Introduction
Pseudomonas aeruginosa (P. aeruginosa)
is the most common bacterial pathogen, causing a wide range of
acute and chronic infections. The bacteria are widely distributed
and are characteristically multidrug resistant. Once infected,
clinical therapies are complex. P. aeruginosa is the main
cause of nosocomial infection (1,2). The
main successful feature in the infected host is the production of a
large array of secretory factors, including proteases, exotoxins,
phospholipases and pigments (3,4). A
number of these secretory factors have been shown to have
biological effects on host cells that may contribute to the
pathogenesis of P. aeruginosa-associated lung disease. Among
these factors is the redox active phenazine derivative, pyocyanin
(3–5), a cytotoxic pigment and virulence
factor (6) that has been isolated
from the sputum of infected patients at levels as high as 27.3
μg/ml. One of the biological effects is a change in the expression
and/or activity of cytokines (5).
It has been reported that the pigment modifies several host cell
responses, including the inhibition of neutrophil superoxide
generation and catalase activity in human lung epithelial cells
(7), the regulation of neutrophil
apoptosis, the impairment of apoptotic cell engulfment (8,9) and
the inhibition of lymphocyte proliferation, which is responsible
for the production of several important proinflammatory mediators.
Thus, it is of significance to study the pathogenesis of a host
infected by pyocyanin.
Alveolar macrophages are defense and inflammation
regulation cells that switch on numerous mediators of inflammation
and cytokines and subsequently cause acute lung injury. Thus, the
intervention of NF-κB has become a therapeutic focus. However, the
human medullary system U937 cell line is characteristic of
monoblasts and pedomonocytes. U937 cells activated with phorbol
12-myristate 13-acetate (PMA) were induced and differentiated to
form macrophages. Thus, differentiated U937 cells are the ideal
model for studying human macrophages.
Pseudomonas infections are characterized by a
marked influx of polymorphonuclear cells (neutrophils) and
excessive inflammatory response. Chemokines are a superfamily of
chemotactic cytokines with >28 recognized family members, and
are important in inflammatory conditions due to their ability to
attract inflammatory cells (10).
They can be divided into the CXC and CC subfamilies (11,12).
The CXC chemokines, of which interleukin-8 (IL-8) is the prototype,
predominantly act on neutrophils (11).
Previous studies have identified a secretory factor
of Pseudomonas with the properties of pyocyanin that
increases the release of IL-8 by airway epithelial cells, in
vitro and in vivo(13,14).
However, the involvement of the protein kinase C (PKC) and NF-κB
pathways in Pseudomonas pyocyanin-induced IL-8 production in
U937 cells remained undefined. In this study, we examined the
effect of pyocyanin on the release of IL-8 and the activation of
NF-κB in U937 human monocytes stimulated with pyocyanin.
Furthermore, we also observed the effect of PKC and NF-κB blockers
on the expression of IL-8 chemokines and the precise contributions
of PKC kinase activation and NF-κB transactivation to IL-8
expression of U937 cells.
Materials and methods
The present study was performed in the Department of
Microbiology laboratory, Liaoning Medical University (Jinzhou,
China) between October 2009 and March 2012. This study was
performed with the approval of the Ethical Committee of Liaoning
Medical University (Jinzhou, China; no. 201232) and conducted
according to the principles of the Declaration of Helsinki. Written
consent was obtained from all participants.
Chemicals and reagents
RPMI-1640, fetal bovine serum (FBS) and antibiotics
were purchased from Gibco-BRL (Carlsbad, CA, USA). Stocks of the
selective PKC inhibitor, calphostin C (Cal C), were purchased from
Calbiochem-Behring Corp. (La Jolla, CA, USA). IL-8 assay kits were
purchased from R&D Systems (Minneapolis, MN, USA). PMA was
purchased from Merck Biosciences (San Diego, CA, USA). Phenazinem
ethosulfate (molecular formula,
C14H14N2O4S) was from
Amresco LLC (Solon, OH, USA). All other reagents were purchased
from Sigma-Aldrich (St. Louis, MO, USA).
Synthesis of pyocyanin
Pyocyanin was prepared by photolysis of 700 mg
phenazine methosulphate in 1.5 l of Tris buffer (0.1 M, pH 7.0) for
three days, as described previously (14,15).
The solution was made alkaline (pH>11), and pyocyanin was
extracted into chloroform (5 × 400 ml). Crude pyocyanin was
chromatographed on a silica column (60 × 3 cm) and eluted using
chloroform and methanol (85:15 v/v). The blue pigment was
rechomatographed on fresh silica to form a product, which was
judged to be pure according to high-performance liquid
chromatography (HPLC; HPLC-2010; Shimadzu, Kyoto, Japan),
ultraviolet (UV) absorbance (Lambda Bio40, Perkin Elemer, Waltham,
MA, USA) and electrospray mass spectrometry (Finnigan™ LCQ™; Thermo
Scientific, Waltham, MA, USA).
Cell culture and differentiation
U937 cells were purchased from ATCC (Rockville, MD,
USA) and cultured at 37°C in a humidified atmosphere with 5%
CO2 in RPMI-1640 medium supplemented with 10% fetal calm
serum (FCS) and 50 μg/ml gentamicin, which itself was supplemented
with 4.5 g/l glucose, 1 mM sodium pyruvate and 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES). The
cell culture was maintained at a cell concentration of
1×106 cells/ml. All cell lines were diluted one day
prior to each experiment. For differentiation into macrophages,
U937 cells were treated with PMA (10 nM) and allowed to adhere for
48 h in a 5% CO2 tissue culture incubator at 37°C, after
which they were washed and fed with PMA-free RPMI-1640 medium.
Treatment with pyocyanin and
inhibitors
PMA-differentiated U937 cells were washed;
subsequently, varying concentrations of pyocyanin (5, 25 and 50 μM)
were added to the U937 cells with the medium, and cells were
incubated in a 5% CO2 atmosphere at 37°C for 24 h. The
same concentrations of pyocyanin (50 μM) were added to the cell
cultures for 8, 16 and 24 h, respectively. Subsequently, the
culture supernatant was collected and stored in a refrigerator at
-70°C. IL-8 concentration was measured by enzyme-linked
immunosorbent assay (ELISA). RNA was subsequently extracted, and
IL-8 mRNA levels were determined. In certain experiments, Cal C and
pyrrolidine dithiocarbamate (PDTC) were added with fresh medium to
the U937 cells, 60 min prior to the addition of pyocyanin.
