Introduction
Dendritic cells (DCs) are the most important antigen
presenting cells, their predominant feature is the ability to
stimulate the proliferation of naive T cells and they are also
important in the immune response. Mature DCs express high levels of
cell surface class II major histocompatibility complex (MHC-II) and
co-stimulatory molecules. On account of their capability of
presenting alloantigens to T cells, DCs stimulate T cell
proliferation to induce an immune response. In contrast, immature
dendritic cells (imDCs), characterized by low expression of both
MHC-II antigens and co-stimulatory molecules, can be instrumental
in the induction of peripheral tolerance. Previous studies have
shown that the capacity of DCs to modulate immune responses relates
to their state of functional maturation (1–3).
Immature dendritic cells (imDCs) are able to capture and process
antigens. Studies have previously indicated that imDCs induce
peripheral tolerance via T cell anergy, immune deviation, promotion
of activated T cell apoptosis and formation of regulatory T cells
(Tregs) (2–6).
To date, studies have shown that nuclear factor-κB
(NF-κB) is important in DC maturation and tolerance induction
(7–9). Moreover, NF-κB activation requires
the action of multiple kinases (7,10),
including IKK2, which has been shown to be essential for DC
maturation. Recipient or donor bone marrow-derived DCs transfected
with IKK2dn to block NF-κB have been observed to prevent DC
maturation (11,12). In addition, recombinant
adenovirus-mediated IKK2dn (Adv-IKK2dn)-DCs prolonged the survival
time of kidney transplants in rats by inducing Treg generation
(11–13). In addition, a number of
observations indicated that donor-derived imDCs transfected with
IKK2dn induced the formation of a unique population of
CD4+CD25− Tregs. These cells are capable of
potently inhibiting naive and activated T cell responses in
vitro and inducing prolongation of kidney allograft survival
in vivo(11,13).
Our previous study demonstrated that
recipient-derived DCs transfected with Adv-IKK2dn inhibit NF-κB
activation and impair DC maturation. In addition, an
Adv-IKK2dn-DC-treated group was demonstrated to exhibit markedly
prolonged renal allograft survival (12). Furthermore, recipient-derived imDCs
transfected by Adv-IKK2dn were shown to generate
CD4+CD25− T cells, which exert immune
tolerance in vitro(14,15).
In the current study, Adv-IKK2dn-CD4+CD25− T
cells were administered to Lewis (LW) rats and their ability to
induce anti-allotolerance in a rat renal transplantation model was
investigated. The results demonstrated that
Adv-IKK2dn-CD4+CD25− T cells may prolong
renal allograft survival in a donor-specific manner and this was
hypothesized to result from high expression levels of interleukin
(Il)-10 and TGF-β.
Materials and methods
Animals and reagents
Male LW (CrlBR), Brown Norway (BN/CrlBR) and Wistar
(WI/CrlBR) rats, 8–10-weeks-old and ~180–200 g, were purchased from
Vital River Laboratories (Beijing, China) and maintained in the
Soochow University animal facility. Procedures involving animals
and their care were conducted in accordance with the institutional
guidelines that were in compliance with Regulations for the
Administration of Affairs Concerning Experimental Animals and
Measures of Jiangsu Province on Administration of Affairs
Concerning Experimental Animals. The recombinant rat granulocyte
macrophage-colony stimulating factor and recombinant rat Il-4 were
purchased from Peprotech, Inc. (Rocky Hill, NJ, USA). Il-2, Il-10,
transforming growth factor-β (TGF-β), interferon-γ (IFN-γ) and
enzyme-linked immunosorbent assay kits were purchased from R&D
Systems (Minneapolis, MN, USA). The CD4+CD25−
Treg isolation kit and MiniMACS separator were purchased from
Miltenyi Biotech (Bergisch Gladbach, Germany). The
replication-deficient Adv encoding a kinase-defective dominant
negative form of human IKK2 plasmid, pACCMVpLpASR(+)-IKK2dn, was
provided by Dr Rain D Martin (University of Vienna, Vienna,
Austria). PAdxsi-GFP-IKK2dn and pAdxsi-GFP-0 were constructed by
SinoGenoMax Co., Ltd (Beijing, China).
DCs transfected with Adv-IKK2dn and
loaded with BN antigen
Bone marrow-derived DCs (from LW rats) were obtained
as described previously (12,14,15).
Cells were harvested at day five of culture and transfected with
Adv-IKK2dn or an empty adenovirus (Adv-0) at a multiplicity of
infection of 50 (12). Cells were
subsequently cultured for a further two days and pulsed with BN
antigens. BN spleen cell lysate (antigen) was prepared by 6
repetitions of freezing (5 min in a dry ice-ethanol bath) and
thawing (10 min in a 37°C bath) and added at a ratio of 1:5,
DC:Spleen cells (used to prepare lysate) for the final 48 h of DC
culture (Adv-IKK2dn-DC loaded with BN antigen). Subsequently, cells
were harvested and used as stimulators for the mixed lymphocyte
reaction.
