular structures in the EPCDll-4+ group was higher
compared with the EPC and EPCnull groups. Tubules in the
EPCDll-4+ group were qualitatively different and more
complex compared with those in the control wells. Despite the
number of emerging sprouts being greater in the
EPCDll-4− group, they had an irregular and disorganized
shape, suggesting that knockdown of the Dll-4 gene impacts on EPCs
ability to form functional tubules (Fig. 3).
Improvement of LV contractile
function
To assess cardiac function, echocardiographic
studies were performed 2 weeks post-transplantation. EF and FS were
improved in the EPCs, EPCnull and EPCDll-4+
groups when compared with the PBS group (P<0.01 or P<0.001).
Furthermore, EF and FS were higher in the EPCDll-4+
group compared with the EPCs and EPCnull groups
(P<0.05; Fig. 4). However,
there was no statistical significance in enhancing cardiac function
between EPCDll-4− and PBS therapy (P>0.05). These
results indicate that transplantation of the Dll-4-overexpressing
EPCs had an improved therapeutic effect for improving the LV
function of MI animals compared with transplantation of untreated
EPCs. By contrast, pre-inhibition of Dll-4 in the transplanted
cells resulted in a poor effect.
 | Figure 4Improvement of LV contractile
function. The LV, EF and FS were compared among these groups 2
weeks post-transplantation. Dll-4, Delta-like-4; LV, left
ventricular, EF, ejection fraction; FS, ejection fraction; EPCs,
endothelial progenitor cells; EPCnull, EPCs infected
with mock vectors; EPCDll-4+, Delta-like-4
(Dll-4)-overexpressing EPCs; EPCDll-4−, Dll-4-inhibited
EPCs; #P<0.01 vs. PBS group; $P<0.001
vs. PBS group; ‡P<0.01 vs. EPCs group;
^P<0.01 vs. EPCnull group;
§P<0.001 vs. EPCDll-4+ group group. |
Transplantation of Dll-4-overexpressing
EPCs increases to form mature micovessels in ischemic
myocardium
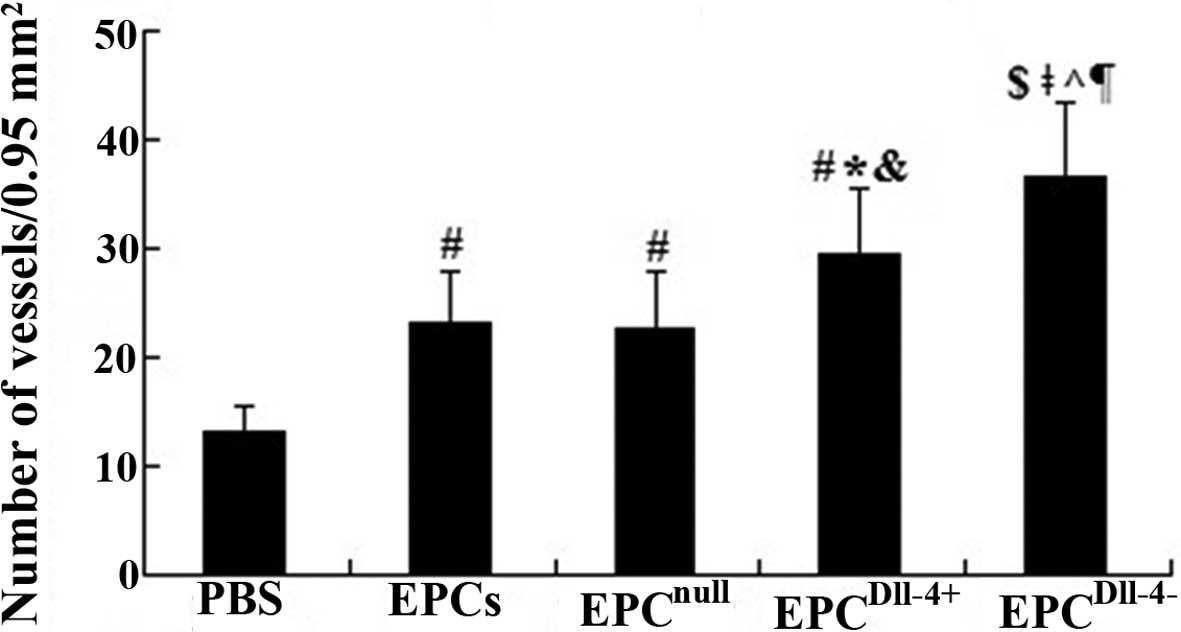
Semiquantitative analysis showed that, 2 weeks
following cell transplantation, the number of blood vessels in
peri-infarcted and infarcted tissue was significantly increased in
the EPC (23.1±4.8) and EPCnull (22.6±5.2) groups when
compared with the PBS group (13.2±2.4) (P<0.01). The microvessel
number was further increased in the EPCDll-4+ group and
an increase in immature vessel proliferation was observed in the
peri-infarcted and infarcted area of mouse heart 2 weeks following
treatment with EPCDll-4−, however, mature vessels with a
lumen were barely detected (Fig.
