Introduction
Loss of endothelial integrity and impaired capacity
for neovascularization are hypothesized to contribute to
cardiovascular diseases, such as atherosclerosis and ischemic
events in the limbs, retina and myocardium (1). Recent studies have shown that
endogenous re-endothelialization and postnatal neovascularization
rely on the migration, proliferation and sprouting of preexisting
mature endothelial cells, as well as on the activity of endothelial
progenitor cells (EPCs) (2,3).
EPCs promote re-endothelialization or stimulate angiogenesis
directly by differentation and proliferation, and also indirectly
with secretory factors that mobilize endothelial and progenitor
cells to be involved in angiogenesis and reconstruction (4,5).
Therefore, EPCs may be used as a potential therapeutic strategy in
vascular disorders.
It has been recognized that EPCs are a heterogeneous
population and, according to their morphology, function and growth
potential, are classified into at least two different populations
in ex vivo culture systems: Pro-angiogenic cells, namely
early EPCs, and endothelial colony forming cells (ECFCs), also
termed late EPCs. Early EPCs appear within 4 to 7 days of culture,
are spindle-shaped and have a limited proliferation potential. Late
EPCs develop after two to three weeks of culture and have a
cobblestone appearance (6).
Previous data suggest that late EPCs are closer to mature
endothelial cells in phenotype but show notable proliferative,
migrational and tube-forming capabilities; while early EPCs may
contribute to endothelialization and neovascularization by the
secretion of vasoactive substances, such as cytokines, stromal
cell-derived factor-1 (SDF-1), nitric oxide (NO) and prostaglandin
I2(6–8).
Although the treatment of cardiovascular patients
with EPCs may be a potential therapeutic option, the numbers of
cells that are directly obtained from bone marrow, peripheral blood
and umbilical cord blood are not large enough for use in a clinical
setting. For example, the animal study results from Iwaguro et
al(9) indicated that up to 12
L of autologous blood may be required to harvest sufficient EPCs in
order to induce angiogenesis in patients following intravenous cell
infusion. Thus, ex vivo culture and expansion of EPCs may be
a promising strategy to overcome the clinical problem of limited
cell numbers. However, EPCs which were cultured in different
culture conditions exhibited different morphologies, surface
markers and biologic functions. It is known that the culture medium
is the key in the determination of cell characteristics. At
present, the common media used for ex vivo expansion of
EPCs, include M-199 with 20% fetal bovine serum (FBS) and 0.05
mg/ml bovine pituitary extract (10,11);
M-199 with 10% FBS, 20 ng/ml vascular endothelial growth factor
(VEGF) and 10 ng/ml basic fibroblast growth factor (bFGF) (12,13);
and endothelium growth medium (EGM)-2 MV medium (supplemented with
EGM-2 bullet kit, including 5% fetal calf serum, vascular
endothelial growth factor (VEGF), R3-insulin-like growth factor 1
(R3-IGF-1), human recombinant epidermal growth factor (rhEGF),
human recombinant fibroblast growth factor (rhFGF)-B, Gentamicin,
Amphotericin-B (GA-1000), hydrocortisone and ascorbic acid)
(14,15). Therefore, the purpose of the
present study was to investigate and compare the effects of these
different culture media on the early and late EPC morphology and
cell functions (proliferation, adhesion, migration,
differentiation, secretion and tube formation).
Materials and methods
Isolation of bone marrow mononuclear
cells and cell culture
Whole bone marrow was isolated from the femurs and
tibias of Sprague-Dawley rats (weight, 150–175 g), which were
obtained from Weifang Medical University, Shandong, China. The bone
marrow mononuclear cells (MNCs) were fractionated by density
gradient centrifugation (Histopaque®-1083,
Sigma-Aldrich, St. Louis, MO, USA). MNCs were plated on dishes
precoated with fibronectin (Roche, Mannheim, Germany) and were
cultured in M1 [M-199 (HyClone, Logan, UT, USA) with 20% FBS
(HyClone) and bovine pituitary extract (Sigma Aldrich Chemie,
Schnelldorf, Germany)], M2 [M-199 with 10% FBS, 20 ng/ml VEGF
(PeproTech, Rocky Hill, NJ, USA) and 10 ng/ml bFGF (Sigma Aldrich
Chemie)] or EGM-2MV (EGM-2 with EGM-2 bullet kit, including, 5%
fetal calf serum, VEGF, R3-IGF-1, rhEGF, rhFGF-B, GA-1000,
hydrocortisone and ascorbic acid) media. After four days in
culture, unattached cells were removed by a single washing step
with phosphate-buffered saline (PBS), following which, fresh medium
was added. This study was conducted in accordance with the
recommendations of the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. All animal protocols
were approved by the local ethics committee at Weifang Medical
University (Weifang, China; permit no. 5876).