Primary mixed lymphocyte reaction (MLR)
and separated T cells
Following 48 h, cells were harvested and used as
stimulators for the primary MLR, and the LW spleen T cells were
used as responders. The DC:T cell ratio was 1:100. Cultures were
prepared in triplicate in 24-well round-bottom microculture plates
(200 μl/well with 1×106 T cells) and maintained for 72 h
in 5% CO2 at 37°C. Uninfected and Adv-0-DC groups served
as controls. Following 72 h T cells were separated by
magnetic-activated cell sorting (Miltenyi Biotec, Bergisch
Gladbach, Germany). Separation was achieved by removing
CD4+ T cells using an LD separation column (composed of
ferromagnetic spheres covered with a cell-friendly coating) by
negative selection. CD25 expression was measured by flow cytometry
(Beckman-Coulter, Fullerton, CA, USA). The
CD4+CD25+ T cells were then removed by
positive selection using an MS separation column (necessary for the
isolation of cells which are only minimally labeled with MACS
MicroBeads, while leaving enough epitopes free for concurrent
antibody staining), which yielded CD4+CD25− T
cells. Following this, CD4+CD25− T cells were
collected for subsequent experiments.
Renal transplantation
Renal transplantation was performed as previously
described (16). Male BN and LW
rats were used as donors and recipients, respectively. LW rats were
administered with an intravenous injection of naive CD4+
T cells (CD4+ T cell group), Adv0-DC-generated
CD4+CD25− T cells
(Adv-0-CD4+CD25− T cell group),
Adv-IKK2dn-DC-generated CD4+CD25− T cells
(Adv-IKK2dn-CD4+CD25− T cell group) or an
equal volume of normal saline (control group) seven days prior to
allotransplantation. In the third party donor group (Wistar donor
group) Wistar rats as donors were treated the same as the
Adv-IKK2dn-CD4+CD25−T cell group prior to
transplantation. Following transplantation, the survival time of
recipients was observed, the T lymphocyte proliferation in
recipients was measured, the levels of serum Il-2, Il-10, IFN-γ and
TGF-β were detected, and the serum creatinine levels were
monitored.
Statistical analysis
Data are presented as the mean ± SD and were
analyzed by one-way analysis of variance. Survival curves were
established using the Kaplan-Meier method. Graft survival between
