Introduction
Nerve repair and regeneration of the peripheral
nerve following injury, is a long and complex biological process,
and is not able to be surgically treated. The immune response
induced by peripheral nerve injury inhibits nerve repair and
regeneration (1). The strength of
the local immune response is proportional to the severity of the
nerve injury. The more severe the nerve injury the stronger the
immune response, which in turn, results in a decreased ability of
nerve regeneration and functional recovery (2,3).
Immunosuppressants, such as FK506, have been widely used for axon
regeneration (4–9). However, FK506 is associated with the
side effect of nervous system disorder. Varying dosages of
immunosuppressants may result in multifocal cerebrospinal
meningitis, optic neuritis, inflammation of the spinal nerve root,
tremors and discoordination (10,11).
Patients who have received an organ transplant also exhibit other
mild side effects such as insomnia, tremors, headaches and
photophobia, or more apparent side effects, such as clouding of
consciousness, epileptic seizures, comas and dysarthroses (5,12).
Studies have shown that natural immunosuppressive
drugs have fewer side effects and more complex underlying
mechanisms (13–15). Their mechanism of action is related
to the immune system and the regulatory neuroendocrine-immune
network (16). Previous, studies
concerning traditional Chinese medicines with immunosuppressive
potential for the purpose of a high benefit-risk ratio have located
novel candidates (13,14,17,18),
one of which is 7,8-dihydroxycoumarin. 7,8-Dihydroxycoumarin is an
effective monomer isolated from Thymelaeaceae Daphne plants,
with a molecular formula of
C9H6O4(18). 7,8-Dihydroxycoumarin is involved in
cleaning necrosis-facilitating substances, balancing water
electrolytes, neurotransmitter and energy metabolism, and the
secretion of neurotrophic factor, thus it is involved in the
functional recovery of neurons (18,19).
7,8-Dihydroxycoumarin exhibited certain protective and inhibitive
effects on the cardiovascular system, and is capable of passing
through the blood brain barrier (18–20).
Following injury to the peripheral nerve, Wallerian degeneration
occurs at the distal end of the injured nerve, and retrograde
degeneration is observed at the proximate end (21,22).
The key to controlling the degenerative process is to suppress the
immune inflammatory response (1)
as contaminant inflammation in nerve injury not only affects nerve
repair and regeneration, but also leads to pathological
neuralgia.
Nuclear factor (NF)-κB, a nucleoprotein factor is
capable of binding to a specific κB enhancer sequence of the
immunoglobulin κ light chain gene, and has a wide range of
expression in the nervous system (23,24).
NF-κB is able to regulate immune and inflammatory responses, as
well as the activity, plasticity and neuropathic pain of neurons
following injury (25). Brambilla
et al(26) demonstrated
that the immunosuppression of NF-κB in astrocytes may alleviate the
inflammatory response following spinal cord injury and promote the
functional recovery of the spinal cord. However, to the best of our
knowledge, there have been no studies regarding the effect of
7,8-dihydroxycoumarin on the expression of NF-κB in peripheral
nerve regeneration following injury.
In this study, the sciatic nerve injury BALB/c mouse
model was treated with 7,8-dihydroxycoumarin, in order to observe
the expression of NF-κB in spinal motor neurons and the effects of
7,8-dihydroxycoumarin on peripheral nerve regeneration following
injury.
Materials and methods
Materials and animals
7,8-Dihydroxycoumarin powder (purity, 95.5%;
Fig. 1) was purchased from Xidian
Pharmaceutical Co., Ltd. (Changchun, China). The powder was
dissolved in 0.9% saline solution, sterilized through a membrane
filter (Φ 0.2 μm) and stored at −20°C for later use. In total 160
healthy adult male BALB/c mice (weight, 20±2 g) were donated by the
Laboratory Animal Center of the Fundamental Medical College of
Jilin University (Changchun, China) and were raised at room
temperature with a routine diet and access to water ad
libitum. The mice were randomized into control (treated with
physiological saline), high-dose, medium-dose and low-dose groups,
respectively, with 40 mice per group. The animal experimental
protocols were approved by the Jilin University Laboratory Animal
Ethnics Committee (27).
