Introduction
Traditional Chinese medicine (TCM) has been used as
effective antitumor agents for numerous years in ancient China
(1). However, it is difficult to
identify the active ingredient due to the complex composition.
Thus, the use of TCM has been severely restricted in clinical
practices following the introduction of western antineoplastic
methods, including radiotherapy and chemotherapy. The therapeutic
efficacy of arsenic trioxide (As2O3) has been
shown previously in the treatment of acute promyelocytic leukemia
(APL), of which the mechanism has also been identified (2–6).
Consequently, TCM has become an important area of study again and
attracted considerable attention in aspects of cancer treatment,
particularly in the treatment of malignant hematologic diseases.
Numerous studies have been conducted thus far, yielding a number of
achievements (7–11).
As2O3 and realgar (arsenic
sulfide, As2S2) belong to a group of Chinese
arsenic drugs (12) and have a
number of similarities. For example, realgar and
As2O3 exhibit a good antitumor effect.
Realgar is the principal constituent in the Realgar-Indigo
naturalis formula (RIF), together with Indigo naturalis, Salvia
miltiorrhiza and Radix Pseudostellariae. RIF shows satisfactory
efficacy in treating human APL (13). Realgar has also been shown to
induce apoptosis of chronic myelogenous leukemia (CML) cells
through the degradation of BCR-ABL(14,15).
Results of recent studies have shown that
As2O3 induced apoptosis of chronic
lymphocytic leukemia (CLL) cells (16,17).
This effect on CLL cells may be mediated by suppressing the
phosphoinositide 3-kinase/Akt survival pathway via
c-jun-NH2 terminal kinase activation and PTEN
upregulation (17). However,
whether realgar induces apoptosis of CLL cells remains unknown.
CLL is the most common adult leukemia in western
countries. It is a highly heterogeneous disease. Immunoglobulin
heavy chain variable mutation status, cytogenetic changes and
expression of CD38 and ZAP-70 differ among patients. Although the
survival rate of CLL has improved over the past decade, with the
exception of allogeneic bone marrow transplantation, no treatment
is sufficiently effective to completely cure the disease.
Particularly for CLL with a 17p deletion, it is almost impossible
to avoid deterioration. Therefore, the purpose of the present study
was to investigate the effect of realgar on the viability,
proliferation and apoptosis of the human
p53deleted/mutated CLL cell line, MEC-1. In
addition, the potential mechanism mediating the effect were
explored during the study.
Materials and methods
Cell lines
Human p53deleted/mutated CLL cell
line, MEC-1, was suspended in Iscove’s modified Dulbecco’s medium
(IMDM; HyClone Laboratories, Inc., Logan, UT, USA) containing 10%
fetal bovine serum (HyClone), 100 U/ml penicillin and 100 μg/ml
streptomycin. Culturing conditions were maintained at 37°C in a
humidified atmosphere containing 5% CO2.
Reagents
Realgar (purity, 99.53%) was purchased from Alfa
Aesar (Ward Hill, MA, USA). It was dissolved in 0.1 M sodium
hydroxide and the pH was adjusted to 7.35–7.45 using hydrochloric
acid (14,18). The stock solution (1 mM
As2S2) was then passed through a 0.22 μm
filter. IMDM was used to dilute the solution prior to cell
application.
Cell viability assay
A WST-8 assay kit (Beyotime Institute of
Biotechnology, Jiangsu, China) was employed to evaluate the effect
of realgar on cell viability. Logarithmically growing MEC-1 cells
were seeded in a 96-well plate at a density of 1×104
cells/100 μl/well. The cells were incubated with increasing
concentrations of realgar (0, 2, 4, 6, 8, 10 and 20 μM) for 24, 48
and 72 h. Subsequently, 10 μl WST-8 was added to each well and the
cells were incubated for 4 h prior to cell viability measurements
of absorbance at 450 nm. The inhibitory concentration of 50% of
cells (IC50) was obtained using the probit regression
analysis method. Three replicates were established for each
sample.