groups of transplanted animals was analyzed with a log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
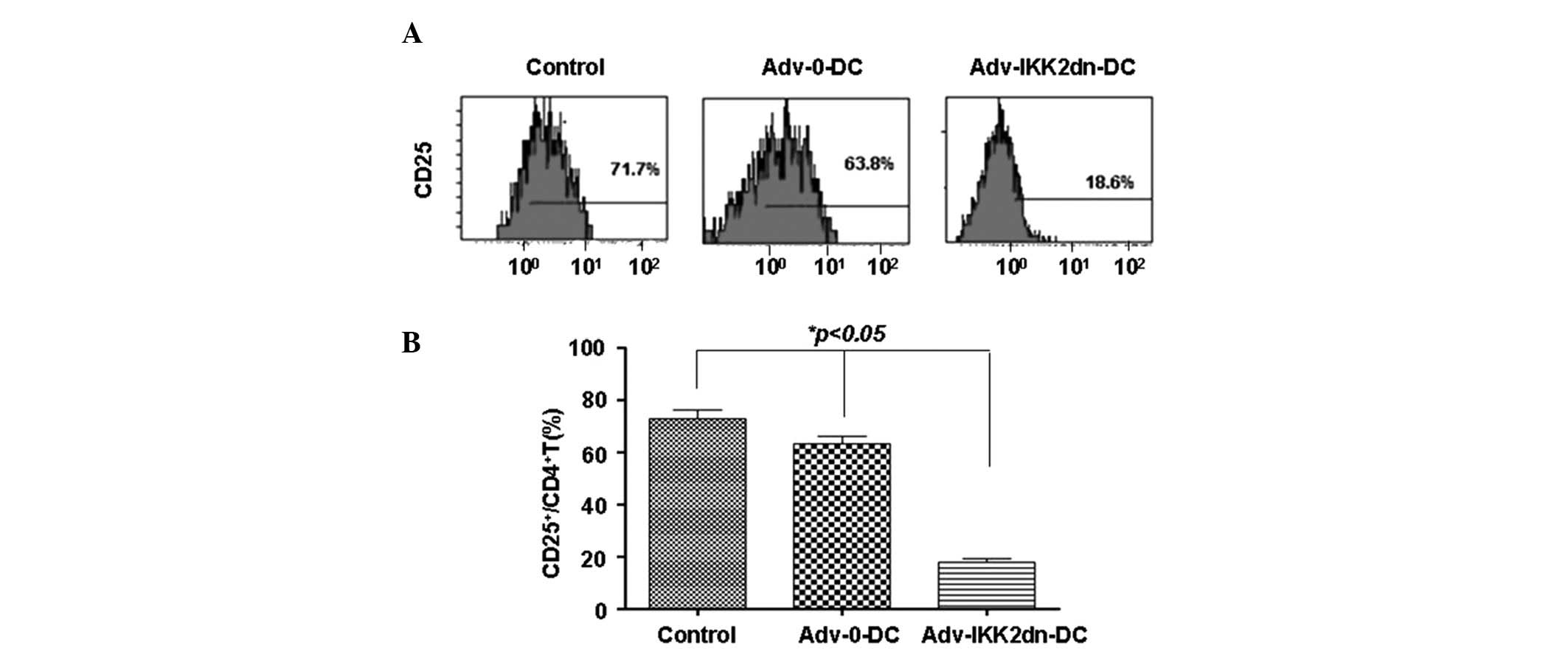
Adv-IKK2dn-DC induced Tregs
CD4+ T cells were isolated by the
negative selection method and CD25 expression was analyzed using
flow cytometry. Results showed that the Adv-IKK2dn-DC group
contained a markedly lower percentage of CD25 (19.1±4.8%, n=6),
compared with the control (77.2±4.8%, n=6) and Adv-0-DC (63.9±2.9%,
n=6) groups; the difference was statistically significant
(P<0.05; Fig. 1). The results
indicted that the majority of CD4+ T cells were
CD25− upon completion of MLR with Adv-IKK2dn-DC. These
observations indicate that
Adv-IKK2dn-CD4+CD25− T cells may be
distinguished from CD4+CD25+T cells, which
express high levels of CD25 (17).
Prolonged kidney graft survival in
Adv-IKK2dn-CD4+CD25− T cell-treated rats
To investigate whether
Adv-IKK2dn-CD4+CD25− T cells exhibited an
immunoregulatory function in vivo, 1×107
CD4+T cells, Adv-0-CD4+CD25− T
cells, Adv-IKK2dn-CD4+CD25− T cells or an
equal volume of normal saline were intravenously infused in LW rats
seven days prior to kidney transplantation. No immunosuppressive
therapy was administered prior to or following transplantation. Rat
survival was monitored daily following transplantation.
Results indicated that allograft survival in the
Adv-IKK2dn-CD4+CD25− T cell-treated group was
prolonged significantly in comparison with the CD4+ T
cell, control, Adv-0-CD4+CD25− T cells and WI
donor groups (Fig. 2). In
addition, compared with the control group (7.2±0.31 days), the
survival time in the WI donor group was not prolonged (7.2±0.48
days; P>0.05; Table I).
Previous results indicated that IKK2dn-transfected DCs are capable
of inducing tolerance and significantly prolonged transplanted
allograft survival (12). The
present results supported the hypothesis that imDCs generate or
activate Tregs (CD4+CD25− T cells) which are
important in the induction and maintenance of immune tolerance. Rat
numbers and survival time in each group are presented in Table I.
 | Table IRat groupings and individual survival
time of kidney transplanted rats. |
Table I
Rat groupings and individual survival
time of kidney transplanted rats.