Animal modeling
All BALB/c mice were anesthetized by intraperitoneal
injection of 1% sodium thiopental with a dosage of 100 mg/kg body
weight. Mice were fixed in the prone position and 1.5
cm-longitudinal incisions were aseptically prepared on the
unilateral rear thigh. The subcutaneous tissues were separated and
the infrapiriformis muscle underwent blunt dissection to expose the
cord of sciatic nerves. The sciatic nerve cords were carefully
separated from the surrounding tissues using blunt glass needles.
Subsequently, the sciatic nerve cords were interrupted at 0.3 cm
below the ischial tuberosity and were microsurgically anastomosed
by 11-0 microsutures (Sharpoint™ MicroSutures; Angiotech
Pharmaceuticals Inc., Vancouver, BC, Canada.), with the aid of a
microscope (magnification, ×12;. OLYMPUS SZX16; Olympus Corp. OCN,
Beijing, China), to close the muscle and skin.
Drug intervention
7,8-Dihydroxycoumarin was dissolved in physiological
saline and administered to the mice intragastrically (ig). The ig
dose was determined by equivalent conversion on the basis of the
clinical dose of 7,8-dihydroxycoumarin (28) and this dose value served as the
medium dose. The high and low doses were determined by geometric
progression. The final high-, medium- and low-doses were 16, 8 and
4 g/kg/day, respectively. Physiological saline of an equivalent
volume served as the control. The injections were administered
daily for seven continuous days.
Sciatic functional index (SFI) test
At four and eight weeks following injury, the
walking gait of each group was observed and analyzed and the SFI
was calculated. The specification of the box was 5cm × 5cm × 8 cm.
A cage with a unilateral door was placed at the remote end of the
box and a piece of white paper, the same length and width as the
box, was placed beneath. The mice were placed into the near end of
the box after their pelma had been dyed with ink, and they were
encouraged to move towards the remote end by tapping the box.
Approximately 6–7 mouse footprints were marked on the paper. The
footprints of the injured foot (E) and normal foot (N) were
recorded and the following three indicators were measured: Podogram
length (PL), the longest distance of footprints from the heel to
toe; the width between the first and fifth toes (TW), the ligature
distance from the first toe to the fifth toe, i.e. the ligature
distance was the same as the width; and the inter-toe distance
(IT)-the ligature distance from the second toe to the fourth toe.
The data group with the largest numerical value was selected for
use. The three variables were placed into the following formula and
SFI was calculated:
SFI=−38.3(EPL−NPL)/NPL+109.5(ETW−NTW)/NTW+13.3(EIT−NIT)/NIT−8.8.
SFI=0 refers to normal and SFI=−100 refers to a complete nerve
injury.
Spinal cord sampling
Five mice from each group were anesthetized by
intraperitoneal injection of 1% sodium thiopental with a dosage of
100 mg/kg body weight at predetermined times (12 h, 24 h, three
days, five days, seven days, two weeks, four weeks and eight weeks
following surgery). The canalis vertebralis was exposed via
a midline incision to the posterior vertebral column. The L4–6
spinal cord segment connected to the injured sciatic nerves was
dissected intact, dissociated and removed. Subsequently the tissue
samples were stored in liquid nitrogen for use in
quantitative-polymerase chain reaction (qPCR) and western blot
analysis.
Five mice from each group were selected and
individually fixed in the supine position. A perfusion needle was
placed to the aortic root by the left ventricular cardiac apex.
Rapid perfusion of 50–100 ml physiological saline was performed to
wash the blood. As the outflow from the auricula dextra
became limpid, perfusion was performed with 300–400 ml fresh 4%
paraformaldehyde in phosphate-buffered saline (PBS). The perfusion
lasted 30 min for tissue fixation. Following the completion of the
perfusion, the canalis vertebralis was exposed. L4–6 spinal
cord segments connected with the injured sciatic nerves were
dissected, dissociated and removed. These tissues were stored until
the terminal deoxynucleotidyl-transferase-mediated dUTP nick-end
labeling (TUNEL) assay was performed.
Western blot analysis detects NF-κB
protein
Tissue samples stored in liquid nitrogen were
removed quickly and ground with a pestle and mortar. Cells were
lysed in an ice-cold radioimmunoprecipitation assay lysis buffer
(Beyotime, Nanjing, China) for 15 min. The proteins were separated
on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
gels and electroblotted onto a polyvinylidine fluoride (PDVF) film.