5-Bromodeoxyuridine (BrdU) cell
proliferation assay
A BrdU cell proliferation ELISA kit (Roche
Diagnostics Co., Indianapolis, IN, USA) was used to evaluate the
inhibitory effects of realgar on cell proliferation, according to
the manufacturer’s instructions. MEC-1 cells with a density of
1×104 cells/ml were exposed to various concentrations
(0, 2, 6 and 10 μM) of realgar for 24 and 48 h. BrdU (10 μl/well)
was then added. Following incubation for an additional 2 h, the
culture medium was removed and the cells were fixed. Following
incubation with the antiBrdU-peroxidase for 1 h and the substrate
reaction, 25 μl of 1 M H2SO4 was applied and
the absorbance was read on an ELISA reader at 450 nm.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) dual staining
Induction of apoptosis was assessed using an annexin
V-FITC apoptosis detection kit (KeyGen Biotech Co., Ltd., Nanjing,
China). MEC-1 cells were treated with 0, 2, 6 and 10 μM realgar.
Dual staining with annexin V-FITC and PI was performed, according
to the manufacturer’s instructions. Following incubation for 24, 48
or 72 h, 5–10×105 cells were collected, washed with PBS,
stained with annexin V-FITC/PI and incubated for 10 min prior to
analysis with a flow cytometer (Becton-Dickinson, San Jose, CA,
USA). The data obtained were processed with FlowJo 7.6 software
(TreeStar, Inc., Ashland, OR, USA). Annexin V-FITC- and PI-negative
cells were identified as viable cells. Cells with annexin
V-FITC-positive and PI-negative staining were considered to be
early apoptotic cells while those with annexin V-FITC- and
PI-positive staining were late apoptotic cells. The addition of
early and late apoptotic cells constituted the apoptotic cells.
Gene expression study by quantitative
polymerase chain reaction (PCR)
Transcriptional levels of BCL2-associated X protein
(BAX), BCL2-like 1 (Bcl-xL), v-myc
myelocytomatosis viral oncogene homolog (avian; c-Myc) and
cyclin-dependent kinase inhibitor 1A (p21) genes, in
realgar-treated and untreated MEC-1 cells, were evaluated by
quantitative PCR. Total RNA was extracted by TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) from MEC-1 cells
following incubation with 0 and 6 μM realgar for 48 h. Reverse
transcription to complementary DNA (cDNA) was performed using
PrimeScript RT reagent kit with gDNA eraser (Takara Biotechnology
Co., Ltd., Dalian, China). Quantitative PCR for the aforementioned
genes was performed using a SYBR Premix Ex Taq II (Tli RNase H
Plus) kit (Takara Biotechnology Co., Ltd.) on a Roche LightCycler
480 (Roche Diagnostics Co.). Actin was used as an internal control.
The sequences of the quantitative PCR primers are listed in
Table I. Isolation of RNA, reverse
transcription and quantitative PCR were performed following the
manufacturer’s instructions. For data analysis the
2−ΔΔCt method was used with the following equations: ΔCt
= Ct (target gene) − Ct (actin); ΔΔCt = ΔCt (realgar-treated cells)
− ΔCt (untreated control). Quantitative PCR for each gene of each
cDNA sample was assayed in triplicate.
 | Table IPrimers used for quantitative
PCR. |
Table I
Primers used for quantitative
PCR.
| Name | Primer sequence
(5′→3′) | Product (bp) |
|---|
| Actin | F:
TGACGTGGACATCCGCAAAG
R: CTGGAAGGTGGACAGCGAGG | 205 |
| BAX | F:
CCCGAGAGGTCTTTTTCCGAG
R: CCAGCCCATGATGGTTCTGAT | 155 |
| Bcl-xL | F:
TCAGAGCTTTGAGCAGGTAG
R: AAGGCTCTAGGTGGTCATTC | 182 |
| c-Myc | F:
GGCTCCTGGCAAAAGGTCA
R: AGTTGTGCTGATGTGTGGAGA | 113 |
| p21 | F:
CGATGGAACTTCGACTTTGTCA
R: GCACAAGGGTACAAGACAGTG | 220 |
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Data are expressed as mean ± SD.