Colony-forming assay
Isolated MNCs were resuspended in different growth
media, and in total, 5×106 MNCs were preplated in
fibronectin-coated 6-well plates in duplicate. After two days, the
non-adherent cells were collected and 1×106 cells were
replated onto a fibronectin-coated 24-well plate. On the fifth day,
the number of colony-forming units (CFUs)/well was counted for each
sample. A colony of EPCs was defined as a central core of round
cells with elongated sprouting cells at the periphery. All colonies
were counted manually in a minimum of three wells by two
independent investigators under blind conditions as described
previously (16).
Cell proliferation assay
Cell proliferative activities were analyzed using
cell counting kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan).
Briefly, EPCs were seeded onto 96-well plates (density, 1,000
cells/100 μl/well), CCK-8 was added to each well according to the
manufacturer’s instructions and incubated for 1 h at 37°C. The
optical density (OD) value at 450 nm was measured using an
enzyme-linked immunoabsorbent assay reader (Bio-Rad 680; Bio-Rad
Laboratories, Hercules, CA, USA).
Cell differentiation assay
Total cellular RNA was isolated with TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and
reverse-transcribed to cDNA using the SYBR®
PrimeScript® RT-PCR kit (Takara Bio Inc., Shiga, Japan)
at 37°C for 15 min. Endothelial cell differentiation markers, such
as von Willibrand factor (vWF) and CD31, and gene expression were
analyzed by SYBR® Premix Ex Taq™ (Takara Bio, Inc.).
Primers used for amplification were as follows: Sense: 5′-GCG TGG
CAG TGG TAG AGT A-3′ and antisense: 5′-GGA GAT AGC GGG TGA AAT A-3′
for vWF; sense: 5′-GAC AGC CAA GGC AGA TGC AC-3′ and antisense:
5′-ATT GGA TGG CTT GGC CTG AA-3′ for CD31; and sense: 5′-GGC ACA
GTC AAG GCT GAG AAT-3′ and antisense: 5′-ATG GTG GTG AAG ACG CCA
GTA-3′ for glyceraldehyde 3-phosphate dehydrogenase, which was used
as a housekeeping gene, in order to normalize the expression target
gene. The thermal cycling conditions were as follows: 30 sec at
95°C for pre-denaturation, 40 cycles for 15 sec at 95°C for
denaturation, 1 min at 59°C for annealing and 10 sec at 72°C for
elongation. At the end of each cycle, the fluorescence emitted by
SYBR-Green I was measured. Following the completion of the cycling
process, samples were immediately subjected to a temperature ramp
for melting curve analysis.
The protein expression of CD31 and vWF was also
determined by a fluorescence-activated cell sorter (FACS, Becton
Dickinson, Franklin Lakes, NJ, USA). Cells were trypsinized and
incubated with CD31 or vWF antibody (eBioscience, San Diego, CA,
USA) for 1 h. For the detection of vWF, the cells were
permeabilized with 0.1% Triton X-100 prior to incubation with the
antibody. Typically, ~20,000 late EPCs were measured for
fluorescence intensity per experiment. In addition, isotype
controls were performed for each sample condition and the mean
fluorescence intensity identified for the isotype control was
subtracted from the mean fluorescent intensity of the
antibody-bound cells.
Cell apoptosis assay
Approximately 1×106 cells were
double-stained with Annexin V-fluorescein isothiocyanate and
propidium iodide (PI) using an Annexin V-FITC Apoptosis Detection
kit (Becton Dickinson) according to the manufacturer’s
instructions. Apoptotic cells (Annexin
V+/PI−) were detected by FACS. The apoptotic
percentage analysis was performed using Cell-Quest software (Becton
Dickinson).
Cell adhesion assay
Cells were washed with PBS, and gently detached with
0.25% trypsin/EDTA. Following centrifugation and resuspension with
serum-free medium, equal cell numbers were seeded on 50 μg/ml
fibronectin-coated culture dishes, and incubated for 1 h at 37°C.
Cultures were washed three times with PBS to remove non-adherent
cells. Then adherent cells were counted independently in six random
high power (×100) microscope fields/well by three blinded
observers.
Cell migration assay
The migratory function of EPCs was analyzed by a
modified Boyden chamber (CoStar, Cambridge, MA, USA) assay.
Briefly, a total of 1×105 EPCs were placed in the upper
chamber and medium containing with SDF-1 was placed in the lower
chamber. The assays were conducted over a 16 h incubation period at
37°C in an incubator equilibrated to 5% CO2. The
membrane was then washed gently with PBS and fixed with 4%
paraformaldeyde. Non-migrating cells were gently removed with
cotton balls from the upper side of the membrane, and the membrane
was then stained by using DAPI. The migration of late EPCs was
analyzed by counting the number of migrated cells in six random
high-power (×100) microscope fields/well.