5). This indicated that Dll-4 may contribute to angiogenesis
and tubulogenesis in ischemic myocardium.
Treatment of Dll-4-overexpressing EPCs
increases the blood flow in ischemic myocardium
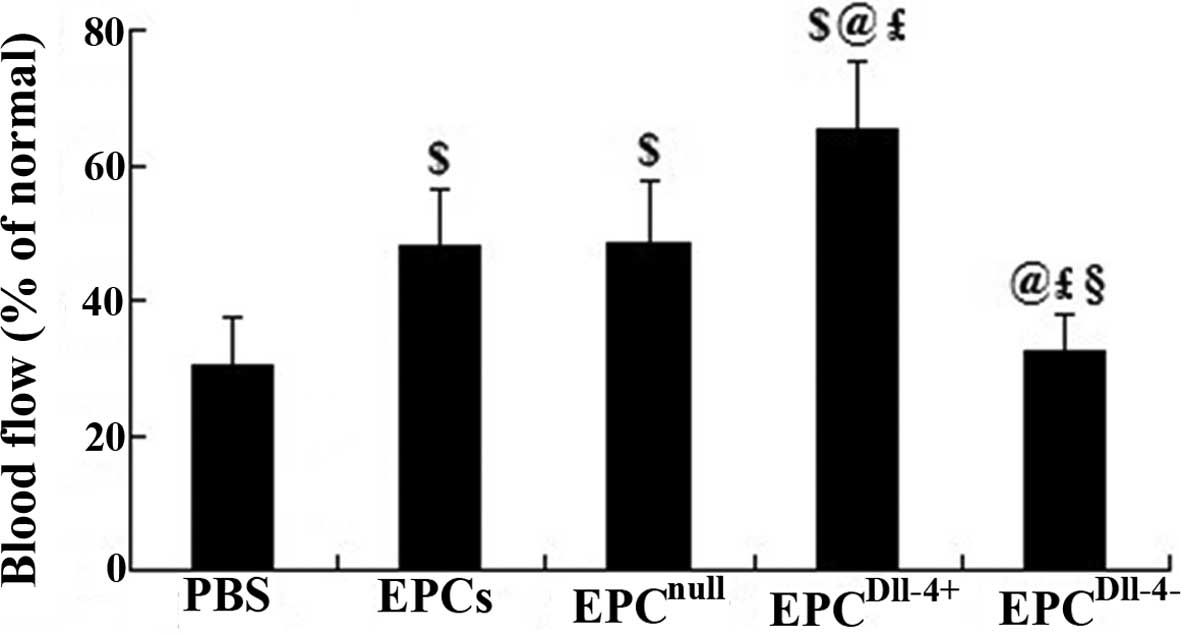
To determine whether new blood vessels translate to
increased coronary blood flow to the infarcted myocardium,
functional microvessels were identified in the infarcted heart
using the fluorescent microsphere method for regional blood flow
assessment 2 weeks following transplantation. There was a
significant decrease in blood flow to the infarcted and
peri-infarcted zone and an increase to ~48% of the normal level was
observed when EPCs and EPCnull treatment was induced.