The significance of differences between groups was determined using
the Student’s t-test or ANOVA. P<0.05 was considered to indicate
a statistically significant difference.
Results
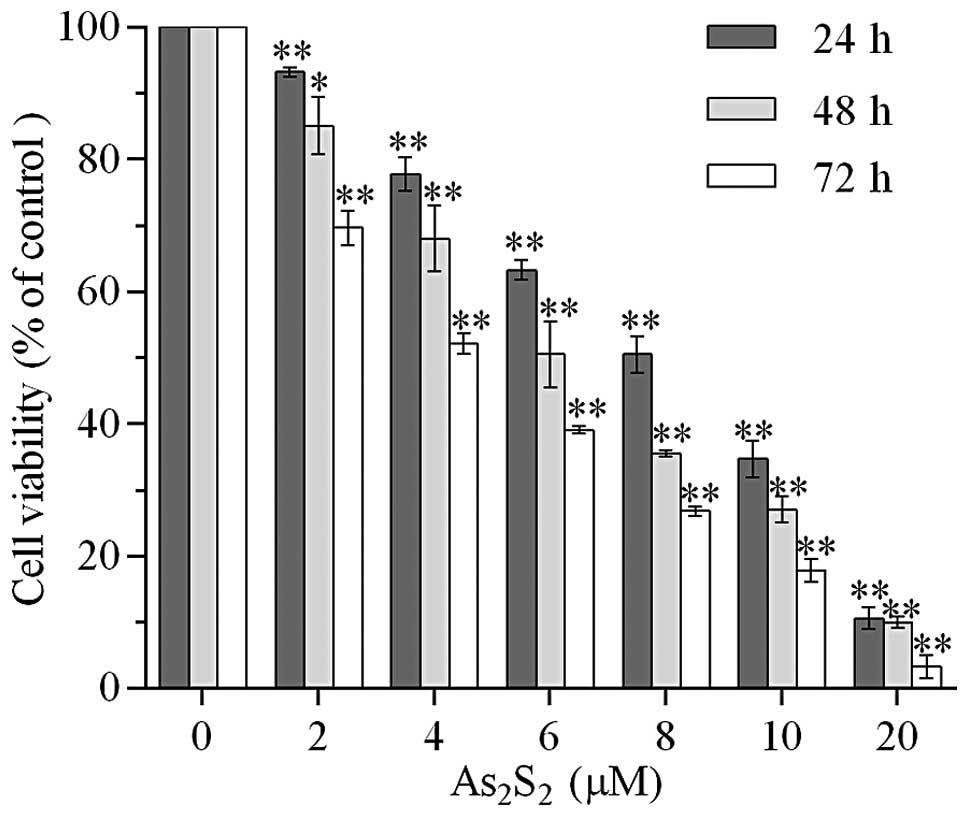
Realgar suppresses the viability of MEC-1
cells
The effect of realgar on cell viability of MEC-1
cells was evaluated using a WST-8 assay. The MEC-1 cells were
incubated with various doses of realgar (0, 2, 4, 6, 8, 10 and 20
μM) for the time periods 24, 48 and 72 h. As shown in Fig. 1, treatment with 2 μM realgar for 24
h resulted in a 6.80±0.70% reduction of viable cells (P<0.01)
compared with the control group. The inhibitory effect of cell
viability was enhanced with an increased realgar dose and
incubation time. The viability rate decreased to 69.67±2.63% when
the treatment time increased to 72 h. There was a significant
difference between the two 2 μM realgar-treated groups (P<0.01).