In vitro tube formation assay
A 96-well plate was coated with 100 μl Matrigel
(Becton Dickinson) and incubated at 37°C for 1 h. Late EPCs/ml
(2×105) were added to each well for 10 h. The enclosed
networks of tubes were photographed from six randomly chosen fields
under a microscope. The averages of the total number and area of
complete tubes, formed by late EPCs, per unit area were compared by
Image-Pro Plus (Media Cybernetics, Rockville, MD, USA).
NO concentration assay
The NO concentration in EPC culture supernatants was
detected using an NO nitrate reductase assay kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China), according to the
manufacturer’s instructions. The NO concentration was calculated
according to the following formula: NO (μmol/l) = standard
concentration (100 μmol/l dilution factory of sample × (OD of NO
measuring tube-OD of blank tube)/(OD of standard-OD of blank).
Statistical analysis
Unless otherwise indicated, results are presented as
the mean ± SE from at least three independent experiments.
Statistical analyses were performed by one-way analysis of
variance, followed by Tukey-Kramer post hoc test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference. All data were analyzed using SPSS software
(version 15.0; SPSS Inc., Chicago, IL, USA).
Results
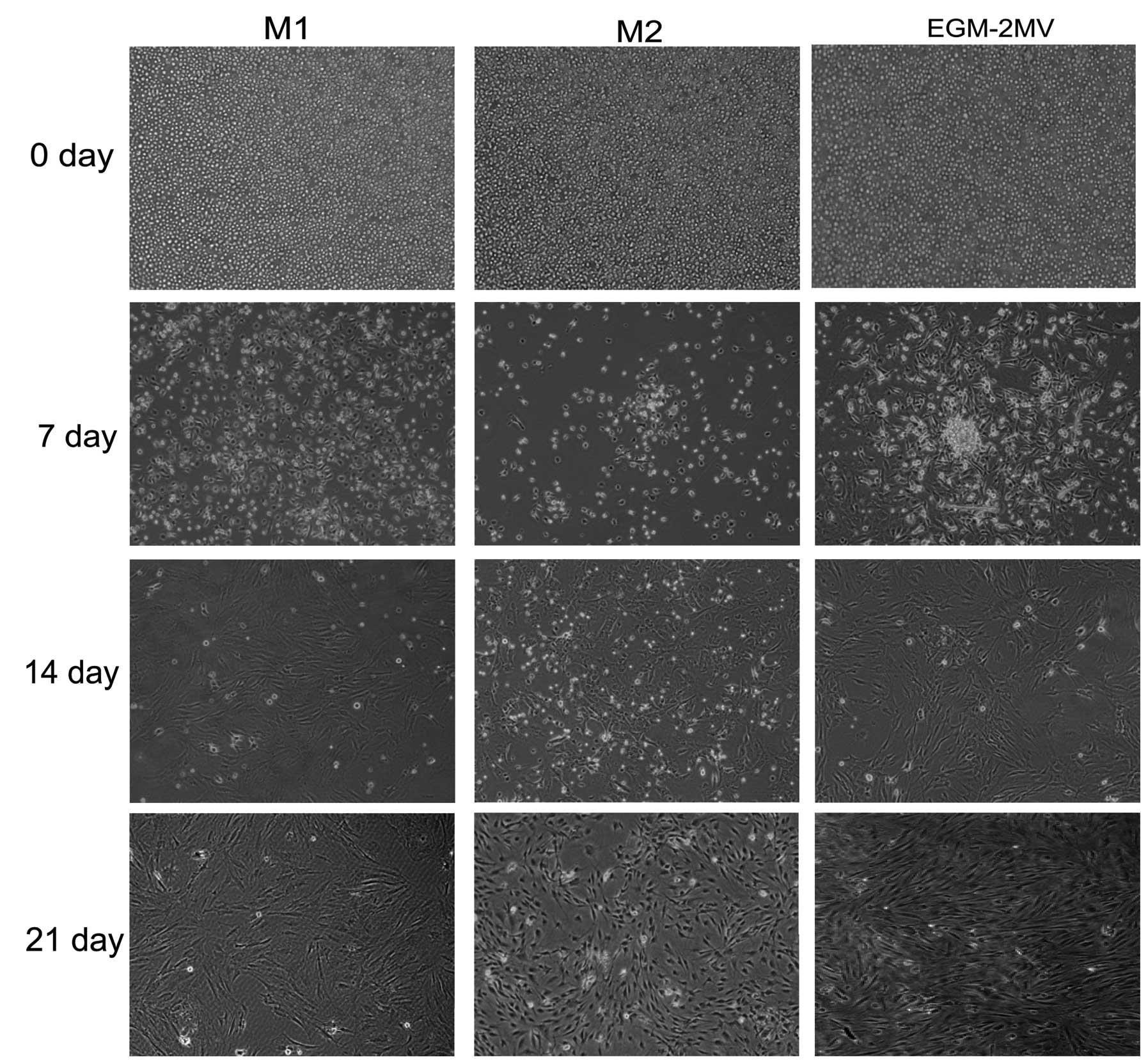
Morphological characteristics of EPCs in
different culture media
The bone marrow MNCs initially cultured in the
different media were round. After changing the medium on day four,
attached cells were observed. Seven days following plating, the
primary culture of MNCs started to differentiate into early EPCs
(17) and tended to form colonies
with the round cells at the center and the typical spindle cells at
the periphery in M2 and EGM-2MV media, although the sizes of the
colonies were different. The colonies grown in M2 media were
smaller than those in EGM-2MV media. However, the attached cells in
M1 media showed a fusiform shape. After 3 weeks, the 3rd or 4th
generations of EPCs, namely late EPCs, with a cobblestone-like
morphology similar to mature endothelial cells were grown to
confluence in all media (Fig.