Notably, transplantation of EPCDll-4+ further increased
the blood flow to ~65.1% that of the normal level. However, no
evident improvement was detected when treated with PBS or
EPCDll-4− (Fig. 6).
Discussion
In terms of EPCs, abundant evidence supports their
involvement in capillary growth and the formation of collateral
vessels. These consequent effects have led to improved perfusion
and functional recovery in animal models of myocardial and
peripheral ischemia (4,18). In early clinical trials, the
therapeutic administration of EPCs to patients with MI or chronic
angina has been associated with positive trends in perfusion
(2). However, low survival and the
angiogenic potential of treated cells in ischemic myocardium
affects the outcome of EPC transplantation for the treatment of
ischemic disease. Cell sheet grafts with genetically engineered
properties to prolong stem cell survival and promote blood vessel
networks integrated with pre-existing coronary may provide a
potential approach to repair dead or injured myocardium (19). The present study suggests that EPCs
may be efficiently transfected with lentiviral vectors encoding
Dll-4 without any adverse effect on the cell viability and that
EPCDll-4+ exhibit a higher resistance against oxidative
stress and the transfected EPCs survive for a longer period of time
in the ischemic area.
Vascular network formation is coordinated by VEGF
and Dll-4. Dll-4 may act downstream of VEGF as a ‘brake’ on
VEGF-mediated angiogenic sprouting (20) and it may act to prevent
overexuberant angiogenic sprouting, promoting the timely formation
of a well-differentiated vascular network (21). Inhibition of Dll-4 induces the
proliferation of immature vascular networks and results in poor
tissue perfusion (11–13). The current in vitro study
demonstrated that the number of tubular structures in the
EPCDll-4+ group was higher compared with the EPC and
EPCnull groups. In in vivo experiments, a
significantly greater capacity was observed when productive vessels
were formed and further increased blood flow to the infarcted and
peri-infarcted zone in the groups receiving EPCDll-4+,
with the exception of the group transplanted with untreated EPCs.
Two weeks following cell transplantation, the cardiac function in
the Dll-4-overexpressing EPCs group gradually recovered, whereas
the PBS and untreated EPCs groups did not exhibit such effects,
suggesting that tubulogenesis and enhancement of tissue perfusion
played a key role in the improvement of cardiac function in MI
animals. EPCDll-4+ also promotes the formation of mature
vessels in the ischemic myocardium and enhances cardiac function.
Thus, treated EPCs are hypothesized to be superior to the untreated
EPCs. By contrast, specific knockdown of Dll-4 attenuates the
ability of EPCs to form mature vascular structures in the in
vitro angiogenesis assay. Injection of Dll-4-inhibited EPCs
into MI animals, resulted in the formation of a dense capillary
network, which appeared irregular and disorganized, thus, unable to
supply ischemic myocardium with adequate perfusion. These
observations were consistent with a previous study showing that
Dll-4 blockade causes functional defects in angiogenesis following
ischemia in mouse limp (22).
With the exception of Notch, the signaling pathways
of mTOR are also closely linked to stem cell development and
neovascularization. In ECs, mTOR may be necessary for EPC
development since inhibition of mTOR pathways with rapamycin may
lead to EPC death, which may result from inhibiting growth factor
signaling (23). As an important
component of cardiac tissue protection and regeneration,
angiogenesis may be regulated by mTOR (24). Inhibition of mTOR may lead to a
sequence of events, including elevated matrix metalloproteinase-1
and the blockade of tissue inhibitor of metalloprotease-3 resulting
in impaired angiogenesis (25).