MTT assay
Cell viability was assessed using the 3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
assay kit (Sigma-Aldrich) according to the manufacturer’s
instructions.
Measurement of IL-8
Cells were cultured in 24-well tissue culture plates
until they achieved 80–90% confluence. Cells were cultured in
serum-free medium without growth supplements for 24 h prior to
treatment. The medium was harvested 24 h following treatment and
stored at −20°C until they were assayed. IL-8 levels were
determined by ELISA according to the manufacturer’s
instructions.
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the U937 cells as
described by Chomczynski (16). At
the end of the incubation period, cells were washed with 1 ml
ice-cold PBS and solubilized with 1 ml TRIzol (Invitrogen,
Carlsbad, CA, USA). RNA was treated with chloroform, centrifuged at
12,000 × g for 15 min at 4°C and finally precipitated with ethanol.
RNA was extracted and redissolved in diethylpyrocarbonate-treated
water, and the outside diameter at 260 nm determined its
concentration. In order to synthesize complementary (c)DNA, 2.5 μg
of RNA was resuspended in a 10-μl final volume of the reaction
buffer and incubated for 30 min at 42°C. The reaction was stopped
by denaturing the enzyme at 95°C for 5 min. PCR was performed as
follows: Synthesized cDNA (10 μl) was added to 40 μl of PCR mixture
containing 5 μl of 5X PCR buffer, 1.0 μl of primers (GenBank
accession IL-8 sense, 5′-AGATGTCAGTGCATAAAGACA-3′ and
antisense, 5′-TGAATTCTCAGCCCTCTTCAAAAA-3′, 201 bp;
and GenBank accession β-actin sense, 5′-GGCATGGGTCAGAAG
GATYCC-3′ and antisense, 5′-ATGTCACGCACGATTTC
CCGC-3′, 501 bp) and 0.25 μl DNA polymerase. Amplification
was performed with 35 cycles, which was the optimal condition for
linearity and permitted semiquantitative analysis of signal
strength (IL-8: temperature profile, 35 cycles of denaturation at
94°C for 45 sec, annealing at 55.3°C for 45 sec and extension at
72°C for 1 min; β-actin: 94°C for 3 min, 94°C for 45 sec, 59°C for
45 sec, 72°C for 1 min, 35 cycles, 72°C for 10 min). Amplified PCR
products were separated by electrophoresis on 1.5% agarose gel
(UltraPure, Sigma-Aldrich) containing 0.05 μg/ml ethidium bromide.
mRNA expression was visualized using a gel imaging system, analyzed
using molecular analyst software (ChemiImager 5500; Alpha Innotech,
Santa Clara, CA, USA) and standardized by the β-actin housekeeping
gene signal to correct any variability in gel loading. The ratio
between the optical density of β-actin and the test gene was
calculated to evaluate relative changes in the test gene.
Western blotting
Cytosol protein preparation and
western blotting
Cells were lysed in a buffer containing 20 mM HEPES
(pH 7.9), 1% Triton-X100, 0.4 M NaCl, 2.5% glycerol, 1 mM EDTA, 1
mM phenol methyl sulfonil fluoride (PMSF), 0.5 mM NaF, 0.02 mg/ml
leupeptin, 0.02 mg/ml aprotinin, 0.1 mg/ml trypsin inhibitor and
0.5 mM dithiothreitol (DTT), as described by Maceyka et
al(17). Protein content was
quantitated by a Bradford assay (Pierce Biotechnology Inc.,
Rockford, IL, USA). Protein (20 μl) of the supernatant was mixed
with 5X sodium dodecyl sulfate (SDS) sample buffer (100 mM Tris-HCl
(pH 6.8), 5% SDS, 25% glycerol, 0.01% bromophenol blue) and
separated on 10% SDS-polyacrylamide gels. The proteins were
transferred onto 0.45-mm nitrocellulose membranes (Schleicher &
Schuell, Dassel, Germany) in a buffer containing 25 mM Tris-HCl (pH
8.3), 0.5% SDS, 192 mM glycine and 20% methanol. Equal protein
loading was monitored by Ponceau S membrane staining. The membranes
were blocked with 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.3%
Tween-20 (TBST) containing 5% BSA for at least 1 h at room
temperature. Blots were subsequently incubated overnight at 4°C
with indicated diluted primary antibodies (1:200; rabbit anti-human
IκBα polyclonal antibodies; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA). Following three washing steps in TBST (10 min
each), blots were incubated for 1 h at room temperature with a
peroxidase-linked secondary antibody (1:2,000; goat anti-rabbit
polyclonal IgG antibodies; Santa Cruz Biotechnology, Inc.). Signals
were developed using 3,3′-diaminobenzidine (DAB) coloration system
(Sigma-Aldrich) according to the manufacturer’s instructions.
Nuclear protein preparation and
western blotting
Cells were scraped into ice-cold PBS, pH 7.4, and
centrifuged at 800 × g for 5 min. The supernatant was discarded and
the cells in the pellet were lysed with a buffer containing 10 mM
HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM ethylene glycol
tetraacetic acid (EGTA), 10X IGEPAL, 1.0 mM DTT and 0.5 mM PMSF.
The cell lysate was chilled on ice for 15 min and subsequently
centrifuged at 12,000 × g for 30 min. The pellet was resuspended in
a buffer containing 20 mM HEPES (pH 7.9), 0.4 mM NaCl, 1.0 mM EDTA,
0.1 mM EGTA, 1.0 mM DTT and 1.0 mM PMSF. The preparation was kept
on ice for 15 min, vortexed several times and centrifuged for 5 min
(12,000 × g at 4°C). The supernatant, containing nuclear protein,
was divided into aliquots, snap frozen and stored at −80°C. Protein
concentration was measured using the Bradford reagent (Bio-Rad,
Hercules, CA, USA). Proteins were separated on 10%
SDS-polyacrylamide gel and transferred onto nitrocellulose
membranes. Immunoreactive proteins were detected by incubating the
blots with diluted p65 monoclonal antibodies (1:200; rabbit
anti-human NF-κB p65; Santa Cruz Biotechnology, Inc.) at 4°C
overnight, followed by incubation with diluted horseradish
peroxidase-conjugated secondary antibody (1:2,000; goat ant-rabbit
IgG antibodies; Santa Cruz Biotechnology, Inc.) and visualized with
the DAB coloration system. The resulting images were analyzed with
Scion Image software (Scion Corporation, Frederick, MD, USA).