1).
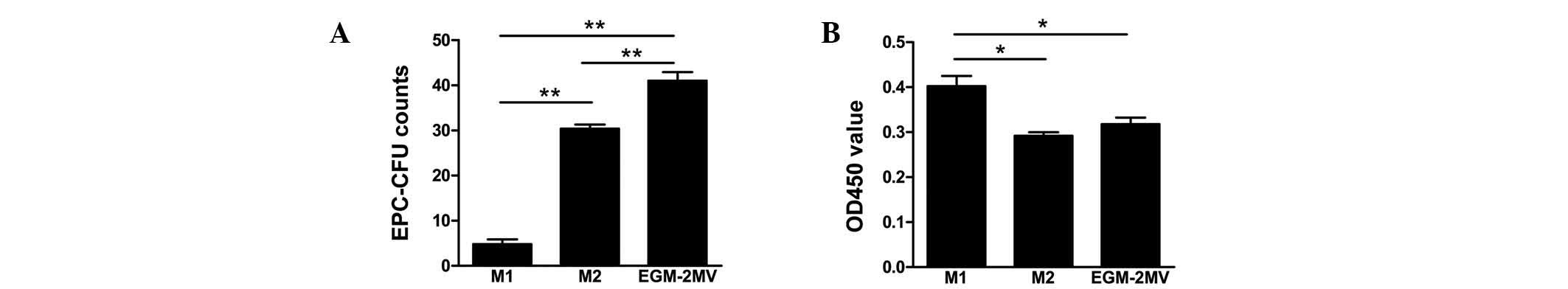
EPC colony-forming capacity and
proliferation in different culture media
Subsequent to seeding MNCs on wells, cells were
incubated with the different culture media. Cells in EGM-2MV media
exhibited the greatest colony forming capacity, followed by M2 and
finally M1 media (Fig. 2A). The
ability of late EPCs in M2 media to expand was low. However, late
EPCs in M1 media reached confluence in a short period of time.
Furthermore, the effects of different culture media on late EPC
proliferation were analyzed by a CCK-8 assay. In comparison to
cells grown in M1 media, those in M2 and EGM-2MV media did not
exhibit such a high level of cell proliferation (Fig. 2B).
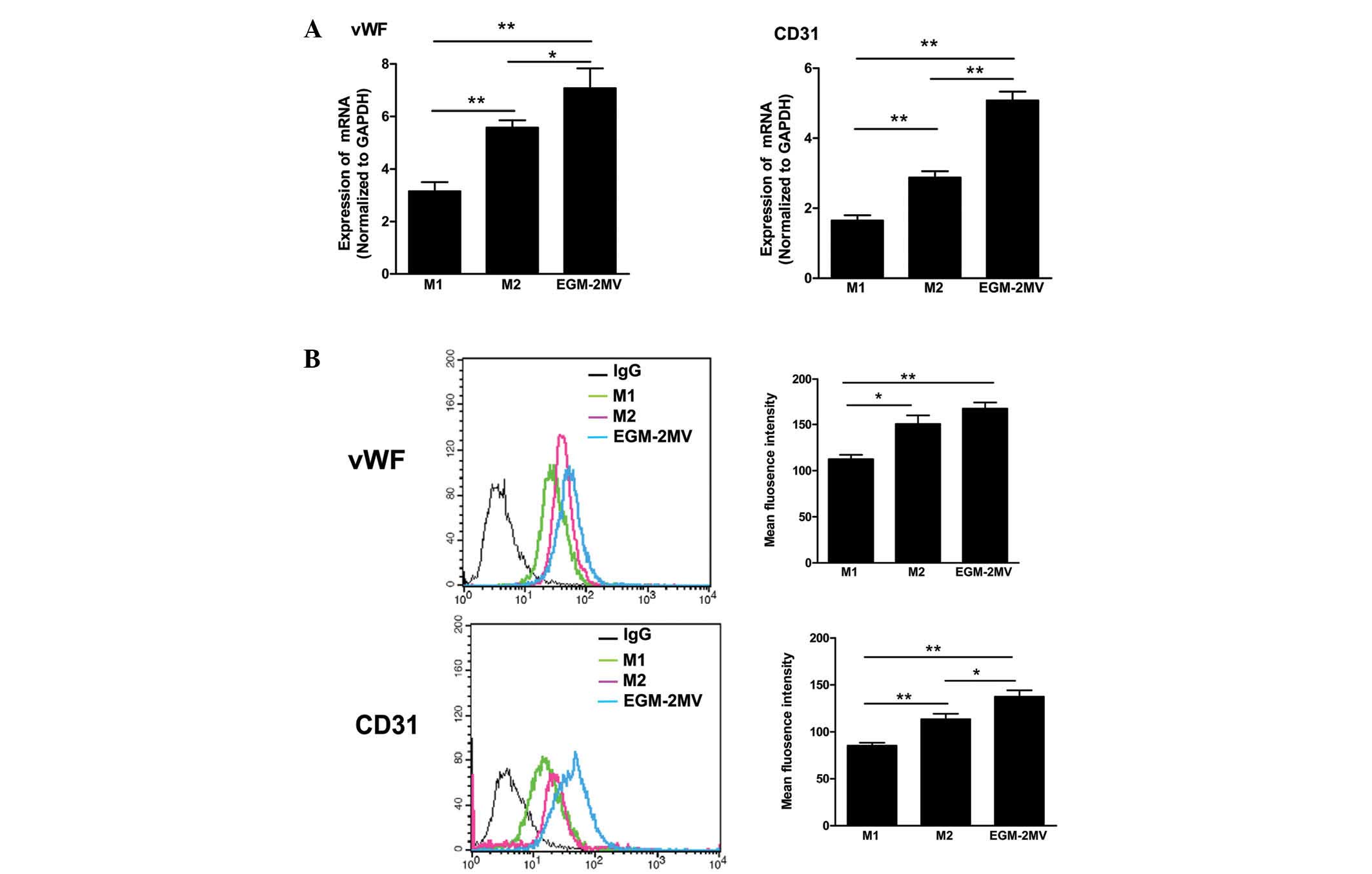
Late EPC differentiation in different
culture media
To identify the effects of different culture media
on late EPC differentiation, the gene and protein expression of
endothelial cell differentiation markers, such as vWF and CD31, was
analyzed by qPCR and FACS. A number of cells were observed to
express vWF and CD31 in each group, consistent with our previous
results, which showed that cultured EPCs express endothelial cell
differentiation markers (18).
Moreover, late EPCs cultured in EGM-2MV media exhibited the highest
gene and protein expression levels of CD31 and vWF (Fig. 3).
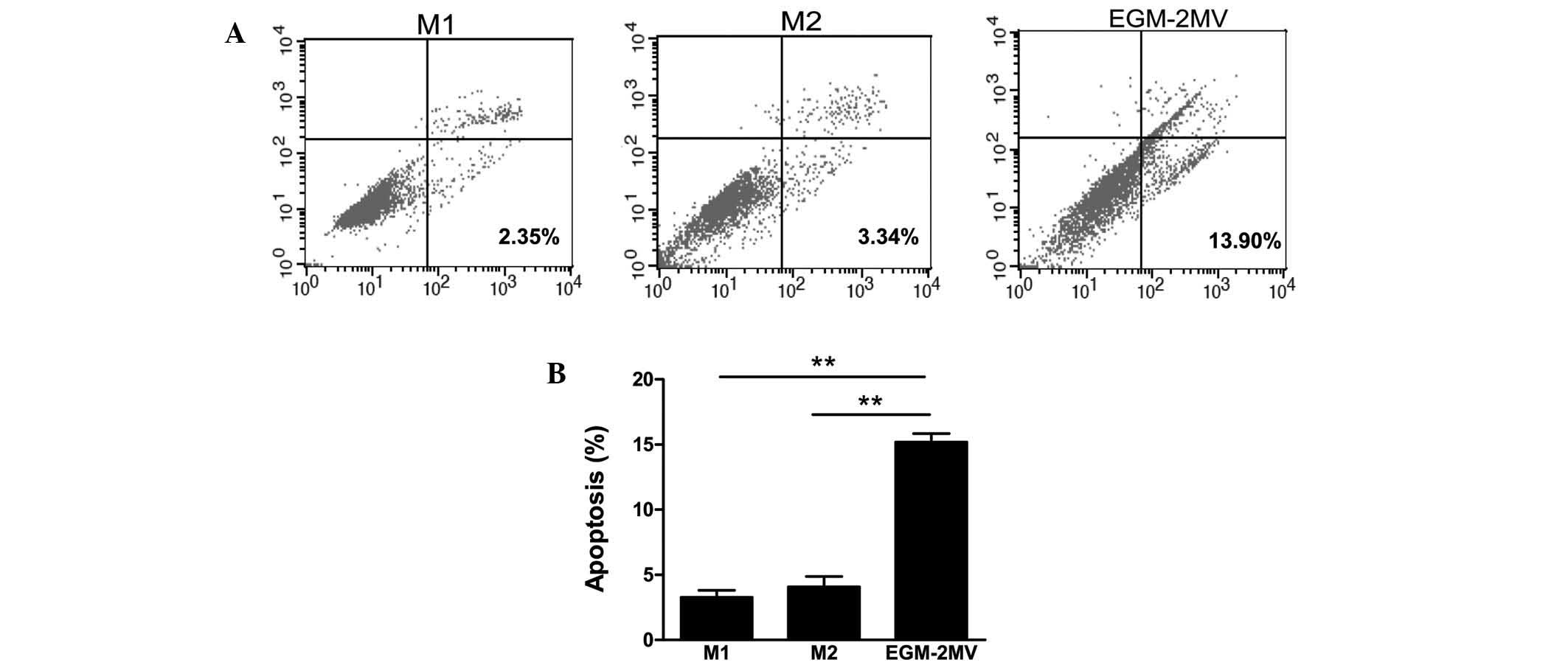
EPC apoptosis in different culture
media
To determine the effects of different culture media
on cell apoptosis, late EPCs were incubated in M1, M2 or EGM-2MV
media. The cells were harvested and the apoptotic cells were
quantified by FACS following Annexin V and PI staining. As shown in
Fig. 4, late EPCs cultured with
EGM-2MV medium exhibited significantly increased numbers of
apoptotic cells, followed by M2 and finally M1 media.
EPC adhesion, migration and angiogenic
properties in different culture media
As adhesion to the extracellular matrix is
hypothesized to be important during novel blood vessel growth