The PDVF film was incubated in rabbit anti-mouse NF-κB antibodies
(diluted 1,000 times with PBS containing 1% bovine serum albumin;
Beyotime) overnight at 4°C and rinsed four times with 0.01 mol/l
PBS, for five min per rinse. The color was developed using a
western blotting 3,3′-diaminobenzidine tetrahydrochloride (DAB)
testing kit (Beyotime). X-ray film exposure was performed and the
samples were scanned and analyzed.
qPCR analysis
NF-κB and reduced glyceraldehyde-phosphate
dehydrogenase (GAPDH) primers were designed by Beacon Designer 7
software (Premier Biosoft Int., Palo Alto, CA, USA) and synthesized
by Sangon Biotech (Shanghai, China). Primer sequences are shown in
Table I. Total RNA was extracted
from tissue samples using TRIzol. cDNA was cloned by reverse
transcription using total RNA templates. The NF-κB gene was
detected by qPCR using cDNA templates. GAPDH served as an inner
control for each reaction system. The reaction conditions were as
follows: 95°C for 30 sec; 58°C for 60 sec and 72°C for 60 sec, for
a total of 40 cycles. Subsequently, the relative mRNA content of
NF-κB/GAPDH was determined.
 | Table INF-κB and GAPDH primer sequences. |
Table I
NF-κB and GAPDH primer sequences.
| Primer name | Sequence (5′-3′) |
|---|
| NF-κB-S |
GCAAAGGAAACGCCAGAAGC |
| NF-κB-A |
CACTACCGAACATGCCTCCAC |
| NF-κB-probe |
CGCTCCACTGCCGCCACCGAAG |
| GAPDH-S |
AATGTGTCCGTCGTGGATCTG |
| GAPDH-A |
CAACCTGGTCCTCAGTGTAGC |
| GAPDH-probe |
CGTGCCGCCTGGAGAAACCTGCC |
Apoptosis of neurons (TUNEL method)
The tissues of the L4–6 spinal cord segment were
routinely prepared into 3-μm thick, paraffin-embedded slices
(20). The specimen slices on the
glass slides were immersed in a 4% paraformaldehyde/PBS solution
for 15 min, incubated in 20 μg/ml proteinase K solution for 10 min
at room temperature, immersed in 4% paraformaldehyde/PBS solution
for a further 5 min and then incubated in the balancing saline
solution for 10 min. The TUNEL reaction solution (29) was added dropwise onto the
specimens, a cover slip was added to cover the specimens, followed
by the reaction in a wet box in the dark at 37°C for 1 h. Specimens
were immersed in 2X saline sodium citrate solution for 15 min.
Specimens were incubated in 0.3% H2O2 for 15
min. A DAB mixture was added for 10 min for color development.
Subsequently, specimens were rinsed with deionized water,
dehydrated with gradient ethanol (1 min at 50, 70, 85, 95 and 100%
respectively), hyalinized with xylene twice, for 1 min each time,
and sealed with neutral resin. Five fields were randomly selected
for each slide and the apoptotic cells were observed.
Statistical analysis
SPSS 10.0 statistical software (SPSS Inc., Chicago,
IL, USA) was used for statistical analysis and Student’s t-test was
conducted for comparison. Data are presented as the mean ± SD.
P<0.05 was considered to indicate a statistically significant
difference.