| Group no. | Group | No. of rats | Survival time, days
(no. of rats) | Mean survival time,
days |
|---|
| 1 | Control | 6 | 6, 7 (3), 8
(2) | 7.2±0.31 |
| 2 | CD4+ T
cells | 7 | 9, 10, 12, 13, 15,
16, 19 | 13.4±1.33a |
| 3 |
Adv-0-CD4+CD25− T
cells | 6 | 4 (2), 5 (3),
6 | 4.8±0.31b |
| 4 |
Adv-IKK2dn-CD4+CD25−
T cells | 8 | 21 (2), 24, 29 (2),
30, 33, 41 | 8.5±2.36a,b,c |
| 5 | Wistar donor | 6 | 6 (2), 7 (2), 8,
9 | 7.2±0.48b |
Adv-IKK2dn-CD4+CD25− T cells act via the
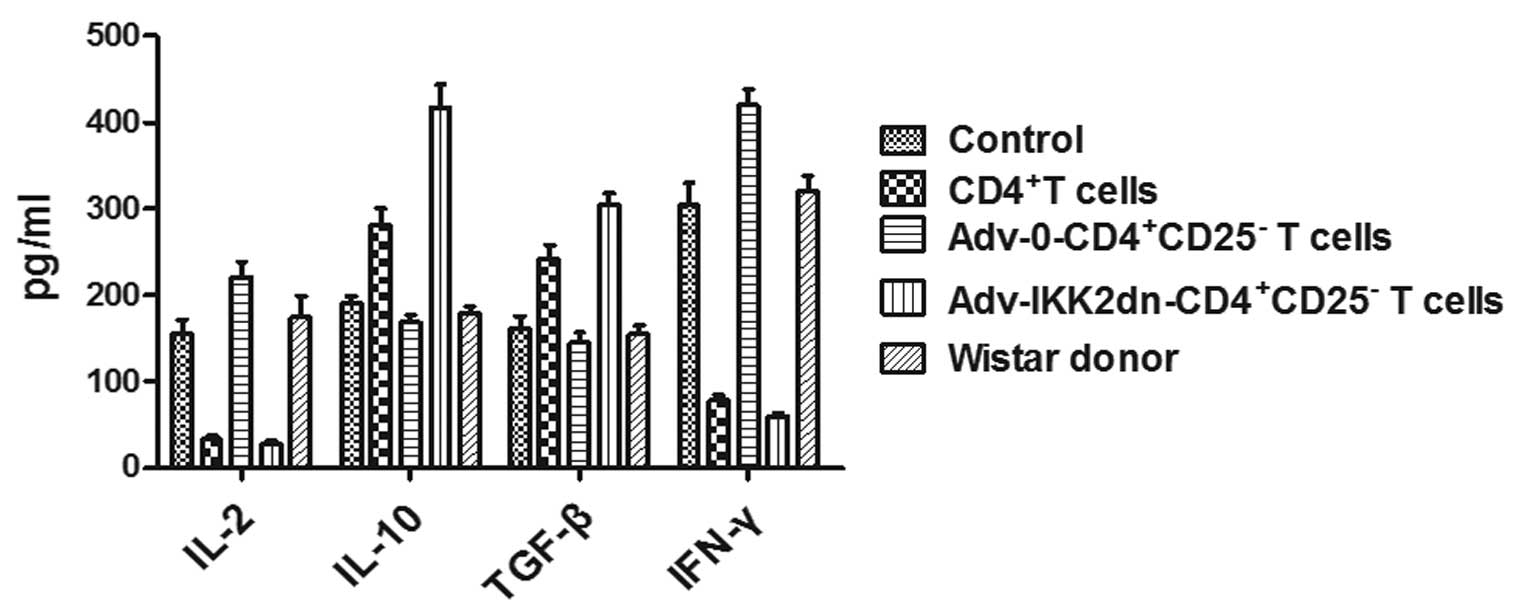
release of cytokines
To detect the mechanism by which
Adv-IKK2dn-CD4+CD25− T cells significantly
prolonged transplanted allograft survival, the serum levels of
Il-2, IFN-γ, TGF-β and Il-10 were tested in different groups on
days 5 and 14 following renal transplantation. On day 5, in
control, Adv-0-CD4+CD25− T cell and the WI
donor kidney transplanted groups, Il-2 and IFN-γ levels were
significantly increased compared with the
Adv-IKK2dn-CD4+CD25− T cell-treated group and
CD4+ T cell-treated group (Fig. 3). Notably, Il-10 and TGF-β
production were significantly higher in the
Adv-IKK2dn-CD4+CD25− T cell and
CD4+ T cell groups compared with the control,
Adv-0-CD4+CD25− T cell and the WI donor
groups, respectively (P<0.05 or P<0.01). Furthermore, the
levels of TGF-β and Il-10 were significantly different between the
Adv-IKK2dn-CD4+CD25− T cell and
CD4+ T cell groups (Fig.
3; P<0.05).
On postoperative day 14 (Fig. 4), the production of Il-2 and IFN-γ
was markedly increased in the CD4+ T cell group,
compared with the Adv-IKK2dn-CD4+CD25− T cell
group, the differences were statistically significant (P<0.05).
In addition, the levels of Il-10 and TGF-β decreased in the
Adv-IKK2dn-CD4+CD25− T cell group (Fig. 4), compared with the CD4+
T cell group, the difference between the two groups was
statistically significant (P<0.05). Thus,
Adv-IKK2dn-CD4+CD25− T cell treatment reduced
Il-2 and IFN-γ production and increased Il-10 and TGF-β secretion
in the serum of allo-kidney transplanted rats. It also indicated
that Adv-IKK2dn-CD4+CD25− T cells
significantly prolonged transplanted allograft survival by
suppressing the anti-allograft T helper (Th) 1 immune response and
enhancing the Th2 response in vivo.
Serum creatinine levels
Following transplantation, serum creatinine levels
were measured on days 5 and 14. On day 5, the serum creatinine
level was markedly increased in the control,
Adv-0-CD4+CD25− T cell and WI donor groups.