(19), the adhesion capacity of
late EPCs cultured with different media was investigated.
Quantitative analysis demonstrated that the number of cells that
adhered to fibronectin was significantly higher in late EPCs
cultured with EGM-2MV than those in the other groups (Fig. 5A).
The migratory function of EPCs in response to SDF-1
is also important during neovascularization and
reendothelialization (19), and
late EPCs have been shown to exhibit a greater migratory capacity
than early EPCs in vitro(20). Thus, the effects of the different
culture media on late EPC migration were analyzed by a modified
Boyden chamber assay using SDF-1 as a chemoattractant. After 16 h,
late EPCs cultured in EGM-2MV exhibited the highest number of
migrating cells among the three different groups (Fig. 5B).
It has been shown that late EPCs, but not early
EPCs, successfully form capillary networks on Matrigel (6). An in vitro angiogenesis assay
was performed with late EPCs to investigate the effects of the
different culture media on EPC neovascularization. The functional
capacity for tube formation of late EPCs on Matrigel was
significantly stronger in the EGM-2MV group compared with the other
groups (Fig. 5C).
NO production of late EPCs in different
culture media
EPCs are able to secrete NO. NO is critical for
regulating EPC functions (21).
Therefore the effects of different culture media on the NO
production in late EPCs were investigated. The culture media were
collected, and the quantity of NO released from the late EPCs were
determined. The results show that the level of NO was highest in
the EGM-2MV group (Fig. 6).
Discussion
EPCs were initially isolated from adult human
peripheral blood in 1997 by Asahara et al(22). Since the discovery, the original
culture-based method to obtain human EPCs from blood has been
adapted to mouse, pig and rat EPCs isolated from bone marrow
(12,14,17,23).
MNCs isolated by density-gradient centrifugation from bone marrow