In addition, 10.59±1.61% cells remained viable when exposed to 20
μM realgar for 24 h (P<0.01). The IC50 at 24, 48 and
72 h was 7.998, 6.380 and 6.219 μM, respectively.
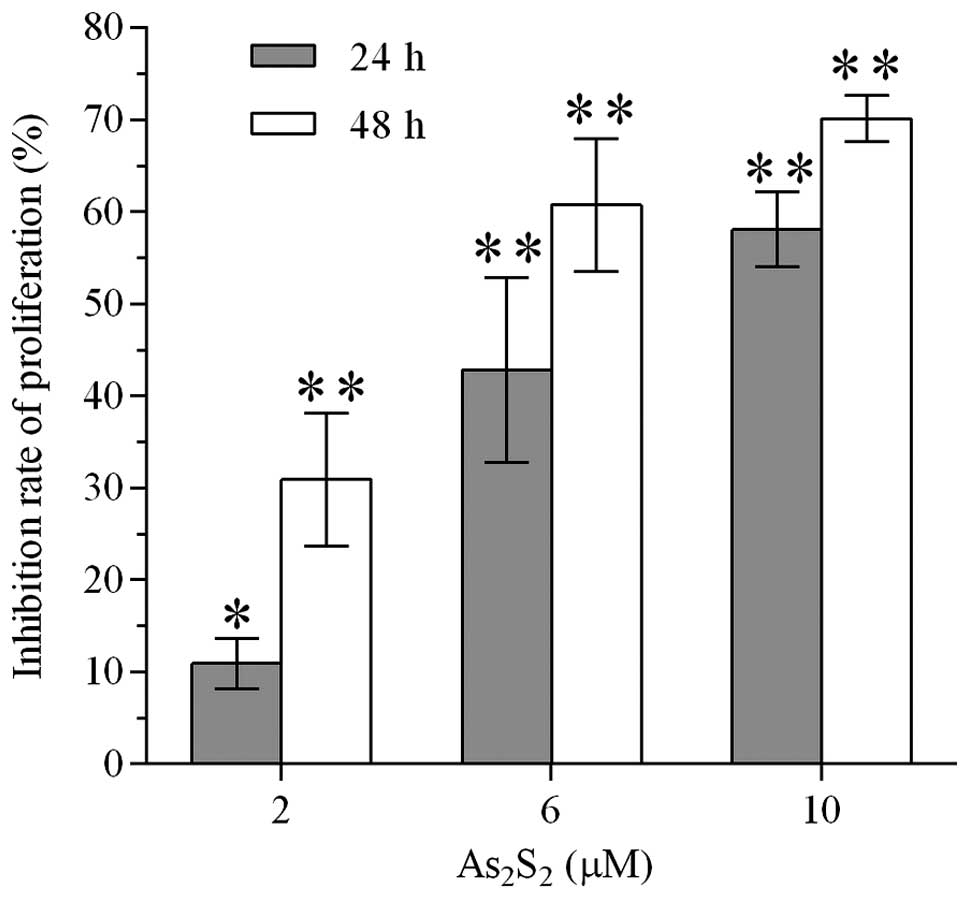
Realgar inhibits the proliferation of
MEC-1 cells
BrdU cell proliferation ELISA was performed to
investigate whether realgar suppressed the proliferation of MEC-1
cells. The inhibition of the proliferation of MEC-1 cells occurred
in a concentration- and time-dependent manner (Fig. 2). Treatment of MEC-1 cells with
realgar at 2, 6 and 10 μM reduced the proliferation of MEC-1 cells
by 5.68, 42.78 and 58.08% following 24 h and 30.90, 60.72 and
70.09% following 48 h, respectively.
Realgar induces apoptosis of MEC-1
cells
The apoptotic effect of realgar on MEC-1 cells was
determined by annexin V-FITC/PI dual staining, followed by flow
cytometry analysis. As shown in Fig.