Results
SFI test
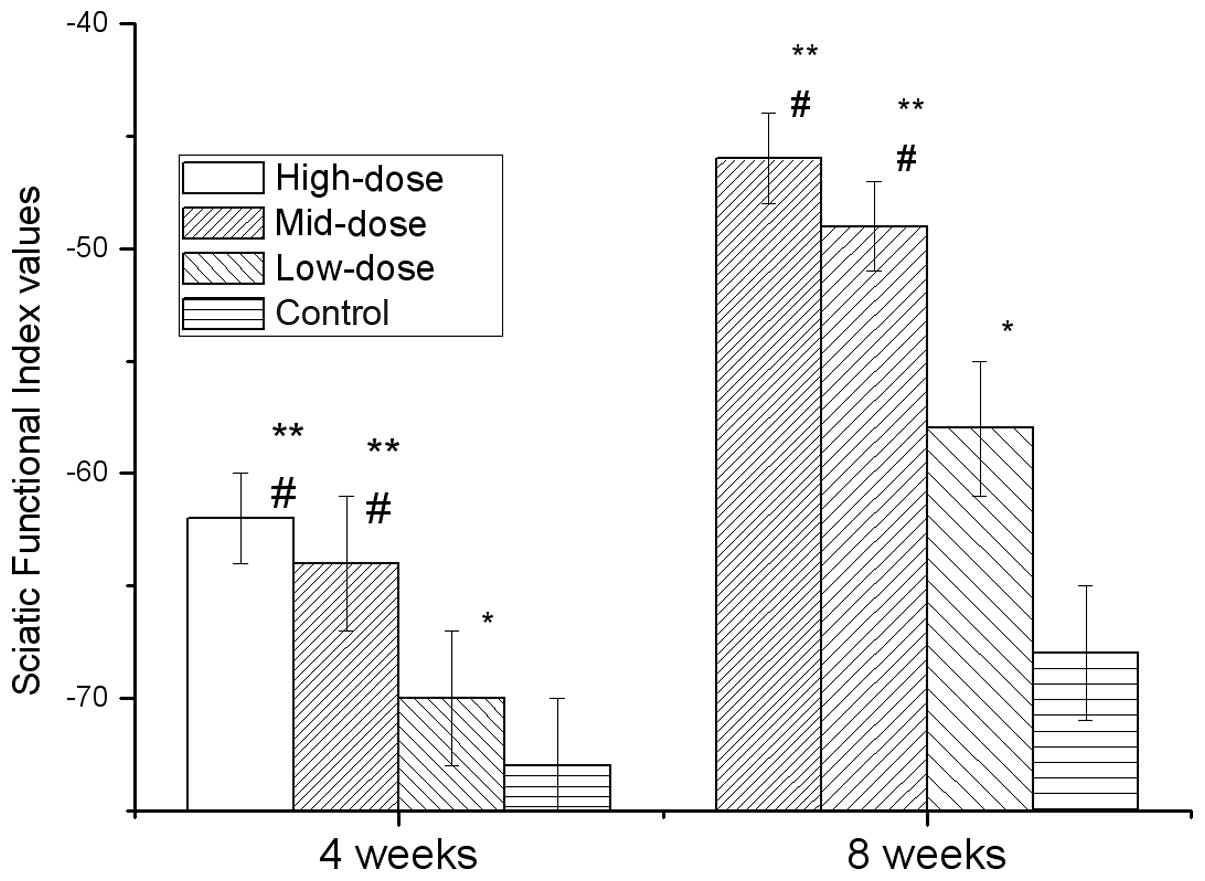
Fig. 2 shows the
SFI of each group at two time points, four and eight weeks, after
modeling. The high-, mid- and low-dose groups exhibited significant
differences compared with the control (P<0.05). The high- and
mid-dose groups exhibited significant differences compared with the
low-dose group (P<0.05), but the high-dose group was not
significantly different compared with the mid-dose group. These
results revealed that the application of 7,8-dihydroxycoumarin
improved the SFI following injury to the sciatic nerve.
Western blot analysis
Fig. 3 shows the
results of the western blot analysis. Grayscale analysis (Table II) demonstrated that the level of
NF-κB protein in each group increased between 12 and 24 h following
injury to the sciatic nerve, then the expression levels of NF-κB in
the high-dose group decreased to a normal level in one week and
expression levels of NF-κB in the medium- and low-dose groups
decreased to normal levels in two and four weeks, respectively.