Immunohistochemistry
Immunohistochemistry, using the
avidin-biotin-peroxidase complex method described previously
(18), was employed to examine the
expression of NF-κB p65 protein following infection with
pyocyanin.
Statistical analysis
Data presented are representative of three to five
experiments. Unless otherwise indicated, data are expressed as the
mean ± SD. Data were analyzed using one-way analysis of variance
(ANOVA) followed by the least significant difference (LSD) test for
multiple comparisons. All analyses were performed using SPSS 13.0
software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Differentiation of U937 cells
The U937 cell of a routine subculture is a single
suspension cell. Following 8 h of undifferentiation, the U937 cells
that had been cultured in the presence of 10 nM PMA were obtained
from an independent small circular suspension cell. They had
started to transform from flat elongated cells into
irregular-shaped adherences; they were amoeba-like in shape
and had adhered to the bottom of the container. Following 48 h of
cultivation, 85% of the cells grew in an adherent manner. To date,
differentiation of U937 cells by treatment with PMA has been
accomplished.
Cell viability assay
To assess the effect of pyocyanin on cell viability,
MTT assays were performed on cells incubated with a range of
pyocyanin concentrations (5–100 μM) for 24 h. Cell viability was
unaffected by pyocyanin (5–75 μM). A 5–6% loss of cell viability
was observed at 100 μM pyocyanin (data not shown). Therefore,
incubation with pyocyanin at concentrations of 5–50 μM were used,
producing increases over the basal level.
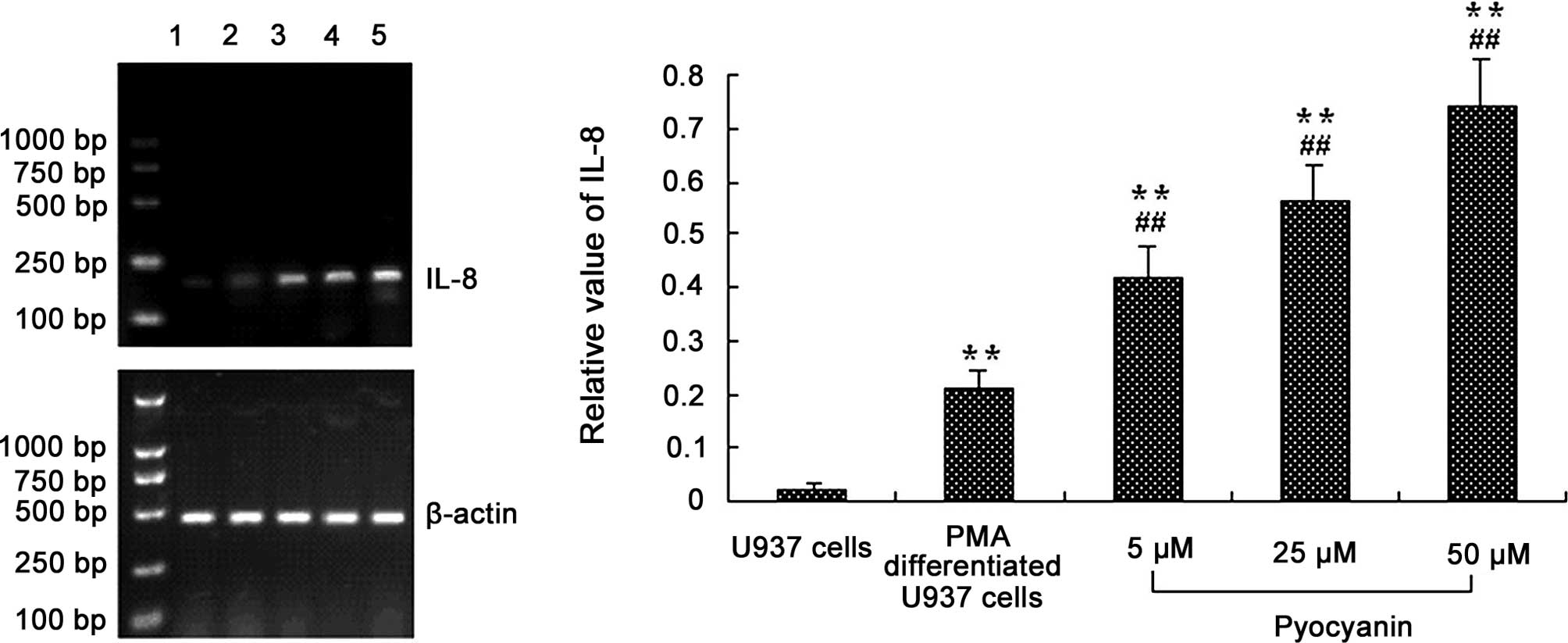
Effect of pyocyanin on IL-8 mRNA
In order to further explore the expression of IL-8
mRNA in cells infected by pyocyanin, various concentration of
pyocyanin (5, 25 and 50 μM) were added for the indicated times and
analyzed by RT-PCR using specific primers for IL-8 according to the
manufacturer’s instructions. The results demonstrated that
pyocyanin induced IL-8 mRNA expression in differentiated U937 cells
in a concentration-dependent manner after 2 h. The medium alone did
not express IL-8 mRNA, and PMA-differentiated U937 cells produced
trace amounts of IL-8 mRNA (Fig.
1).