3, apoptosis of MEC-1 cells was induced by realgar in a dose-
and time-dependent manner. Following treatment with 2 μM realgar
for 24 h, the percentage of apoptotic cells increased between
6.78±0.60 and 9.26±1.25% (P<0.05). With an increase in
incubation time or concentration of realgar, the induction of
apoptosis was significantly enhanced. Percentages of apoptotic
cells, following incubation with 2 μM realgar for 48 h and 6 μM for
24 h, were 14.44±1.54 (P<0.01) and 36.09±2.48% (P<0.01),
respectively.
Realgar upregulates mRNA levels of BAX
while downregulating mRNA levels of c-Myc
To further investigate whether realgar-induced
apoptosis was dependent on the mitochondrial apoptosis pathway, the
effect of realgar on the mRNA levels of BAX,
Bcl-xL, c-Myc and p21 genes was
measured by quantitative PCR. Following treatment with 6 μM realgar
for 48 h, mRNA expression of BAX in MEC-1 cells was
upregulated almost 2-fold while c-Myc mRNA expression was
reduced by more than half (Fig.
4). However, no significant difference in the expression of
Bcl-xL and p21 was observed between the
realgar-treated and untreated MEC-1 cells.
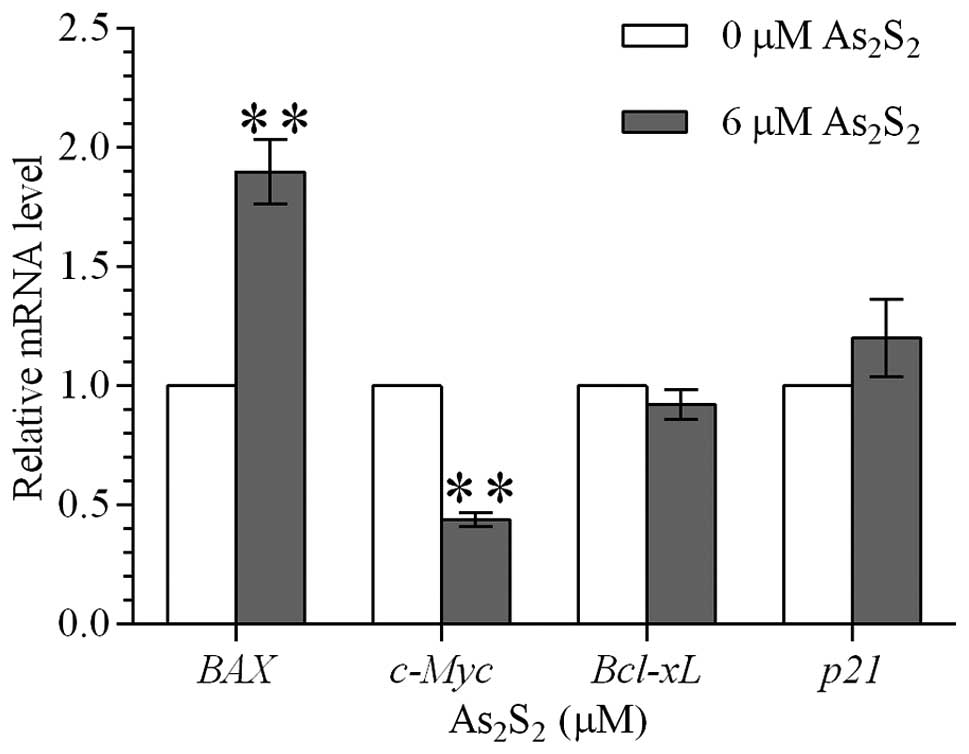 | Figure 4Effect of realgar on the
transcriptional levels of BAX, Bcl-xL,
c-Myc and p21 genes in MEC-1 cells. Relative mRNA
levels of BAX, Bcl-xL, c-Myc and
p21 genes were assessed by quantitative PCR in MEC-1 cells
following treatment with 0 and 6 μM realgar for 48 h. The
2−ΔΔCt method was used to calculate the relative mRNA
expression to the internal control (actin). mRNA expression
of BAX in MEC-1 cells was upregulated, whereas c-Myc mRNA
expression was downregulated. Values are presented as mean ± SD.