However, the NF-κB levels in the control group remained elevated
after eight weeks. The expression levels of NF-κB between the
post-surgical times of 12 h, 24 h, three days, five days and one
week in each experimental group exhibited statistically significant
differences (P<0.05). NF-κB expression levels in the high- and
medium-dose groups were significantly inhibited compared with the
control group (P<0.01).
 | Table IIRelative grayscale of NF-κB/GAPDH
blots in different groups (n=5). |
Table II
Relative grayscale of NF-κB/GAPDH
blots in different groups (n=5).
| Time |
|---|
|
|
|---|
| Group | 12 h | 1 day | 3 days | 5 days | 1 week | 2 weeks | 4 weeks | 8 weeks |
|---|
| High-dose |
0.01±0.03a |
0.27±0.00a |
0.68±0.03a |
1.21±0.02b |
0.53±0.02a |
0.18±0.03a |
0.09±0.03a |
0.03±0.01b |
| Mid-dose |
0.01±0.01a |
0.39±0.01a |
0.79±0.03a | 1.33±0.02 |
0.62±0.04a |
0.66±0.03b |
0.16±0.01b |
0.03±0.01b |
| Low-dose |
0.01±0.02a |
0.49±0.01a |
1.11±0.02b | 1.60±0.02 |
1.89±0.02b |
0.67±0.04b | 0.29±0.03 |
0.03±0.01b |
| Model control | 1.25±0.01 | 2.21±0.01 | 2.01±0.02 | 1.74±0.24 | 3.30±0.03 |
1.02±0.03b | 0.23±0.02 | 0.07±0.02 |
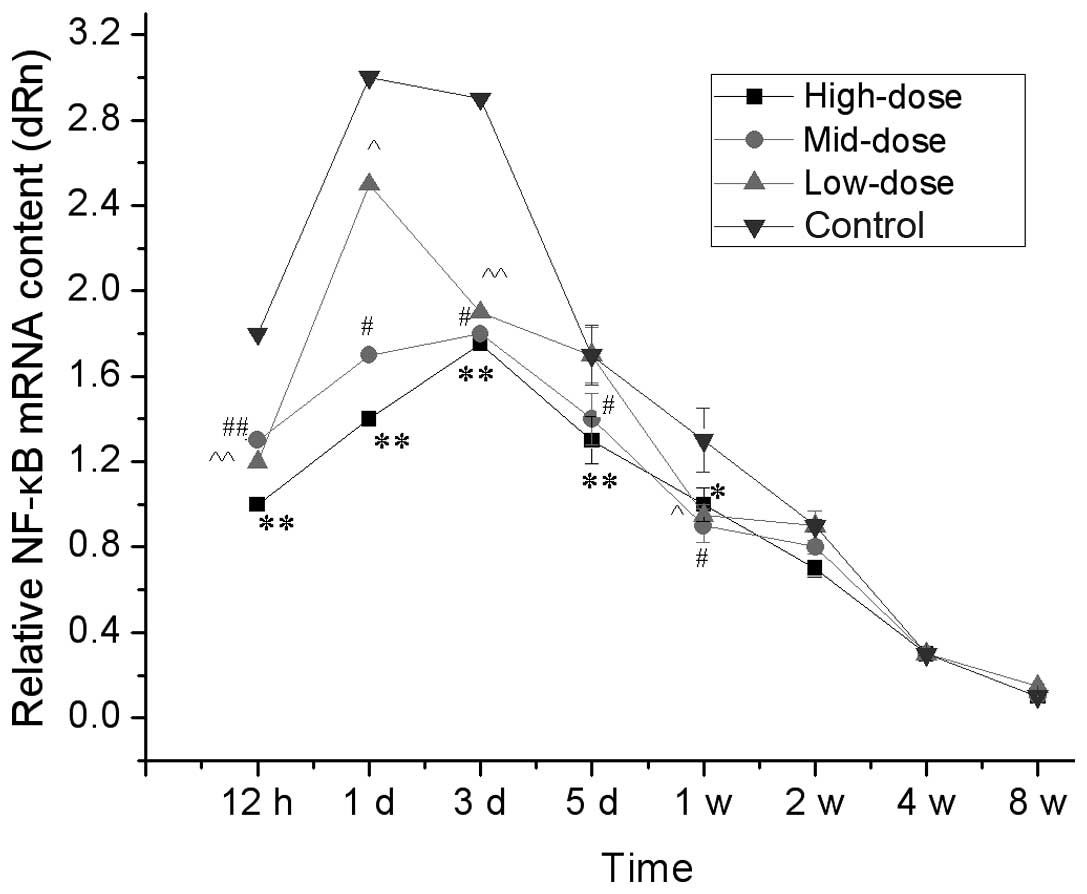
qPCR analysis
qPCR analysis of relative NF-κB mRNA in the spinal
cord are shown in Fig. 4. Between
12 to 24 h following injury to the sciatic nerve, NF-κB mRNA
contents in the spinal cord segments connected with the injured
sciatic nerve were increased in the short term and the increments
in the high- and medium-dose groups were significantly less than
those in the low-dose and control groups (P<0.05). After one
week, the NF-κB mRNA content was not significantly different
between the groups. These results demonstrated that the high- and
medium-dose of 7,8-dihydroxycoumarin suppressed the expression of
NF-κB.