Moreover, loss of mTOR activity may also lead to a blockage of
endothelial proliferation and angiogenesis (26) as well as proliferation of EPCs
(23). In the current study, the
expression of Notch downstream target molecule, Hey-1 and the
phosphorylated status of mTOR and p70S6K (one of mTOR downstream
effectors) were significantly increased under a higher expression
of Dll-4 in EPCs. EPCDll-4+transplantation also led to a
similar variation as the mouse ischemic myocardium was extracted
for western blot analysis. Notably, an opposite effect was observed
in in vitro cultered EPCDll-4− and the animals
treated with EPCnull. A previous study by Chan et
al observed that Notch signals may positively regulate the
activity of the mTOR pathway in T-cell acute lymphoblastic leukemia
(27). Overexpression of Dll-4 was
thus hypothesized to increase Hey-1 expression via interaction with
the Notch-1 or -4 receptor (28,29)
and in turn activate the mTOR/p70S6K-mediated angiogenesis
signaling pathways.
In conclusion, the present study suggests that the
intravenous injection of Dll-4-overexpressing EPCs stimulates
angiogenesis and tubulogenesis effectively and increases blood flow
to the ischemic zone through the activation of
Notch/Hey-1/mTOR/p70S6K signaling pathways. The combined strategy
of EPCs transplantation with Dll-4 therapy may be proposed as a
promising approach for the treatment of ischemic heart disease.
Abbreviations:
|
EPCs
|
endothelial progenitor cells
|
|
Dll
|
Delta-like
|
|
Jag
|
Jagged
|
|
NICD
|
Notch intracellular domain
|
|
bHLH
|
basic helix-loop-helix
|
|
Hes
|
hairy/enhancer of split
|
|
Hey
|
Hes-related protein
|
|
ECs
|
endothelial cells
|
|
PBS
|
phosphate-buffered saline
|
|
VEGF
|
vascular endothelial growth factor
|
|
shRNAs
|
short hairpin RNAs
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
MI
|
myocardial infarction
|
|
LV
|
left ventricular
|
|
LVID
|
left ventricular internal
dimensions
|
|
LVIDD
|
LVID in diastole
|
|
LVIDS
|
LVID in systole
|
|
EF
|
ejection fraction
|
|
FS
|
fractional shortening
|
|
ANOVA
|
analysis of variance
|
|
mTOR
|
mammalian target of rapamycin
|
References
|
1
|
Karamysheva AF: Mechanisms of
angiogenesis. Biochemistry (Mosc). 73:751–762. 2008. View Article : Google Scholar
|
|
2
|
Lawall H, Bramlage P and Amann B:
Treatment of peripheral arterial disease using stem and progenitor
cell therapy. J Vasc Surg. 53:445–453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gimble JM, Bunnell BA and Guilak F: Human
adipose-derived cells: an update on the transition to clinical
translation. Regen Med. 7:225–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalka C, Masuda H, Takahashi T, Kalka-Moll
WM, Silver M, Kearney M, Li T, Isner JM and Asahara T:
Transplantation of ex vivo expanded endothelial progenitor cells
for therapeutic neovascularization. Proc Natl Acad Sci USA.
97:3422–3427. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roca C and Adams RH: Regulation of
vascular morphogenesis by Notch signaling. Genes Dev. 21:2511–2524.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leslie JD, Ariza-McNaughton L, Bermange
AL, McAdow R, Johnson SL and Lewis J: Endothelial signalling by the
Notch ligand Delta-like 4 restricts angiogenesis. Development.
134:839–844. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Phng LK and Gerhardt H: Angiogenesis: a
team effort coordinated by notch. Dev Cell. 16:196–208. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scehnet JS, Jiang W, Kumar SR, et al:
Inhibition of Dll4-mediated signaling induces proliferation of
immature vessels and results in poor tissue perfusion. Blood.