*P<0.05 and **P<0.01, vs. control.
BAX, BCL2-associated X protein; Bcl-xL, BCL2-like 1;
c-Myc, v-myc myelocytomatosis viral oncogene homolog
(avian); p21, cyclin-dependent kinase inhibitor 1A; PCR,
polymerase chain reaction. |
Discussion
Studies have been conducted on the antineoplastic
effect of realgar. Realgar has been shown to have antiproliferative
and pro-apoptotic effects on a number of cancer cell lines,
including rat glioma C6 cells, mouse melanoma B16 cells and the
cervical cancer cell line SiHa (19–21).
More studies on the antineoplastic effect of realgar have been
carried out in a few cells types of malignant hematologic diseases.
The majority of these studies have focused on promyelocytic
leukemia HL-60 cells, APL cell line NB4 and CML cell line K562, as
well as peripheral blood or bone marrow cells gained from APL and
CML patients. Consequently, considerable success has been achieved.
Oxidative stress, membrane toxicity and protein tyrosine kinase may
be involved in the process of realgar-induced apoptosis (14,22).
Realgar has been reported to induce apoptosis in human histocytic
lymphoma U937 cells through caspase, MAPK and mitochondrial
pathways (23,24). However, no studies focusing on the
effect of realgar on CLL cells have been conducted prior to this
study.
CLL is a malignant disease of B lymphocytes,
initially recognized as the result of accumulation of rest cells.
However, CLL was later found to be a disease of activated
monoclonal cells that proliferated in particular microenvironments
(25,26). The molecular mechanism leading to
the imbalance between apoptosis and proliferation has attracted
considerable attention and associated pathways have been explored
widely in order to identify new therapies targeted to cure CLL. In
the present study, arsenic compound realgar was used to dispose
MEC-1 cells. The MEC-1 cell line was established from the
peripheral blood of a CLL patient in prolymphocytoid transformation
(27). Several cytogenetic
aberrations were detected, including del(17)(p11.2pter) (27). It was found that realgar, not only
suppressed viability and proliferation, but also induced apoptosis
of MEC-1 cells.
Further investigations were performed analyzing the
effect various doses and incubation times had on MEC-1 cells.
Following exposure to various concentrations of realgar for
different time periods, cell viability and proliferation were
inhibited in MEC-1 cells in a dose- and time-dependent manner. The
effect of realgar on apoptosis was also evaluated in MEC-1 cells
and found to induce apoptosis in a dose- and time-dependent
manner.
Potential mechanisms involved in the realgar-induced
apoptosis were explored. mRNA expression of BAX and
c-Myc was upregulated and downregulated, respectively,
following realgar treatment. BAX belongs to the Bcl-2
family of pro-apoptotic genes and is an important member of the
mitochondrial apoptosis pathway (28). Protein encoded by the c-Myc
gene functions as a transcription factor that participates in
significant processes, including cell cycle progression, apoptosis
and cell transformation. Changes in mRNA expression levels of
BAX and c-Myc indicated that realgar-induced
apoptosis of MEC-1 cells may be dependent on the mitochondrial
pathway.
In summary, the inhibitory effect of realgar on the
CLL cell line, to the best of our knowledge, was explored for the
first time. Based on the findings of the present study, it may be
concluded that realgar inhibited viability and proliferation and
induced apoptosis of MEC-1 cells in a dose- and time-dependent
manner. This phenomenon may depend on the mitochondrial apoptosis
pathway, as the upregulation of BAX expression and
downregulation of c-Myc expression was observed in MEC-1
cells following realgar treatment. The results of the present study
may be beneficial for the identification of a new target therapy
for CLL. In addition, more studies are required to explore the
detailed mechanism involved in the process.