were cultured with endothelial medium, which induced the MNCs to
differentiate into EPCs.
Recent studies have suggested that the culture
medium may be involved in the number and function of EPCs (17,24).
Therefore, in the present study, three common media were selected
to culture EPCs isolated from rat bone marrow. The results have
shown that EPCs exhibit different biological properties in
different media.
In the conventional EPC culture, two predominant
cell types have been shown to emerge from MNC cultures: Early and
late EPCs (6). In the present
study, after seven days, the primary culture of MNCs
differentiating into early EPCs (17) showed different morphological
phenotypes and exhibited different colony-forming capacities. The
attached cells in M1 medium did not form the obvious clone and
showed a fusiform shape. However, the cells cultured with M2 and
EGM-2MV tended to form colonies with round cells at the center and
the typical spindle cells at the periphery, with the colonies in M2
medium being smaller than those in EGM-2MV. A study by Yang et
al(25) indicated that larger
colonies exhibit greater differentiation than smaller colonies. In
line with these results it was demonstrated that after three weeks,
the 3rd or 4th generations of EPCs, namely late EPCs, cultured in
EGM-2MV exhibited the highest gene and protein expression levels of
CD31 and vWF, suggesting that endothelial differentiation may occur
more robustly in cells cultured in EGM-2MV. In the present study it
was also observed that late EPCs cultured in EGM-2MV exhibit a
greater number of apoptotic cells. Ramasamy et al(26) demonstrated that elevated expression
of stem cell-associated transcription factors is correlated with a
reduction in cell apoptosis. As EGM-2MV is the medium that most
strongly supports the differentiation of EPCs towards endothelial
cells, it is reasonable that a greater number of apoptotic cells
were observed in late EPCs in the EGM-2MV medium.