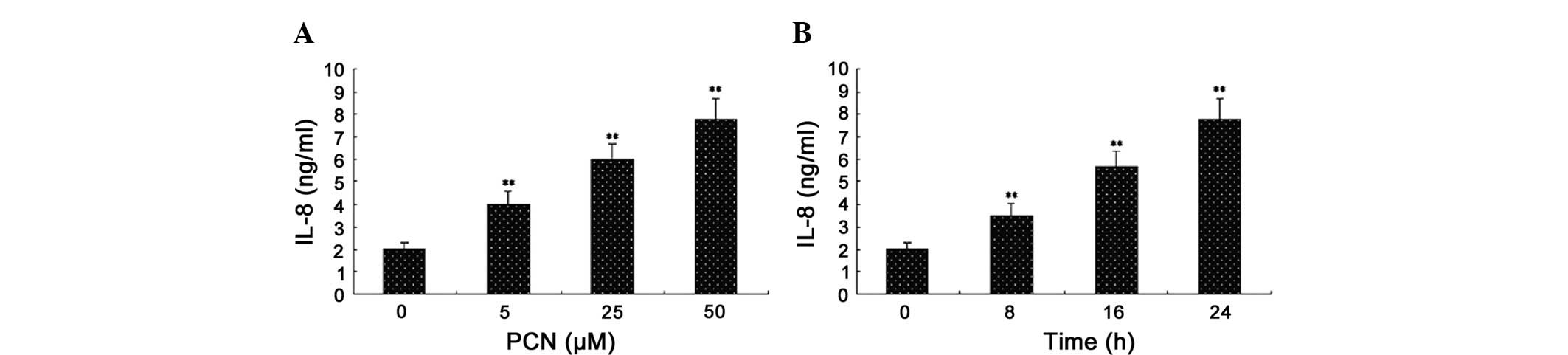
Pyocyanin increases the release of IL-8
in PMA-differentiated U937 cells
Previous studies have identified that pyocyanin
stimulates the production of IL-8 by lung macrophage cells
(19) and epithelial cells
(5,20). Based on the physical properties of
pyocyanin, we hypothesized that pyomyacin was capable of
stimulating the production of IL-8 in differentiated U937 cells. In
order to assess this hypothesis, we exposed human differentiated
U937 cells to purified pyocyanin and measured the release of IL-8.
Following the addition of various concentrations of pyocyanin (5,
25 and 50 μM) and stimulating PMA-differentiated U937 cells for 24
h, the supernatants were collected and analyzed by ELISA. The same
concentrations of pyocyanin (50 μM) were added to the cell cultures
for 8, 16 and 24 h, respectively. The results revealed that
pyocyanin increased the release of IL-8 in differentiated U937
cells in a concentration- and time-dependent manner. Measurable
increases in IL-8 release were observed with pyocyanin
concentrations as low as 5 μM; a 50-μM concentration showed the
strongest cellular response to stimulation (Fig. 2). Increases in IL-8 above control
levels were observed as early as 8 h following the addition of
pyocyanin (50 μM), and these levels continued to increase relative
to the controls between 24 and 48 h (data not shown). Longer times
were not assessed.
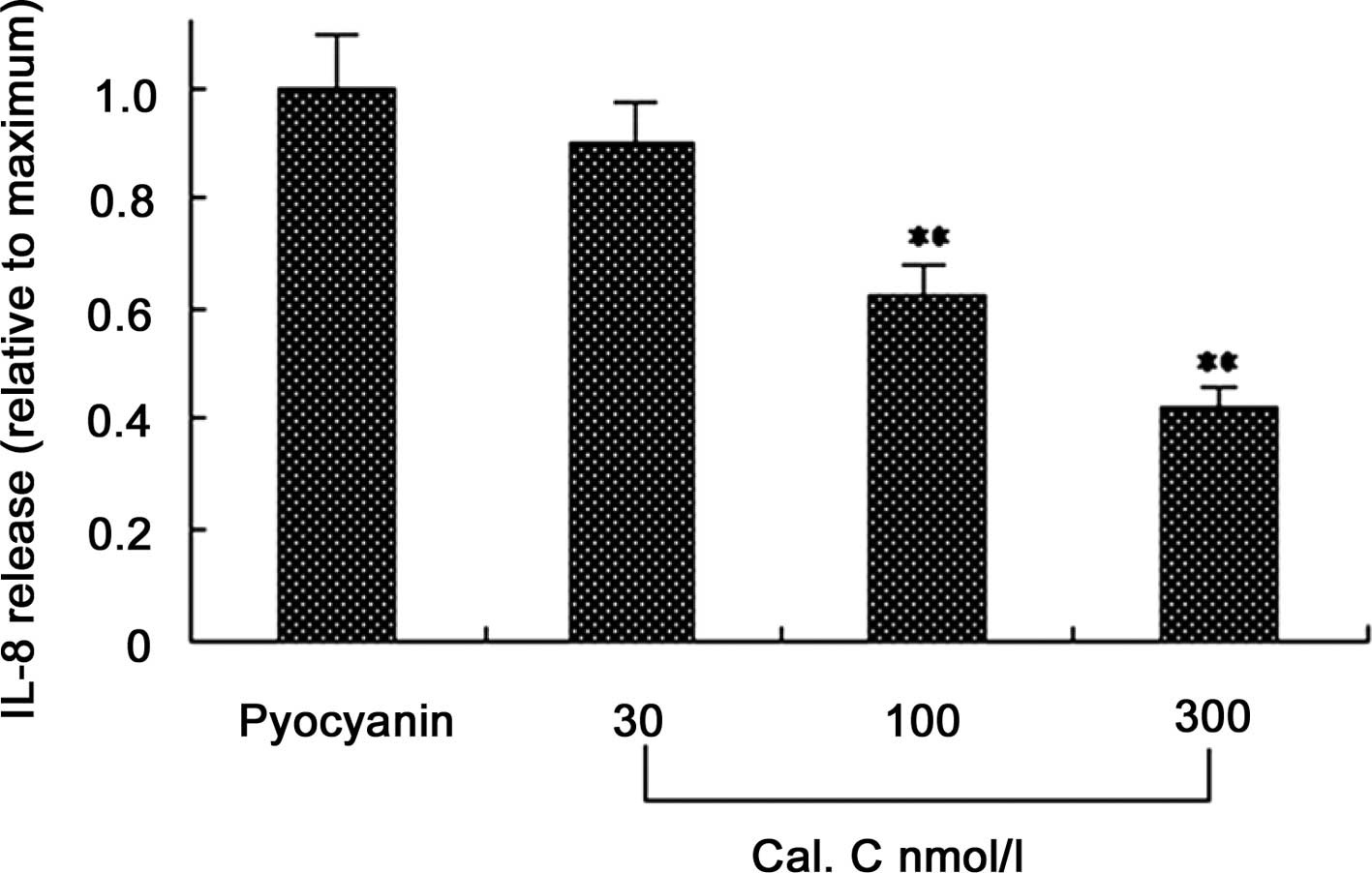
Effect of PKC inhibitors on
pyocyanin-induced IL-8 release
A number of studies have revealed that the PKC
signaling pathway mediates a variety of stimulating factors that
induce IL-8 expression. To explore the pathogenesis of pyocyanin,
we hypothesized that pyocyanin may induce the expression of IL-8 in
U937 cells through the PKC signaling pathway. In certain
experiments, different concentrations of a PKC blocker (Cal C; 30,
100 and 300 nmol/l) were added in a fresh medium to U937 cells 60
min prior to the addition of pyocyanin. After 24 h, the
supernatants were collected and IL-8 concentrations were detected
by ELISA. The results showed that Cal C significantly decreased the
secretion of IL-8, and as the concentration of Cal C increased, the
secretion of IL-8 decreased, indicating that pyocyanin may
stimulate U937 cells to express IL-8 cytokines through the PKC
signaling pathway (Fig. 3).