Acknowledgements
The study was supported by grants from the National
Natural Science Foundation (no. 81270598), Natural Science
Foundations of Shandong Province (nos. Y2007C053, 2009ZRB14176 and
ZR2012HZ003), Technology Development Projects of Shandong Province
(nos. 2007GG10 and 2010GSF10250) and the Program of Shandong
Medical Leading Talent and Taishan Scholar Foundation of Shandong
Province.
Abbreviations:
|
TCM
|
traditional Chinese medicine
|
|
As2O3
|
arsenic trioxide
|
|
APL
|
acute promyelocytic leukemia
|
|
As2S2
|
realgar, arsenic sulfide
|
|
CML
|
chronic myelogenous leukemia
|
|
CLL
|
chronic lymphocytic leukemia
|
|
IMDM
|
Iscove’s modified Dulbecco’s
medium
|
|
IC50
|
inhibitory concentration of 50%
|
|
BrdU
|
5-bromodeoxyuridine
|
|
FITC
|
fluorescein isothiocyanate
|
|
PI
|
propidium iodide
|
|
cDNA
|
complementary DNA
|
References
|
1
|
Man S, Gao W, Wei C and Liu C: Anticancer
drugs from traditional toxic Chinese medicines. Phytother Res.
26:1449–1465. 2012.PubMed/NCBI
|
|
2
|
Soignet SL, Maslak P, Wang ZG, et al:
Complete remission after treatment of acute promyelocytic leukemia
with arsenic trioxide. N Engl J Med. 339:1341–1348. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu J, Chew EH and Holmgren A: Targeting
thioredoxin reductase is a basis for cancer therapy by arsenic
trioxide. Proc Natl Acad Sci USA. 104:12288–12293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu J, Liu YF, Wu CF, et al: Long-term
efficacy and safety of all-trans retinoic acid/arsenic
trioxide-based therapy in newly diagnosed acute promyelocytic
leukemia. Proc Natl Acad Sci USA. 106:3342–3347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim J, Lee JJ, Kim J, Gardner D and Beachy
PA: Arsenic antagonizes the Hedgehog pathway by preventing ciliary
accumulation and reducing stability of the Gli2 transcriptional
effector. Proc Natl Acad Sci USA. 107:13432–13437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang XW, Yan XJ, Zhou ZR, et al: Arsenic
trioxide controls the fate of the PML-RARalpha oncoprotein by
directly binding PML. Science. 328:240–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mahieux R, Pise-Masison C, Gessain A, et
al: Arsenic trioxide induces apoptosis in human T-cell leukemia
virus type 1- and type 2-infected cells by a caspase-3-dependent
mechanism involving Bcl-2 cleavage. Blood. 98:3762–3769. 2001.
View Article : Google Scholar
|
|
8
|
Bae-Jump VL, Zhou C, Boggess JF and Gehrig
PA: Arsenic trioxide (As2O3) inhibits
expression of estrogen receptor-alpha through regulation of the
mitogen-activated protein kinase (MAPK) pathway in endometrial
cancer cells. Reprod Sci. 15:1011–1017. 2008.
|
|
9
|
Kang YH and Lee SJ: The role of p38 MAPK
and JNK in Arsenic trioxide-induced mitochondrial cell death in
human cervical cancer cells. J Cell Physiol. 217:23–33. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahn RW, Chen F, Chen H, et al: A novel
nanoparticulate formulation of arsenic trioxide with enhanced
therapeutic efficacy in a murine model of breast cancer. Clin
Cancer Res. 16:3607–3617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raju GP: Arsenic: a potentially useful
poison for Hedgehog-driven cancers. J Clin Invest. 121:14–16. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu XM, Liu F and Ma R: Application and
assessment of Chinese arsenic drugs in treating malignant
hematopathy in China. Chin J Integr Med. 16:368–377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiang Y, Wang XB, Sun SJ, et al: Compound
huangdai tablet as induction therapy for 193 patients with acute
promyelocytic leukemia. Zhonghua Xue Ye Xue Za Zhi. 30:440–442.