TUNEL detects the apoptosis of
neurons
The results of neuron apoptosis following TUNEL
staining are shown in Table III.
The cell count of neuron apoptosis peaked at one week following
injury and decreased at two, four and eight weeks. The apoptotic
cell count of the high- and mid-dose groups at each time point was
significantly lower than that of the low-dose and control groups
(P<0.05).
 | Table IIIApoptosis cell count of neurons
(n=5). |
Table III
Apoptosis cell count of neurons
(n=5).
| Time (weeks) |
|---|
|
|
|---|
| Group | 1 | 2 | 4 | 8 |
|---|
| High-dose | 6.61±0.32a | 3.33±0.21a | 2.76±0.20a | 2.08±0.18a |
| Mid-dose | 6.82±0.43a | 4.18±0.32a | 3.91±0.30a | 3.02±0.20a |
| Low-dose | 9.72±0.31 | 8.18±0.32 | 6.18±0.32 | 5.18±0.32 |
| Model control | 10.11±0.03 | 8.86±0.02 | 7.12±0.01 | 6.31±0.02 |
Discussion
In this study, 7,8-dihydroxycoumarin was injected
into the BACB/c mice following sciatic nerve injury, and the mice
were tracked and tested for 8 weeks. The western-blot analysis and
qPCR analysis results respectively revealed that the expression
levels of NF-κB protein in the L4–6 spinal cord segment of the
high- and mid-dose groups were lower than that of the low-dose and
control groups. The SFI test results demonstrated that the nerve
function index increased during the regeneration of injured nerves;
the SFIs of the high- and mid-dose groups were significantly higher
than that of the low-dose and control groups, and the SFI of the
low-dose group was significantly higher than that of the control
group. This indicates that 7,8-dihydroxycoumarin promotes the
functional recovery of injured nerves (18,19).
The low-dose group was incapable of significantly inhibiting NF-κB
protein expression (it was not significantly different when
compared with the control group), but significantly promoted the
functional recovery of injured nerves. These results suggested that
the application of 7,8-dihydroxycoumarin following nerve injury
promotes nerve regeneration and functional recovery (18,19).
Following the continuous injection of
7,8-dihydroxycoumarin for eight weeks, the expression levels of
NF-κB in the high- and mid-dose groups were not significantly
different compared with the low-dose and control groups, yet the
SFI, which refers to the degree of recovery following nerve injury,
exhibited significant differences. These results suggest that the
nerve repair and regeneration process of high- and mid-dose groups
are completed after eight weeks, thus the NF-κB expression levels
were gradually reduced, to approximately that of the low-dose and
control groups. Thus, at eight weeks the neural function appeared
to have been well recovered. The neuron apoptosis count of results
following TUNEL staining reveal that the neuron apoptosis count of
each group peaks one week after injury and declines at two, four
and eight weeks. The apoptotic cell count of the high-dose group at
each point was significantly lower than that of the low-dose and
control groups.
NF-κB is a key nuclear transcription factor that
exists in eukaryotes and is present in the cytoplasm as inactive
homologous or heterologous dimers, which form complexes with its
inhibitory proteins, IκBs (25).