However, in Adv-IKK2dn-CD4+CD25− T
cell-treated and CD4+ T cell-treated groups, serum
creatine remained at a low level (Fig.
5). On day 14, the serum creatinine level was markedly elevated
in the CD4+ T cell-treated group. However, the
Adv-IKK2dn-CD4+CD25− T cell-treated group
remained at a low level (Fig. 5).
There were statistically significant differences between these two
groups (P<0.01). Thus,
Adv-IKK2dn-CD4+CD25− T cells are important in
maintaining stable renal function. These observations indicate that
Adv-IKK2dn-CD4+CD25− T cells may extend the
length of rat renal allograft survival.
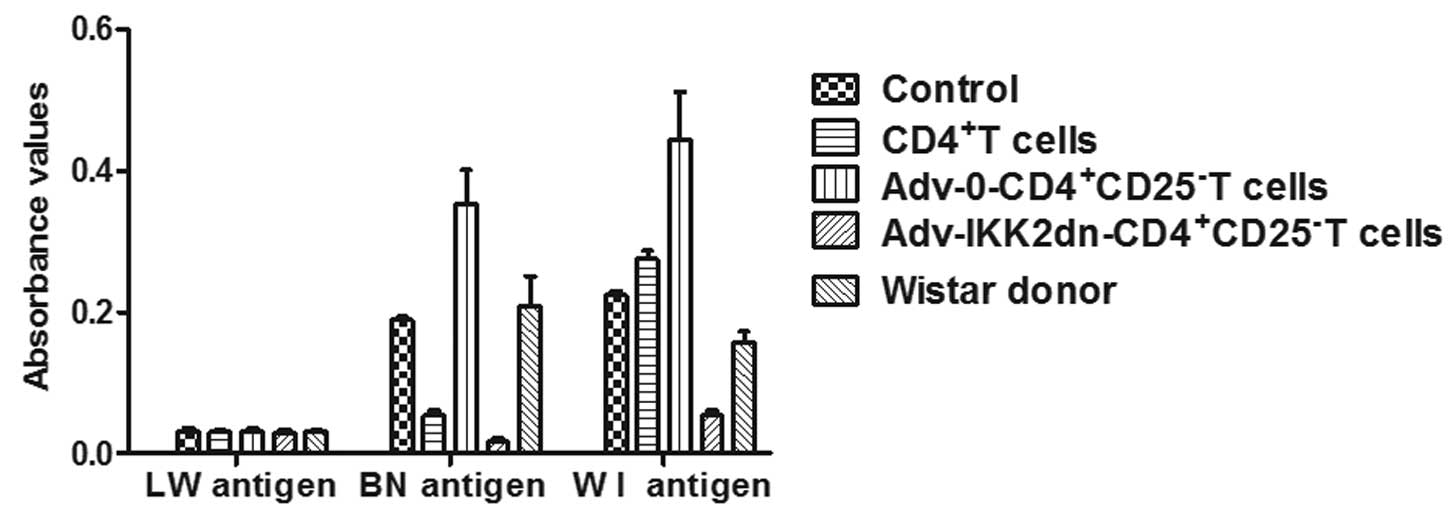
Co-culture MLR
A three-day MLR was performed with syngeneic (LW) or
allogeneic (alloantigen-specific BN or third-party WI) irradiated
splenocytes (antigen) and T cells obtained from the lymph nodes of
rats that had undergone transplantation following different
treatments. T cells in each group remained in a low response state
to the LW antigen stimulation. T lymphocyte proliferation
stimulated by the BN antigen in the
Adv-IKK2dn-CD4+CD25− T cell group was
significantly lower compared with that in the control,
Adv-0-CD4+CD25− T cells and the WI donor
groups (Fig. 6; P<0.01).
Compared with the control group, the ability of T lymphocyte
proliferation in the WI donor group was not reduced (Fig. 6; P>0.05). Each group maintained
a high T cell proliferation response to the WI antigen stimulation
(Fig. 6). The results indicate
that Adv-IKK2dn-CD4+CD25− T cells are capable
of suppressing the proliferative responses of naive syngeneic T
cells towards donor-specific BN antigens.
Discussion
imDCs are hypothesized to generate or activate Tregs
(6,18–20).
Tregs are important in the induction and maintenance of immune
tolerance (17,21–23).
However, the mechanisms underlying their ability to suppress
immunity remain to be fully defined and are commonly disputed. The
current study demonstrated that regulatory cells generated by
Adv-IKK2dn-DC are a unique population of
CD4+CD25− T cells, unlike the
CD4+CD25+ Tregs. A previous study observed
that imDCs may form a Treg subset that is different from
CD4+CD25+T Tregs in vitro. These cells
are termed type 1 T regulatory cells (Tr1). At variance with
CD4+CD25+Treg cells, Tr1 cells exert
suppressor activity cytokine-independently, but are mainly
dependent on direct cell-cell contact and through the T cell
receptor to activate inhibitory cells and the membrane surface
molecule CTLA-4 is important role in this process (24). A previous study indicated that
imDCs transfected by IKK2dn induced the
CD4+CD25− Tregs and these cells were capable
of inducing prolongation of kidney allograft survival in
vivo(13).