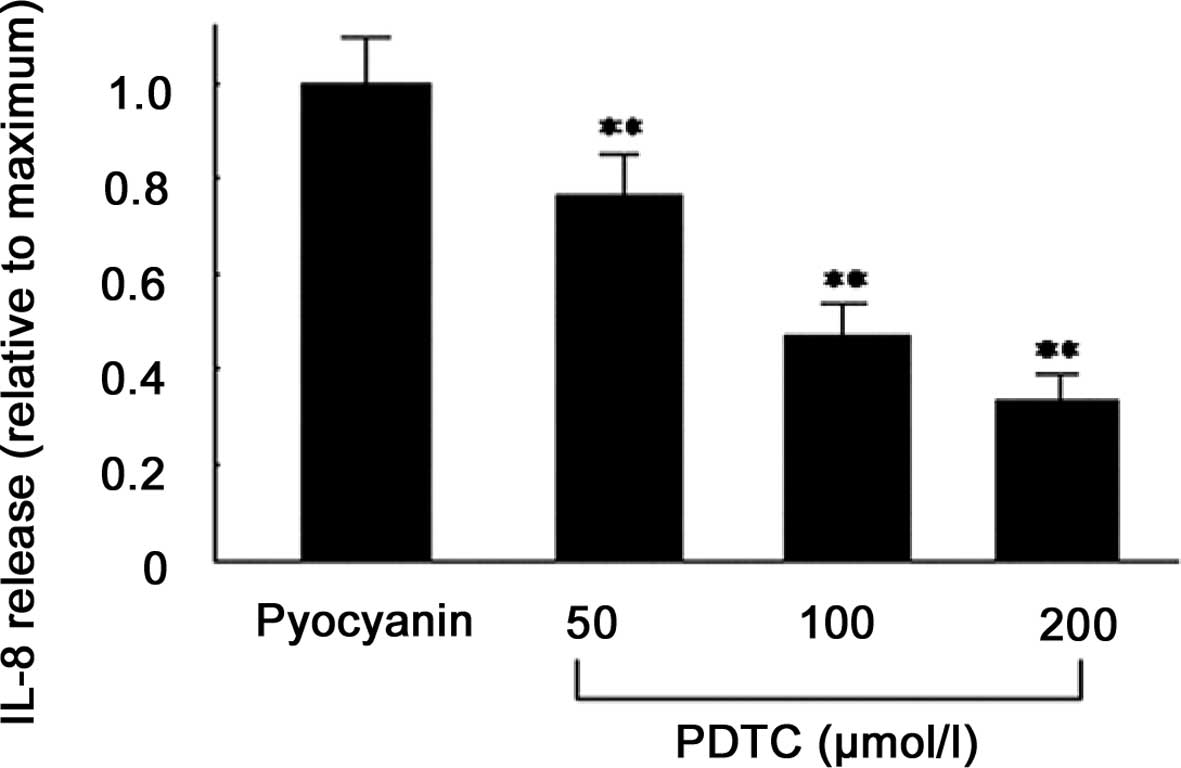
Effect of NF-κB inhibitors on
pyocyanin-induced IL-8 release
In order to further investigate which PKC downstream
pathway is involved in pyocyanin-induced IL-8 production, varying
concentrations of NF-κB blockers (PDTC; 50, 100 and 200 μmol/l)
were added in a fresh medium to PMA-differentiated U937 cells 60
min prior to the addition of pyocyanin. After 24 h, the
supernatants were collected and IL-8 concentrations were detected
by ELISA. The results demonstrated that PDTC significantly
decreased IL-8 secretion, and with increasing concentrations, IL-8
secretion decreased further, indicating that pyocyanin stimulated
PMA-differentiated U937 cells to express IL-8 cytokines via the
NF-κB signaling pathway (Fig.
4).