Cell proliferation is markedly affected by culture
conditions, particularly the addition of cytokines. When the
required cytokines were not added to the culture media, cell
profanation was slower and cell death occurred. In the present
study it was demonstrated that early EPCs cultured in EGM-2MV
exhibited the highest colony forming capacity, followed by those
cultured in M2 and finally in M1 media. However, M1 greatest effect
on the promotion of proliferation of late EPCs. These results
indicated that the requirement of cytokines is different during EPC
maturation. Jianguo et al(17) demonstrated that combinations of
cytokines increased the rate of proliferation of EPCs. It is
therefore reasonable that M1 was identified to exhibit the greatest
effect on late-EPC proliferation as the bovine pituitary extract
contained more than one type of cytokine.
The Matrigel model is a global assay, which analyzes
multiple cellular processes involved in blood vessel growth, such
as cell migration, adhesion and differentiation. In the present
study, it was demonstrated that the number cell that adhered to
fibronectin and migrated in response to SDF-1 was significantly
higher in late EPCs cultured with EGM-2MV. Thus, EGM-2MV medium was
also identified to markedly increase the number of cell extensions
formed within cell networks.
In the present study, it was also observed that the
production of NO in late EPCs was greatest when cultured in EGM-2MV
medium. NO is a predominant vasodilator and a key survival factor
for the endothelium. Endothelial dysfunction is characterized by
low bioavailability of endothelium-derived NO, which itself is an
independent predictor of future cardiovascular events (27). Ozuyaman et al(28) demonstrated that NO stimulates EPC
mobilization from bone marrow stem cell niches to the peripheral
circulation, implying that they participate in the
neovascularization process (28).
Moreover, NO is essential for the survival, migration and
angiogenesis of EPCs (29). This
indicates the importance of NO in maintaining EPC function. These
results suggest the possibility that the increased NO production in
EGM-2MV cultured cells contributes to the promotion of EPC
functions, such as migration, adhesion and tube formation in
vitro. The biological properties of bone marrow-derived early
and late EPCs were observed in vitro. However, further
investigation is required to determine the functions of EPCs
cultured in the different media in vivo.
In conclusion, ex vivo culture and expansion
of EPCs may be a promising strategy to overcome the clinical
problem of limited cell numbers; however, cell culture condition,
for example cell media, affect the biological properties of bone
marrow-derived early and late EPCs.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 30900290 and 31270993); the
Natural Science Foundation of Shandong Province (grant nos.
ZR2009CQ027 and ZR2010HQ046); the Program for New Century Excellent
Talents in University (grant no. NCET-10-0922); the Foundation of
Shandong Educational Committee (grant no. J09LF06); and the
Project-sponsored by SRF for ROCS, SEM. The authors would like to
thank Dr Emil Avsar for his critical reading of the manuscript.
References
|
1
|
Vita JA: Endothelial function.
Circulation. 124:e906–e912. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Möbius-Winkler S, Höllriegel R, Schuler G
and Adams V: Endothelial progenitor cells: implications for
cardiovascular disease. Cytometry A. 75:25–37. 2009.
|
|
3
|
Ben-Shoshan J and George J: Endothelial
progenitor cells as therapeutic vectors in cardiovascular
disorders: from experimental models to human trials. Pharmacol
Ther. 115:25–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Urbich C, Aicher A, Heeschen C, et al:
Soluble factors released by endothelial progenitor cells promote
migration of endothelial cells and cardiac resident progenitor
cells. J Mol Cell Cardiol. 39:733–742. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang TJ, Yang YJ, Xu B, et al:
Atorvastatin accelerates both neointimal coverage and
re-endothelialization after sirolimus-eluting stent implantation in
a porcine model: new findings from optical coherence tomography and
pathology. Circ J. 76:2561–2571. 2012. View Article : Google Scholar
|
|
6
|
Hur J, Yoon CH, Kim HS, et al:
Characterization of two types of endothelial progenitor cells and
their different contributions to neovasculogenesis. Arterioscler
Thromb Vasc Biol. 24:288–293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brown MA, Wallace CS, Angelos M and
Truskey GA: Characterization of umbilical cord blood-derived late
outgrowth endothelial progenitor cells exposed to laminar shear
stress. Tissue Eng Part A. 15:3575–3587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aburakawa Y, Kawabe J, Okada M, et al:
Prostacyclin stimulated integrin-dependent angiogenic effects of
endothelial progenitor cells and mediated potent circulation
recovery in ischemic hind limb model. Circ J. 77:1053–1062. 2013.