In the present study, recipient DCs transfected by
IKK2dn were observed to guide naive T cells to differentiate into
CD4+CD25− Tregs
(Adv-IKK2dn-CD4+CD25− T cells) in
vitro. Notably, Adv-IKK2dn-CD4+CD25− T
cells administered in vivo to syngeneic naive recipient rats
prolonged the survival of LW kidney allograft (Fig. 2; Table
I), without the requirement for immunosuppression. However,
Adv-IKK2dn-CD4+CD25− T cells exhibited no
effect on the survival of a third-party (WI) kidney allograft
(Fig. 2; Table I). Co-culture MLR was used to
investigate whether the suppression effector function of
Adv-IKK2dn-CD4+CD25− T cells is
antigen-specific (Fig. 6). T cells
from Adv-IKK2dn-CD4+CD25− T cell-treated
transplanted rats were unresponsive to donor allo-antigens (BN) and
partially responsive to third-party (WI) antigens (Fig. 6). Therefore, the regulation of
suppression by Adv-IKK2dn-CD4+CD25− T cells
was hypothesized to be antigen-specific. These results are
concordant with the hypothesis of Aiello et al(13).
It is also important to determine the mechanisms
underlying the suppressive function of
Adv-IKK2dn-CD4+CD25− T cells. There are two
possible types of suppression mechanisms by which Tregs regulate
the immune system, via cell contact or cytokine and/or other
soluble factors, including TGF-β and Il-10 (25–27).
It has been established that Tregs secrete two suppressive
cytokines, Il-10 and TGF-β, and may also secrete Il-4. Il-10
inhibits Th1 cells and Th1 type factor proliferation (28), particularly IFN-γ synthesis. TGF-β
inhibits immune molecules, macrophage activation and the Th1-type
inflammatory response (29). In
the current study, Il-2, IFN-γ, TGF-β and Il-10 serum levels in
different groups were investigated on days 5 and 14. In vivo
studies indicated that the
Adv-IKK2dn-CD4+CD25− T cell-treated group
exhibited significantly reduced Il-2 and IFN-γ production and
increased Il-10 and TGF-β expression in the serum of allo-kidney
transplanted rats (Figs. 3 and
4). These observations indicated
that Adv-IKK2dn-CD4+CD25− T cells prolong
renal allograft survival and it is hypothesized that this occurs
due to high expression levels of Il-10 and TGF-β, which is
concordant with previous studies (30–31).
In addition to the cytokine pathway, it has been hypothesized that
DnIKK2-Tregs expressing high levels of inducible nitric oxide
synthase are immunoregulatory (13).
In organ transplantation immunity, Th1 cells induce
acute rejection, causing graft inactivation, while Th2 cells have a
protective effect on the graft (32). The Th1-type factors, Il-2 and
IFN-γ, increase the risk of rejection by promoting the immune
response between the recipient and the transplanted kidney. Type
Th2 factors, particularly Il-4 and Il-10 may inhibit the
transplantation immune response and promote the formation of
tolerance between the recipient and the transplanted kidney.
Previous studies have shown that large amounts of effect cytokines
are detected in the acute rejection of transplanted organs, while
immune tolerance of transplanted organs mainly produces regulatory
cytokines (33,34). The current study demonstrated that
renal transplanted rats in the
Adv-IKK2dn-CD4+CD25− T cell group did not
survive long-term. It was hypothesized that the reason for this was
due to a time-dependent decrease in the Th2 cytokine secretion of
Adv-IKK2dn-CD4+CD25− T cells, leading to
increased Th1 cytokine secretion and finally inducing rejection.
These observations indicate that the inhibitory effect may be
mediated by the release of soluble mediators.
The results of the present study indicate that
recipient-derived imDCs transfected by IKK2dn induced
CD4+CD25− T cells prolong renal allograft
survival, secrete high levels of cytokine Il-10 and TGF-β.
Adv-IKK2dn-CD4+CD25− T cells inhibited the
transformation of recipient T cells to effector T cells and
promoted differentiation into Th2 and Th3 cells. The results
indicate that Tregs are important in immune regulation and act by
releasing regulatory cytokines. The present study revealed
mechanisms underlying the involvement of DCs transfected by IKK2dn
in inducing immune tolerance. The current study hypothesizes a
clinical use for recipient DCs in cadaveric renal transplantation
to induce tolerance.
Acknowledgements
This study was supported by grants from the
National Natural Science Fund Projects of China (grant no.