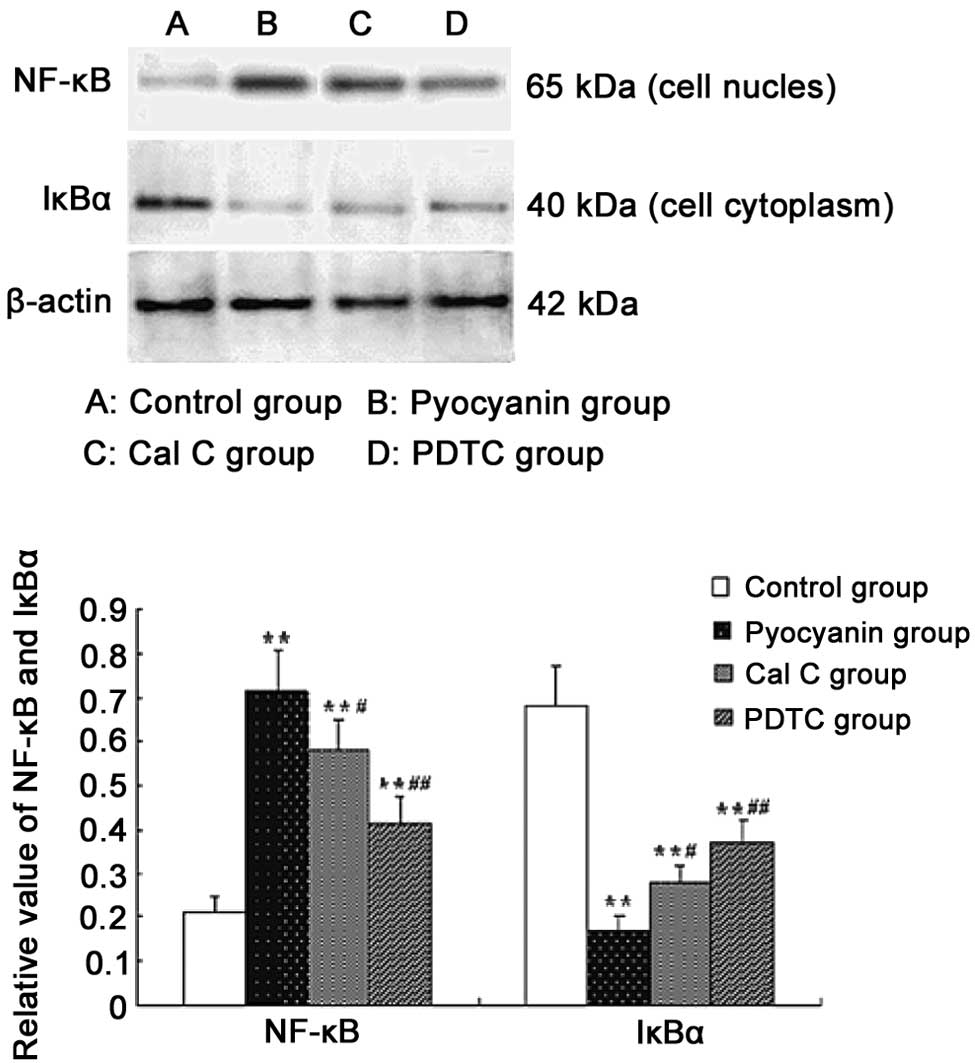
Effect of PKC and NF-κB inhibitors on
pyocyanin-induced expression of NF-κB protein
To establish whether the NF-κB signaling pathway
participates in pyocyanin-induced IL-8 expression in
PMA-differentiated U937 cells, we added pyocyanin (50 μM) to
stimulate PMA-differentiated U937 cells and tested the effects of
PKC and NF-κB inhibitors. For these experiments, the cells were
pretreated for 60 min with the PKC inhibitor, Cal C (100 nmol/l) or
NF-κB inhibitor, PDTC (100 μmol/l) and stimulated for 1 h with 50
μM pyocyanin; inhibitors were present throughout the experiments.
Cell protein was collected and the levels of protein for NF-κB and
IκB were assayed using western blotting, as described previously.
The results showed that protein expression of NF-κB was more marked
with the degradation of IκB after 1 h in PMA-differentiated
pyocyanin-induced U937 cells. The PKC inhibitor, Cal C, or NF-κB
inhibitor, PDTC, reduced the protein expression of NF-κB (Fig. 5). The data shown in Fig. 5 are representative of three
independent experiments.
Effect of PKC and NF-κB inhibitors on
pyocyanin-induced NF-κB activation
In order to establish whether the NF-κB signaling
pathway is involved in pyocyanin-induced IL-8 expression in
PMA-differentiated U937 cells, we added pyocyanin (50 μM) to
stimulate PMA-differentiated U937 cells and tested the effects of
PKC and NF-κB inhibitors. For these experiments, the cells were
pretreated for 60 min with the PKC inhibitor, Cal C (100 nmol/l) or
NF-κB inhibitor, PDTC (100 μmol/l), and subsequently stimulated for
1 h with 50 μM pyocyanin. The results of immunohistochemical
testing demonstrated that NF-κB exists in the cytoplasm and, under
normal conditions in its inactive form, is stained with
well-distributed light dye. Increased NF-κB activity with nuclear
localization was observed following stimulation with pyocyanin.
Furthermore, after PMA-differentiated U937 cells were cultured with
the PKC inhibitor, Cal C, or NF-κB inhibitor, PDTC for 60 min prior
to in vitro pyocyanin infection, a decrease in NF-κB nuclear
translocation was observed (Fig.
6).
 | Figure 6Effect of protein kinase C (PKC) and
nuclear factor (NF)-κB inhibitors on pyocyanin-induced NF-κB
activation. 3,3′-Diaminobenzidine dyeing was used (magnification,
×400). (A) Normal negative control group; (B) normal control group;
(C) pyocyanin group; (D) calphostin C (Cal C) group; (E)
pyrrolidine dithiocarbamate (PDTC) group. For these experiments,
cells were pretreated for 60 min with the PKC inhibitor, Cal C (100
nmol/l) or NF-κB inhibitor, PDTC (100 μmol/l) and subsequently
stimulated for 1 h with 50 μm pyocyanin. The results of the
immunohistochemical methods revealed that NF-κB exists in the
cytoplasm, which was stained with well-distributed light dye, in
its inactive form under normal conditions. Increased NF-κB activity
with nuclear localization was observed following stimulation with
pyocyanin. Furthermore, after phorbol 12-myristate
13-acetate-differentiated U937 cells were cultured together with
Cal C or PDTC, respectively, for 60 min prior to pyocyanin
infection in vitro, it was found that NF-κB nuclear
translocation decreased (A–D). Representative data of three
independent experiments are shown. |
Discussion
Macrophages are critical in development, physiology,
innate and adaptive immunity and in the pathogenesis of a variety
of infectious, immunological and degenerative disease processes.
They possess numerous specialized cellular functions, including
phagocytosis, chemotaxis, antibody-dependent cell cytotoxicity,
antigen presentation and the specific expression of a repertoire of
cytokines, chemokines and cell surface markers. Several myelocytic
cell lines have been established that, when differentiated with a
variety of agents, make the study of these diverse functions
tractable. The U937 cell line, established from a patient with
histiocytic lymphoma, has properties consistent with an immature
monocyte (21) and may be induced
by phorbol esters to undergo differentiation to a macrophage. Thus,
U937 cell lines are useful model systems for the study of
macrophage differentiation (22–24).
CXC chemokines, of which IL-8 is the prototype, act
predominantly on neutrophils and are activated in a variety of
disease states and are thus likely to play an important role in
P. aeruginosa infections. A variety of different factors are
capable of inducing the production of IL-8 (25,26),
of which the main substances include microbial lipopolysaccharide,
bacteria, viruses, cell factors, such as TNF-α, IL-1β and PMA, and
air pollution particles. Furthermore, UV-inactivated P.
aeruginosa are capable of inducing the production of IL-8 and
IL-6 by mononuclear cells (27).