109:4753–4760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Noguera-Troise I, Daly C, Papadopoulos NJ,
Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD and Thurston
G: Blockade of Dll4 inhibits tumour growth by promoting
non-productive angiogenesis. Nature. 444:1032–1037. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ridgway J, Zhang G, Wu Y, et al:
Inhibition of Dll4 signalling inhibits tumour growth by
deregulating angiogenesis. Nature. 444:1083–1087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu L, Wen T, Zheng XY, Yang DG, Zhao SP,
Xu DY and Lü GH: Remnant-like particles accelerate endothelial
progenitor cells senescence and induce cellular dysfunction via an
oxidative mechanism. Atherosclerosis. 202:405–414. 2009. View Article : Google Scholar
|
|
15
|
Chen JJ and Zhou SH: Mesenchymal stem
cells overexpressing MiR-126 enhance ischemic angiogenesis via the
AKT/ERK-related pathway. Cardiol J. 18:675–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Urbich C, Aicher A, Heeschen C, Dernbach
E, Hofmann WK, Zeiher AM and Dimmeler S: Soluble factors released
by endothelial progenitor cells promote migration of endothelial
cells and cardiac resident progenitor cells. J Mol Cell Cardiol.
39:733–742. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang F, Zhu X, Hu XQ, et al: Mesenchymal
stem cells modified with miR-126 release angiogenic factors and
activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis
and cell survival. Int J Mol Med. 31:484–492. 2013.PubMed/NCBI
|
|
18
|
Murohara T: Angiogenesis and
vasculogenesis for therapeutic neovascularization. Nagoya J Med
Sci. 66:1–7. 2003.PubMed/NCBI
|
|
19
|
Deuse T, Peter C, Fedak PW, et al:
Hepatocyte growth factor or vascular endothelial growth factor gene
transfer maximizes mesenchymal stem cell-based myocardial salvage
after acute myocardial infarction. Circulation. 120(Suppl 11):
S247–S254. 2009. View Article : Google Scholar
|
|
20
|
Suchting S, Freitas C, le Noble F,
Benedito R, Bréant C, Duarte A and Eichmann A: The Notch ligand
Delta-like 4 negatively regulates endothelial tip cell formation
and vessel branching. Proc Natl Acad Sci USA. 104:3225–3230. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lobov IB, Renard RA, Papadopoulos N, Gale
NW, Thurston G, Yancopoulos GD and Wiegand SJ: Delta-like ligand 4
(Dll4) is induced by VEGF as a negative regulator of angiogenic
sprouting. Proc Natl Acad Sci USA. 104:3219–3224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Al Haj Zen A, Oikawa A, Bazan-Peregrino M,
Meloni M, Emanueli C and Madeddu P: Inhibition of
delta-like-4-mediated signaling impairs reparative angiogenesis
after ischemia. Circ Res. 107:283–293. 2010.PubMed/NCBI
|
|
23
|
Miriuka SG, Rao V, Peterson M, Tumiati L,
Delgado DH, Mohan R, Ramzy D, Stewart D, Ross HJ and Waddell TK:
mTOR inhibition induces endothelial progenitor cell death. Am J
Transplant. 6:2069–2079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maiese K, Chong ZZ, Shang YC and Hou J:
FoxO proteins: cunning concepts and considerations for the
cardiovascular system. Clin Sci (Lond). 116:191–203. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lemaitre V, Dabo AJ and D’Armiento J:
Cigarette smoke components induce matrix metalloproteinase-1 in
aortic endothelial cells through inhibition of mTOR signaling.
Toxicol Sci. 123:542–549. 2011. View Article : Google Scholar
|
|
26
|
Humar R, Kiefer FN, Berns H, Resink TJ and
Battegay EJ: Hypoxia enhances vascular cell proliferation and
angiogenesis in vitro via rapamycin (mTOR)-dependent signaling.
FASEB J. 16:771–780. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chan SM, Weng AP, Tibshirani R, Aster JC
and Utz PJ: Notch signals positively regulate activity of the mTOR
pathway in T-cell acute lymphoblastic leukemia. Blood. 110:278–286.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shutter JR, Scully S, Fan W, Richards WG,
Kitajewski J, Deblandre GA, Kintner CR and Stark KL: Dll4, a novel
Notch ligand expressed in arterial endothelium. Genes Dev.
14:1313–1318. 2000.PubMed/NCBI
|
|
29
|
Hellström M, Phng LK, Hofmann JJ, et al:
Dll4 signalling through Notch1 regulates formation of tip cells
during angiogenesis. Nature. 445:776–780. 2007.PubMed/NCBI
|