Following different types of internal and external stimuli (certain
cytokines, growth factors, immunity receptors, ischemia, anoxia and
injury), NF-κB dissociates from IκBs and is rapidly transferred
into the nucleus where it binds to the target gene promoter or
enhancer κB motif to modulate the synthesis of the mRNA of target
genes. This results in the participation of NF-κB in various
biological processes, such as immune response, inflammation,
apoptosis and cell proliferation (27). NF-κB has multiple regulatory
actions on gene transcription, such as binding to specific
sequences of the immunoglobulin gene κ-light chain enhancer κB, it
participates in inflammatory nerve lesions, pathologic nerve pain
and functional change that inhibits axonal regeneration and
promotes neuronal apoptosis following nerve injury (25). Protecting neuronal soma and
avoiding irreversible degeneration are prerequisites for successful
regeneration following nerve injury to aid nerve regeneration by
promoting injured neuron survival and inhibiting neuron
degeneration (25,26). In the present study, the potential
nerve repair accelerator, 7,8-dihydroxycoumarin, was used. The
application of 7,8-dihydroxycoumarin following injury to the
peripheral nerve in mice was capable of restraining the expression
of NF-κB protein in a dose-dependent manner. The results of the SFI
test demonstrated that the nerve function recovery with
7,8-dihydroxycoumarin was markedly improved compared with the
control group. Therefore, it is hypothesized that
7,8-dihydroxycoumarin reduces neuron cell apoptosis in the course
of repair and regeneration by inhibiting NF-κB expression in L4–6
spinal cord neurons following nerve injury, this provides favorable
conditions for nerve repair and regeneration and is protective in
nerve regeneration following injury.
The present study has certain shortcomings. The
local immune response following nerve injury has not yet been
tested. Furthermore, further studies are required regarding the
cell signaling pathways related to the local inflammatory reaction
following nerve injury. In conclusion, 7,8-dihydroxycoumarin is a
potential drug for use in peripheral nerve repair and
regeneration.
Acknowledgements
The study was funded by the Jilin Provincial Science
& Technology Development (Project no. 20100751).
References
|
1
|
Iwatsuki K, Arai T, Ota H, Kato S, Natsume
T, Kurimoto S, Yamamoto M and Hirata H: Targeting anti-inflammatory
treatment can ameliorate injury-induced neuropathic pain. PLoS One.
8:e577212013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Camara-Lemarroy CR, Gonzalez-Moreno EI,
Guzman-de la Garza FJ and Fernandez-Garza NE: Arachidonic acid
derivatives and their role in peripheral nerve degeneration and
regeneration. ScientificWolrdJournal. 2012:1689532012.PubMed/NCBI
|
|
3
|
Stern S, Sinske D and Knöll B: Serum
response factor modulates neuron survival during peripheral axon
injury. J Neuroinflammation. 9:782012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Que J, Cao Q, Sui T, Du S, Kong D and Cao
X: Effect of FK506 in reducing scar formation by inducing
fibroblast apoptosis after sciatic nerve injury in rats. Cell Death
Dis. 4:e5262013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Azizi S, Mohammadi R, Amini K and Fallah
R: Effects of topically administered FK506 on sciatic nerve
regeneration and reinnervation after vein graft repair of short
nerve gaps. Neurosurg Focus. 32:E52012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan Y, Sun HH, Hunter DA, Mackinnon SE and
Johnson PJ: Efficacy of short-term FK506 administration on
accelerating nerve regeneration. Neurorehabil Neural Repair.
26:570–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Wang W, Wei G, Wang G, Zhang W and
Ma X: Immunophilin FK506 loaded in chitosan guide promotes
peripheral nerve regeneration. Biotechnol Lett. 32:1333–1337. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang MS and Gold BG: FK506 increase the
regeneration of spinal cord axons in a predegenerated peripheral
nerve autograft. J Spinal Cord Med. 22:287–296. 1999.PubMed/NCBI
|
|
9
|
Jost SC, Doolabh VB, Mackinnon SE, Lee M
and Hunter D: Acceleration of peripheral nerve regeneration
following FK506 administration. Restor Neurol Neurosci. 17:39–44.
2000.PubMed/NCBI
|
|
10
|
Phan DQ and Schuind F: Tolerance and
effects of FK506 (tacrolimus) on nerve regeneration: a pilot study.