View Article : Google Scholar
|
|
9
|
Iwaguro H, Yamaguchi J, Kalka C, et al:
Endothelial progenitor cell vascular endothelial growth factor gene
transfer for vascular regeneration. Circulation. 105:732–738. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taniguchi E, Kin M, Torimura T, et al:
Endothelial progenitor cell transplantation improves the survival
following liver injury in mice. Gastroenterology. 130:521–531.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Loomans CJ, de Koning EJ, Staal FJ, et al:
Endothelial progenitor cell dysfunction: a novel concept in the
pathogenesis of vascular complications of type 1 diabetes.
Diabetes. 53:195–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang N, Li D, Jiao P, et al: The
characteristics of endothelial progenitor cells derived from
mononuclear cells of rat bone marrow in different culture
conditions. Cytotechnology. 63:217–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye C, Bai L, Yan ZQ, Wang YH and Jiang ZL:
Shear stress and vascular smooth muscle cells promote endothelial
differentiation of endothelial progenitor cells via activation of
Akt. Clin Biomech (Bristol, Avon). 23(Suppl 1): S118–S124. 2008.
View Article : Google Scholar
|
|
14
|
Zhang X, Cui X, Cheng L, et al: Actin
stabilization by jasplakinolide affects the function of bone
marrow-derived late endothelial progenitor cells. PLoS One.
7:e508992012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tepper OM, Galiano RD, Capla JM, et al:
Human endothelial progenitor cells from type II diabetics exhibit
impaired proliferation, adhesion, and incorporation into vascular
structures. Circulation. 106:2781–2786. 2002. View Article : Google Scholar
|
|
16
|
Chen YH, Lin SJ, Lin FY, et al: High
glucose impairs early and late endothelial progenitor cells by
modifying nitric oxide-related but not oxidative stress-mediated
mechanisms. Diabetes. 56:1559–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jianguo W, Tianhang L, Hong Z, et al:
Optimization of culture conditions for endothelial progenitor cells
from porcine bone marrow in vitro. Cell Prolif. 43:418–426. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui X, Zhang X, Guan X, et al: Shear
stress augments the endothelial cell differentiation marker
expression in late EPCs by upregulating integrins. Biochem Biophys
Res Commun. 425:419–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Urbich C and Dimmeler S: Endothelial
progenitor cells: characterization and role in vascular biology.
Circ Res. 95:343–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoon CH, Hur J, Park KW, et al:
Synergistic neovascularization by mixed transplantation of early
endothelial progenitor cells and late outgrowth endothelial cells:
the role of angiogenic cytokines and matrix metalloproteinases.
Circulation. 112:1618–1627. 2005. View Article : Google Scholar
|
|
21
|
Hamed S, Brenner B and Roguin A: Nitric
oxide: a key factor behind the dysfunctionality of endothelial
progenitor cells in diabetes mellitus type-2. Cardiovasc Res.
91:9–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Asahara T, Murohara T, Sullivan A, et al:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang QR, Wang BH, Huang YH, Dai G, Li WM
and Yan Q: Purification and growth of endothelial progenitor cells
from murine bone marrow mononuclear cells. J Cell Biochem.
103:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Muscari C, Gamberini C, Basile I, et al:
Comparison between culture conditions improving growth and
differentiation of blood and bone marrow cells committed to the
endothelial cell lineage. Biol Proced Online. 12:90232010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang J, Ii M, Kamei N, et al:
CD34+ cells represent highly functional endothelial
progenitor cells in murine bone marrow. PLoS One. 6:e202192011.
|
|
26
|
Ramasamy R, Tong CK, Yip WK, Vellasamy S,
Tan BC and Seow HF: Basic fibroblast growth factor modulates cell
cycle of human umbilical cord-derived mesenchymal stem cells. Cell
Prolif. 45:132–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Desjardins F and Balligand JL: Nitric
oxide-dependent endothelial function and cardiovascular disease.
Acta Clin Belg. 61:326–334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ozüyaman B, Ebner P, Niesler U, et al:
Nitric oxide differentially regulates proliferation and
mobilization of endothelial progenitor cells but not of
hematopoietic stem cells. Thromb Haemost. 94:770–772.
2005.PubMed/NCBI
|
|
29
|
He T, Peterson TE, Holmuhamedov EL, et al:
Human endothelial progenitor cells tolerate oxidative stress due to
intrinsically high expression of manganese superoxide dismutase.
Arterioscler Thromb Vasc Biol. 24:2021–2027. 2004. View Article : Google Scholar : PubMed/NCBI
|