However, the mechanism by which pyocyanin induces U937 cells to
produce IL-8 remains unknown. Therefore, in this study, we examined
the effect of pyocyanin on the release of IL-8 and the activation
of NF-κB in U937 human monocytes stimulated with pyocyanin.
Our results demonstrated that undifferentiated U937
cells are incapable of secreting IL-8, and PMA-differentiated U937
cells are capable of secreting a small quantity of IL-8, but
pyocyanin is capable of inducing IL-8 mRNA expression and IL-8
protein secretion in a concentration- and time-dependent manner in
PMA-differentiated U937 cells, indicating that pyocyanin may induce
inflammation.
The previously discussed results suggest that PMA
provides U937 cells with the ability to produce IL-8, which
demonstrates that mononuclear cells with a degree of
differentiation may affect the production of IL-8. This indicates
that the pyocyanin itself is an important stimulant for
inflammation. However, its mechanism is unclear. Based on a review
of current published literature (4,13,14),
the majority of studies only observe inflammation of P.
aeruginosa bacterial products in epithelial cells and
macrophages, and the lack of inflammatory response signal
transduction mechanism in U937 cells. This study approaches PKC and
NF-κB signal transduction and further explores the pyocyanin
mechanism.
PKC is a family of protein kinase enzymes that are
involved in controlling the function of other proteins through the
phosphorylation of the hydroxyl groups of serine and threonine
amino acid residues on these proteins. The majority of unstimulated
PKC is located in the cell cytosol and, upon stimulation,
translocates to the plasma membrane (28). The basic signal transduction
pathway involves the allosteric activation of PKC by the
intracellular messengers, diacylglycerol and calcium ions. We used
pyocyanin to stimulate PMA-differentiated U937 cells and
subsequently detected IL-8 expression. The result revealed that
pyocyanin may induce IL-8 synthesis in a time- and
concentration-dependent manner, PKC may decrease IL-8 secretion,
and with increasing concentrations of Cal C, IL-8 secretion
decreased, indicating that pyocyanin may stimulate
PMA-differentiated U937 cells to express IL-8 cytokines via the PKC
signaling pathways.
The downstream target proteins of PKC, which have
the most significant function, are the IκB and mitogen-activated
protein kinase, and phosphorylated IκB rapidly decomposes and
releases small NF-κB proteins that act as nuclear transcription
factors for a variety of cells. Therefore, PKC activity directly
affects NF-κB activity, which is important in the transcriptional
regulation of target cells as nuclear transcription factors
(29).
We used pyocyanin to stimulate PMA-differentiated
U937 cells and subsequently detected IL-8 expression. The results
demonstrated that PDTC significantly decreased the secretion of
IL-8. With increasing concentrations of PDTC, IL-8 secretion
decreased, indicating that pyocyanin may stimulate
PMA-differentiated U937 cells to express IL-8 cytokines through the
NF-κB signaling pathways. We also observed the NF-κB p65 nuclear
translocation by using immunohistochemical methods. Additionally,
quantitative analysis was performed on the cytoplasm and nuclear
p65 by the application of western blotting. The experimental
results agree with the classic electrophoretic mobility shift assay
test results, which may be used to reflect the degree of NF-κB
activation (21).
The result of western blotting indicated that
pyocyanin was also capable of significantly promoting the
phosphorylation of IκBα and activation of NF-κB following 60 min of
infection with pyocyanin. Pretreatment of U937 cells with specific
inhibitors of NF-κB (PDTC) and PKC (Cal C) prior to infection with
pyocyanin demonstrated that PDTC and Cal C are capable of
decreasing NF-κB activation. The inhibitory role of Cal C was weak
compared with PDTC. This result demonstrated that other signaling
pathways, excluding the PKC pathway, may still exist.
Immunocytochemistry results revealed that NF-κB
exists in the cytoplasm, stained with well-distributed light dye,
in an inactive form under normal conditions. Increased NF-κB
activity with nuclear localization was observed following
stimulation with pyocyanin. Furthermore, after PMA differentiated
U937 cells were cultured together with the PKC inhibitor Cal C or
NF-κB inhibitor PDTC, respectively, for 60 min prior to the in
vitro infection with P. aeruginosa, NF-κB nuclear
translocation was observed.
In conclusion, we observed that pyocyanin infects
U937 cells in a concentration- and time-dependent manner and is
capable of inducing PMA-differentiated U937 cells to produce IL-8
by activating the PKC and NF-κB signaling pathways. This indicates
that pyocyanin from Pseudomonas has an important role in the
inflammation reaction. Further studies focused on understanding the
interaction between PKC and other cytokine regulators are required.
Knowledge of the mechanisms by which pyocyanin induces
PMA-differentiated U937 cells to produce IL-8 may provide an
improved understanding and further rational approaches for the
control of pyocyanin-induced inflammatory processes.
Acknowledgements
The authors would like to acknowledge the technical
advice and assistance of Dr Rong Jian Su, Dr Hong Xin Wang and Mr.
Zhi Hong Zong. Partial funding for this study was provided by the
Department of Science and Technology, Liaoning Province (no.
201102126). This study was also partially funded by a grant from
Liaoning Medical College.
References
|
1
|
Aloush V, Navon-Venezia S, Seigman-Igra Y,
Cabili S and Carmeli Y: Multidrug-resistant Pseudomonas
aeruginosa: risk factors and clinical impact. Antimicrob Agents
Chemother. 50:43–48. 2006.
|
|
2
|
Tam VH, Chang KT, Abdelraouf K, et al:
Prevalence, resistance mechanisms, and susceptibility of
multidrug-resistant bloodstream isolates of Pseudomonas
aeruginosa. Antimicrob Agents Chemother. 54:1160–1164. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sadikot RT, Blackwell TS, Christman JW and
Prince AS: Pathogen-host interactions in Pseudomonas
aeruginosa pneumonia. Am J Respir Crit Care Med. 171:1209–1223.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vinckx T, Wei Q, Matthijs S and Cornelis
P: The Pseudomonas aeruginosa oxidative stress regulator
OxyR influences production of pyocyanin and rhamnolipids:
protective role of pyocyanin. Microbiology. 156:678–686. 2010.
|
|
5
|
Look DC, Stoll LL, Romig SA, Humlicek A,
Britigan BE and Denning GM: Pyocyanin and its precursor
phenazine-1-carboxylic acid increase IL-8 and intercellular
adhesion molecule-1 expression in human airway epithelial cells by
oxidant-dependent mechanisms. J Immunol. 175:4017–4023. 2005.