J Hand Surg Eur Vol. 37:537–543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jifeng H, Dezhong L, Qiongjiao Y, Huayong
Z and Shipu L: Evaluation of PRGD/FK506/NGF conduits for peripheral
nerve regeneration in rats. Neurol India. 58:384–391. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rustemeyer J and Dicke U: Allografting
combined with systemic FK506 produces greater functional recovery
than conduit implantation in a rat model of sciatic nerve injury. J
Reconstr Microsurg. 26:123–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sibilia J and Griscelli C: Immunotherapy
of inflammatory diseases, 2005. Expert Opin Biol Ther. (Suppl 1):
S1–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng BR, Shen JP, Zhuang HF, Lin SY, Shen
YP and Zhou YH: Treatment of severe aplastic anemia by
immunosuppressor anti-lymphocyte globulin/anti-thymus globulin as
the chief medicine in combination with Chinese drugs. Chin J Integr
Med. 15:145–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue B, Jiao J, Zhang L, Li KR, Gong YT,
Xie JX and Wang XM: Triptolide upregulates NGF synthesis in rat
astrocyte cultures. Neurochem Res. 32:1113–1119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang G: Trends of research on immunology
of Chinese materia medica. Zhongguo Chong Xi Yi Jie He Za Zhi.
19:259–260. 1999.(In Chinese).
|
|
17
|
Cao J, Niu Z, Wang Y, Jiang Y, et al:
Immune reactions and nerve repair in mice with sciatic nerve injury
14 days after intraperitoneal injection of Brazil. Neural Regen
Res. 7:675–679. 2012.PubMed/NCBI
|
|
18
|
Yan L, Zhou X, Zhou X, Zhang Z and Luo HM:
Neurotrophic effects of 7,8-dihydroxycoumarin in primary cultured
rat cortical neurons. Neurosci Bull. 28:493–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du J, Zhao Q, Zhang Y, Wang Y and Ma M:
7,8-dihydroxycoumarin improves neurological function in a mouse
model of sciatic nerve injury. Neural Regen Res. 7:445–450.
2012.PubMed/NCBI
|
|
20
|
Ojala T, Remes S, Haansuu P, Vuorela H,
Hiltunen R, Haahtela K and Vuorela P: Antimicrobial activity of
some coumarin containing herbal plants growing in Finland. J
Ethnopharmacol. 73:299–305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang CH, Wang B, Wendu RL, Bi HE, Cao GF,
Ji C, Jiang Q and Yao J: Protective role of Wallerian degeneration
slow (Wlds) gene against retinal ganglion cell body damage in a
Wallerian degeneration model. Exp Ther Med. 5:621–625.
2013.PubMed/NCBI
|
|
22
|
Li M, Guo W, Zhang P, Li H, Gu X and Yao
D: Signal flow and pathways in response to early Wallerian
degeneration after rat sciatic nerve injury. Neurosci Lett.
536:56–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun SC, Ganchi PA, Ballard DW and Greene
WC: NF-kappa B controls expression of inhibitor I kappa B alpha:
evidence for an inducible autoregulatory pathway. Science.
259:1912–1915. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen F, Castranova V and Shi X: New
insights into the role of nuclear factor-kappa B in cell growth
regulation. Am J Pathol. 159:387–397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meffert MK, Chang JM, Wiltgen BJ, Fanselow
MS and Baltimore D: NF-kappa B functions in synaptic signaling and
behavior. Nat Neurosci. 6:1072–1078. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brambilla R, Bracchi-Ricard V, Hu WH,
Frydel B, Bramwell A, Karmally S, Green EJ and Bethea JR:
Inhibition of astroglial nuclear factor kappaB reduces inflammation
and improves functional recovery after spinal cord injury. J Exp
Med. 202:145–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
The Ministry of Science and Technology of
the People’s Republic of China. Guidance suggestions for the care
and use of Laboratory Animals. 2006.
|
|
28
|
Huang JH, Huang XZ, Chen ZY, Zheng QS and
Sun RY: Dose conversion among different animals and healthy
volunteers in pharmacological study. Chin J Clin Pharmacol
Therapeut. 9:1069–1072. 2004.(In Chinese).
|
|
29
|
Chen G, Gong M, Yan M and Zhang X:
Sevoflurane induces endoplasmic reticulum stress mediated apoptosis
in hippocampal neurons of aging rats. PLoS One. 8:e578702013.
View Article : Google Scholar : PubMed/NCBI
|