2009.(In Chinese).
|
|
14
|
Li JE, Wu WL, Wang ZY and Sun GL:
Apoptotic effect of As2S2 on K562 cells and
its mechanism. Acta Pharmacol Sin. 23:991–996. 2002.
|
|
15
|
Mao JH, Sun XY, Liu JX, et al:
As4S4 targets RING-type E3 ligase c-CBL to
induce degradation of BCR-ABL in chronic myelogenous leukemia. Proc
Natl Acad Sci USA. 107:21683–21688. 2010.PubMed/NCBI
|
|
16
|
Bairey O, Vanichkin A and Shpilberg O:
Arsenic-trioxide-induced apoptosis of chronic lymphocytic leukemia
cells. Int J Lab Hematol. 32(1 Pt 1): e77–e85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Redondo-Muñoz J, Escobar-Díaz E, Hernández
Del Cerro M, et al: Induction of B-chronic lymphocytic leukemia
cell apoptosis by arsenic trioxide involves suppression of the
phosphoinositide 3-kinase/Akt survival pathway via
c-jun-NH2 terminal kinase activation and PTEN
upregulation. Clin Cancer Res. 16:4382–4391. 2010.PubMed/NCBI
|
|
18
|
Yin T, Wu YL, Sun HP, et al: Combined
effects of As4S4 and imatinib on chronic
myeloid leukemia cells and BCR-ABL oncoprotein. Blood.
104:4219–4225. 2004.
|
|
19
|
Zhao QH, Zhang Y, Liu Y, et al: Anticancer
effect of realgar nanoparticles on mouse melanoma skin cancer in
vivo via transdermal drug delivery. Med Oncol. 27:203–212. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
An YL, Nie F, Wang ZY and Zhang DS:
Preparation and characterization of realgar nanoparticles and their
inhibitory effect on rat glioma cells. Int J Nanomedicine.
6:3187–3194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng YX, Liu R, Wang Q, et al:
Realgar-induced apoptosis of cervical cancer cell line Siha via
cytochrome c release and caspase-3 and caspase-9 activation.
Chin J Integr Med. 18:359–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye HQ, Gan L, Yang XL and Xu HB:
Membrane-associated cytotoxicity induced by realgar in
promyelocytic leukemia HL-60 cells. J Ethnopharmacol. 103:366–371.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang XB, Gao HY, Hou BL, Huang J, Xi RG
and Wu LJ: Nanoparticle realgar powders induce apoptosis in U937
cells through caspase MAPK and mitochondrial pathways. Arch Pharm
Res. 30:653–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xi RG, Huang J, Li D, Wang XB and Wu LJ:
Roles of PI3-K/Akt pathways in nanoparticle realgar powders-induced
apoptosis in U937 cells. Acta Pharmacol Sin. 29:355–363. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klein U and Dalla-Favera R: New insights
into the pathogenesis of chronic lymphocytic leukemia. Semin Cancer
Biol. 20:377–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Damle RN, Calissano C and Chiorazzi N:
Chronic lymphocytic leukaemia: a disease of activated monoclonal B
cells. Best Pract Res Clin Haematol. 23:33–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stacchini A, Aragno M, Vallario A, et al:
MEC1 and MEC2: two new cell lines derived from B-chronic
lymphocytic leukaemia in prolymphocytoid transformation. Leuk Res.
23:127–136. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Buggins AG and Pepper CJ: The role of
Bcl-2 family proteins in chronic lymphocytic leukaemia. Leuk Res.
34:837–842. 2010. View Article : Google Scholar : PubMed/NCBI
|