View Article : Google Scholar
|
|
6
|
O’Malley YQ, Abdalla MY, McCormick ML,
Reszka KJ, Denning GM and Britigan BE: Subcellular localization of
Pseudomonas pyocyanin cytotoxicity in human lung epithelial
cells. Am J Physiol Lung Cell Mol Physiol. 284:L420–L430. 2003.
|
|
7
|
O’Malley YQ, Reszka KJ, Rasmussen GT,
Abdalla MY, Denning GM and Britigan BE: The Pseudomonas
secretory product pyocyanin inhibits catalase activity in human
lung epithelial cells. Am J Physiol Lung Cell Mol Physiol.
285:L1077–L1086. 2003.PubMed/NCBI
|
|
8
|
Prince LR, Bianchi SM, Vaughan KM, et al:
Subversion of a lysosomal pathway regulating neutrophil apoptosis
by a major bacterial toxin, pyocyanin. J Immunol. 180:3502–3511.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bianchi SM, Prince LR, McPhillips K, et
al: Impairment of apoptotic cell engulfment by pyocyanin, a toxic
metabolite of Pseudomonas aeruginosa. Am J Respir Crit Care
Med. 177:35–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farberman MM, Ibricevic A, Joseph TD, et
al: Effect of polarized release of CXC-chemokines from wild-type
and cystic fibrosis murine airway epithelial cells. Am J Respir
Cell Mol Biol. 45:221–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mackay CR: Chemokines: immunology’s high
impact factors. Nat Immunol. 2:95–101. 2001.
|
|
12
|
Power CA and Proudfoot AE: The chemokine
system: novel broad-spectrum therapeutic targets. Curr Opin
Pharmacol. 1:417–424. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan NY, Hui WS, Tipoe GL, et al:
Inhibition of pyocyanin-potentiated IL-8 release by steroids in
bronchial epithelial cells. Respir Med. 100:1614–1622. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rada B, Gardina P, Myers TG and Leto TL:
Reactive oxygen species mediate inflammatory cytokine release and
EGFR-dependent mucin secretion in airway epithelial cells exposed
to Pseudomonas pyocyanin. Mucosal Immunol. 4:158–171. 2011.
View Article : Google Scholar
|
|
15
|
Elswaifi SF, Palmieri JR, Hockey KS and
Rzigalinski BA: Antioxidant nanoparticles for control of infectious
disease. Infect Disord Drug Targets. 9:445–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chomczynski P: A reagent for the
single-step simultaneous isolation of RNA, DNA and proteins from
cell and tissue samples. Biotechniques. 15:532–537. 1993.PubMed/NCBI
|
|
17
|
Maceyka M, Payne SG, Milstien S and
Spiegel S: Sphingosine kinase, sphingosine-1-phosphate, and
apoptosis. Biochim Biophys Acta. 1585:193–201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shidham VB, Qi D, Rao RN, et al: Improved
immunohistochemical evaluation of micrometastases in sentinel lymph
nodes of cutaneous melanoma with ‘MCW melanoma cocktail’ - a
mixture of monoclonal antibodies to MART-1, Melan-A, and
tyrosinase. BMC Cancer. 3:152003.PubMed/NCBI
|
|
19
|
Lauredo IT, Sabater JR, Ahmed A,
Botvinnikova Y and Abraham WM: Mechanism of pyocyanin- and
1-hydroxyphenazine-induced lung neutrophilia in sheep airways. J
Appl Physiol. 85:2298–2304. 1998.PubMed/NCBI
|
|
20
|
Denning GM, Iyer SS, Reszka KJ, O’Malley
Y, Rasmussen GT and Britigan BE: Phenazine-1-carboxylic acid, a
secondary metabolite of Pseudomonas aeruginosa, alters
expression of immunomodulatory proteins by human airway epithelial
cells. Am J Physiol Lung Cell Mol Physiol. 285:L584–L592.
2003.PubMed/NCBI
|
|
21
|
Sundström C and Nilsson K: Establishment
and characterization of a human histiocytic lymphoma cell line
(U-937). Int J Cancer. 17:565–577. 1976.PubMed/NCBI
|
|
22
|
Huang ZL and Failla ML: Copper deficiency
suppresses effector activities of differentiated U937 cells. J
Nutr. 130:1536–1542. 2000.PubMed/NCBI
|
|
23
|
Harris P and Ralph P: Human leukemic
models of myelomonocytic development: a review of the HL-60 and
U937 cell lines. J Leukocyte Biol. 37:407–422. 1985.PubMed/NCBI
|
|
24
|
Hewison M, Brennan A, Singh-Ranger R,
Walters JC, Katz DR and O’Riordan JL: The comparative role of
1,25-dihydroxycholecalciferol and phorbol esters in the
differentiation of the U937 cell line. Immunology. 77:304–311.
1992.PubMed/NCBI
|
|
25
|
Delgado MA, Poschet JF and Deretic V:
Nonclassical pathway of Pseudomonas aeruginosa DNA-induced
interleukin-8 secretion in cystic fibrosis airway epithelial cells.
Infect Immun. 74:2975–2984. 2006.PubMed/NCBI
|
|
26
|
Venza I, Cucinotta M, Visalli M, De Grazia
G, Oliva S and Teti D: Pseudomonas aeruginosa induces
interleukin-8 (IL-8) gene expression in human conjunctiva through
the recruitment of both RelA and CCAAT/enhancer-binding protein
beta to the IL-8 promoter. J Biol Chem. 284:4191–4199. 2009.
View Article : Google Scholar
|
|
27
|
Hessle CC, Andersson B and Wold AE:
Gram-positive and Gram-negative bacteria elicit different patterns
of pro-inflammatory cytokines in human monocytes. Cytokine.
30:311–318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Steinberg SF: Structural basis of protein
kinase C isoform function. Physiol Rev. 88:1341–1378. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tak PP and Firestein GS: NF-kappaB: a key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|