81070591).
References
|
1
|
van Duivenvoorde LM, van Mierlo GJ,
Boonman ZF and Toes RE: Dendritic cells: vehicles for tolerance
induction and prevention of autoimmune diseases. Immunobiology.
211:627–632. 2006.PubMed/NCBI
|
|
2
|
Moser M: Dendritic cells in immunity and
tolerance-do they display opposite functions? Immunity. 19:5–8.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morelli AE and Thomson AW: Dendritic
cells: regulators of alloimmunity and opportunities for tolerance
induction. Immunol Rev. 196:125–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muth S, Schütze K, Schild H and Probst HC:
Release of dendritic cells from cognate CD4+ T-cell
recognition results in impaired peripheral tolerance and fatal
cytotoxic T-cell mediated autoimmunity. Proc Natl Acad Sci USA.
109:9059–9064. 2012.PubMed/NCBI
|
|
5
|
Camirand G, Caron NJ, Turgeon NA, Rossini
AA and Tremblay JP: Treatment with anti-CD154 antibody and
donor-specific transfusion prevents acute rejection of myoblast
transplantation. Transplantation. 73:453–461. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sela U, Olds P, Park A, Schlesinger SJ and
Steinman RM: Dendritic cells induce antigen-specific regulatory T
cells that prevent graft versus host disease and persist in mice. J
Exp Med. 208:2489–2496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karin M, Yamamoto Y and Wang QM: The IKK
NF-kappa B system: a treasure trove for drug development. Nat Rev
Drug Discov. 3:17–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng H, Guerau-de-Arellano M, Mehta VB,
Yang Y, Huss DJ, Papenfuss TL, Lovett-Racke AE and Racke MK:
Dimethyl fumarate inhibits dendritic cell maturation via nuclear
factor κB (NF-κB) and extracellular signal-regulated kinase 1 and 2
(ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. J
Biol Chem. 287:28017–28026. 2012.PubMed/NCBI
|
|
9
|
Jimenez F, Quinones MP, Martinez HG,
Estrada CA, Clark K, Garavito E, Ibarra J, Melby PC and Ahuja SS:
CCR2 plays a critical role in dendritic cell maturation: possible
role of CCL2 and NF-kappa B. J Immunol. 184:5571–5581. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cosulich SC, James NH, Needham MR, Newham
PP, Bundell KR and Roberts RA: A dominant negative form of IKK2
prevents suppression of apoptosis by the peroxisome proliferator
nafenopin. Carcinogenesis. 21:1757–1760. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tomasoni S, Aiello S, Cassis L, Noris M,
Longaretti L, Cavinato RA, Azzollini N, Pezzotta A, Remuzzi G and
Benigni A: Dendritic cells genetically engineered with adenoviral
vector encoding dnIKK2 induce the formation of potent
CD4+ T-regulatory cells. Transplantation. 79:1056–1061.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ouyang J, Fan C, Wen D, Hou J, Du Y, Wang
Y and Shi G: Donor antigen loaded IKK2dn gene-modified dendritic
cells prolong allograft survival. Scand J Immunol. 71:336–344.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aiello S, Cassis P, Cassis L, Tomasoni S,
Benigni A, Pezzotta A, Cavinato RA, Cuqini D, Azzollini N, Mister
M, et al: DnIKK2-transfected dendritic cells induce a novel
population of inducible nitric oxide synthase-expressing
CD4+CD25− cells with tolerogenic properties.
Transplantation. 83:474–484. 2007.PubMed/NCBI
|
|
14
|
Fan CB, Zhang DX, Wen DG, Hou JQ, Ouyang J
and Du KL: Screening and function identifying of
CD4+CD25− T cells induced by immature
dendritic cells transfected with IKK2dn. Zhonghua Shi Yan Wai Ke Za
Zhi. 29:1076–1079. 2012.
|
|
15
|
Du KL, Fan CB, Wen DG, Hou JQ, Ouyang J
and Zhang DX: Dominant negative form of IκB kinases 2-transfected
recipient immature dendritic cells induce
CD4+CD25− T cells with tolerogenic
properties. Zhonghua Shi Yan Wai Ke Za Zhi. 29:2439–2441. 2012.
|
|
16
|
Schumacher M, Van Vliet BN and Ferrari P:
Kidney transplantation in rats: an appraisal of surgical techniques
and outcome. Microsurgery. 23:387–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wood KJ and Sakaguchi S: Regulatory T
cells in transplantation tolerance. Nat Rev Immunol. 3:199–210.
2003. View Article : Google Scholar
|
|
18
|
Yates SF, Paterson AM, Nolan KF, Cobbold
SP, Saunders NJ, Waldmann H and Fairchild PJ: Induction of
regulatory T cells and dominant tolerance by dendritic cells
incapable of full activation. J Immunol. 179:967–976. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamazaki S, Inaba K, Tarbell KV and
Steinman RM: Dendritic cells expand antigen-specific
Foxp3+ CD25+CD4+ regulatory T
cells including suppressors of alloreactivity. Immunol Rev.
212:314–329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang H, Cheng EY, Sharma VK, Lagman M,
Chang C, Song P, Ding R, Muthukumar T and Suthanthiran M: Dendritic
cells with TGF-β1 and Il-2 differentiate naive CD4+ T
cells into alloantigen-specific and allograft protective
Foxp3+ regulatory T Cells. Transplantation. 93:580–588.
2012.
|
|
21
|
Brennan TV, Tang Q, Liu FC, Hoang V, Bi M,
Bluestone JA and Kang SM: Requirements for prolongation of
allograft survival with regulatory T cell infusion in
lymphosufficient hosts. J Surg Res. 169:e69–e75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Di Ianni M, Falzetti F, Carotti A, Terenzi
A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI,
Cecchini D, et al: Tregs prevent GVHD and promote immune
reconstitution in HLA-haploidentical transplantation. Blood.
117:3921–3928. 2011.PubMed/NCBI
|
|
23
|
Sagoo P, Ali N, Garg G, Nestle FO, Lechler
RI and Lombardi G: Human regulatory T cells with alloantigen
specificity are more potent inhibitors of alloimmune skin graft
damage than polyclonal regulatory T cells. Sci Transl Med.
3:83ra422011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wakkach A, Fournier N, Brun V, Breittmayer
JP, Cottrez F and Groux H: Characterization of dendritic cells that
induce tolerance and T regulatory 1 cell differentiation in vivo.
Immunity. 18:605–617. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fahlén L, Read S, Gorelik L, Hurst SD,
Coffman RL, Flavell RA and Powrie F: T cells that cannot respond to
TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp
Med. 201:737–746. 2005.PubMed/NCBI
|
|
26
|
Marie JC, Letterio JJ, Gavin M and
Rudensky AY: TGF-beta1 maintains suppressor function and Foxp3
expression in CD4+CD25+ regulatory T cells. J
Exp Med. 201:1061–1067. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kearley J, Barker JE, Robinson DS and
Lloyd CM: Resolution of airway inflammation and hyperreactivity
after in vivo transfer of CD4+ D25+
regulatory T cells is interleukin 10 dependent. J Exp Med.
202:1539–1547. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Darrah PA, Hegde ST, Patel DT, Lindsay RW,
Chen L, Roederer M and Seder RA: Il-10 production differentially
influences the magnitude, quality, and protective capacity of Th1
responses depending on the vaccine platform. J Exp Med.
207:1421–1433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao Q, Wang Y, Zheng D, Sun Y, Wang Y, Lee
VW, Zheng G, Tan TK, Ince J, Alexander SI and Harris DC:
Il-10/TGF-beta-modified macrophages induce regulatory T cells and
protect against adriamycin nephrosis. J AM Soc Nephrol. 21:933–942.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang S, Golshayan D, Tsang J, Lombardi G
and Lechler RI: In vitro expanded alloantigen-specific
CD4+CD25+ regulatory T cell treatment for the
induction of donor-specific transplantation tolerance. Int
Immunopharmacol. 6:1879–1882. 2006.PubMed/NCBI
|
|
31
|
Velásquez-Lopera MM, Eaton VL, Lerret NM,
Correa LA, Decresce RP, García LF and Jaramillo A: Induction of
transplantation tolerance by allogeneic donor-derived
CD4(+)CD25(+)Foxp3(+) regulatory T cells. Transpl Immunol.
19:127–135. 2008.
|
|
32
|
Zhang Y, Wang YL, Liu YW, Li Q, Yuan YH,
Niu WY, Sun LY, Zhu ZJ, Shen ZY and Han RF: Change of peripheral
blood mononuclear cells IFN-gamma, Il-10, and TGF-beta1 mRNA
expression levels with active human cytomegalovirus infection in
orthotopic liver transplantation. Transplant Proc. 41:1767–1769.
2009. View Article : Google Scholar
|
|
33
|
Tomasoni S, Azzollini N, Casiraghi F,
Capogrossi MC, Remuzzi G and Benigni A: CTLA4Ig gene transfer
prolongs survival and induces donor-specific tolerance in a rat
renal allograft. J Am Soc Nephrol. 11:747–752. 2000.PubMed/NCBI
|
|
34
|
Kurlberg G, Haglind E, Schön K, Törnqvist
H and Lycke N: Blockade of the B7-CD28 pathway by CTLA4-Ig
counteracts rejection and prolongs survival in small bowel
transplantation. Scand J Immunol. 51:224–230. 2000. View Article : Google Scholar : PubMed/NCBI
|