Introduction
Human populations are exposed to environmental and
occupational fibrous and granular dust. The number of synthetic or
natural fibres and particles being introduced into the environment
is continuously increasing. Due to the increasing number and
compositional heterogeneity of potentially harmful fibres and
particles, there is a crucial need to understand the mechanisms of
their pathogenicity.
Lately, nano-sized particles with a diameter below
100 nm have become the focus of attention, as they are predicted to
have a higher toxic potential as a result of their high
surface/mass ratios. However, the crystalline structure, surface
properties, solubility and particle size are also known to be
relevant parameters (1). Therefore
an accurate characterization of the particles is essential to allow
an interpretation of the results of a study.
It is reasonable to categorise particles and fibres
by their molecular effects. To identify the molecular
characteristics of particles and fibres we used Union
Internationale Contre le Cancer (UICC) crocidolite and chrysotil
asbestos as fibered dust and titanium dioxide (TiO2) as
well as zirconium dioxide (ZrO2) as representatives for
bio-persistent granular dust. Hematite
(Fe2O3) represents a nano-sized ultrafine
dust with an iron (Fe) content of ~70%. Asbestos is known to be a
carcinogen associated with the induction of lung cancer,
mesothelioma and lung fibrosis (2). DNA damage and apoptosis are important
downstream effects of asbestos, which occur in all the major lung
target cells studied (3). Exposure
to asbestos fibres causes alterations in cell signalling (4) and induction of various
pro-inflammatory molecules, such as cytokines (5,6). The
pathogenicity of various types of asbestos fibres is thought to be
associated with fibre size, geometry and surface composition
(7). The iron content in
particular has to be considered when assessing the toxicity of
asbestos fibres. Crocidolite
(Na2[Fe3+]2[Fe2+]3Si8O22[OH]2)
typically has a high iron content of ~26%, while the iron content
in chrysotile
(Mg6Si4O10[OH]8) ranges
between 1 and 6%, and is primarily present as a surface contaminant
(8).
In order to verify that the evoked effects are not
only due to the iron content of the investigated particles, we used
hematite with an iron content of ~70%. Hematite, the hexagonal
modification of iron (III) oxide (α-Fe2O3) is
the most important industrial iron oxide used.
Titanium dioxide, also known as titanium (IV) oxide,
is the naturally occurring oxide of titanium, which is commercially
used in a wide range of products, such as paint, varnishes, paper
coating and cosmetics (9,10). Micro-sized titanium dioxide is
suggested to be biologically inert (11,12),
although an inflammatory response has been described (10). Particles can generate reactive
oxygen species; particularly in the case of nano-sized particles,
DNA adducts are observed in human lung cells (9,13).
Additionally, increased micronucleus formation and DNA breakage, as
well as activation of DNA damage checkpoint kinases in
nano-TiO2-treated lymphocytes, have been demonstrated
(14).
Zirconium dioxide, also known as zirconia, is used
in various products, such as ceramic materials, scratch resistant
varnishes and coatings, as well as in medical implants (15,16).
The aim of this study was to compare the effects of
well-defined fibres (UICC, crocidolite and chrysotile ‘A’) and
different size particles (titanium dioxide, zirconium dioxide and
hematite) on human bronchial epithelial cells (BEAS-2B). We focused
on the mRNA expression of 84 signalling molecules attributed to
pathways such as ‘DNA damage and repair’, ‘oxidative/metabolic
stress’, ‘growth arrest and senescence’, ‘inflammation’,
‘proliferation and carcinogenesis’, ‘heat shock’ and
‘apoptosis’.
Materials and methods
Materials
Crocidolite asbestos (UICC, South African NB
#4173-111-3) and chrysotil asbestos (UICC, Rhodesian NB #4173-11-2)
were used as standard references for bio-persistent fibrous dust.
Titanium dioxide anatase (Sigma-Aldrich Chemie GmbH, Steinheim,
Germany; AL232033) and zirconium dioxide (Sigma-Aldrich Chemie
GmbH; AL230693) represented bio-persistent granular dust. Hematite,
α-Fe2O3 (Nanopowder 544884, Sigma-Aldrich
Chemie GmbH) was used to represent ultrafine particles.
Characterization of dust materials
For a detailed description of the characterization
method, refer to a former paper (17). Scanning electron microscopy (SEM;
Hitachi S-2700, Chiyoda, Japan) was used to identify particle
geometry as well as the microstructure of the samples. The element
analysis resulted from energy dispersive X-ray (EDX). To optimize
the conductivity (electron beam), all samples were deposited with a
very fine gold (Au) layer using a sputtering technique.
Transmission electron microscopy (TEM) analysis combined with
electron diffraction (detection of crystallinity) was performed
using a transmission electron microscope H-600 (Hitachi, Japan).
Thermogravimetry (TG) measurements (corundum crucibles, heating
rate 5 K/min and synthetic air atmosphere) for controlling
impurities such as water were conducted using a thermo balance TG
209 F1 Iris (NETZSCH-Gerätebau GmbH, Selb, Germany).
Culture conditions
SV-40 virus-transformed BEAS-2B cells were obtained
from the European collection of cell cultures (ECCC, 95102433).
Approximately 10 million cells (10×106) after
trypsinization and counting using a haemocytometer were plated in
75 cm2 flasks (Falcon; Franklin Lakes, NJ, USA). The
cells were grown in 15 ml Gibco® RPMI 1640 media
containing 10–15% fetal calf serum (FCS), 0.5% gentamycin, 1%
L-glutamine and 1% amphotericin. The cultures were maintained at
37°C and 5% CO2. After a 24-h pre-incubation, the cells
were exposed to crocidolite (5 μg/cm2), chrysotil (1
μg/cm2), zirconium dioxide (10 μg/cm2),
titanium dioxide (10 μg/cm2) or hematite (10
μg/cm2) for 48 h. Unexposed cells served as negative
controls. All experiments were repeated 4 times. Cytotoxicity and
genotoxicity analyses were investigated intensively in various cell
systems for crocidolite and chrysotile (18–21),
titanium dioxide (22,23) and hematite (22,24).
Based on the results of the above study, the particle
concentrations did not show any loss of viability in the BEAS-2B
cell line. Additionally, the same concentrations and incubation
times were used in numerous published studies and therefore the
results are comparable.
mRNA extraction and reverse
transcription
After washing twice with PBS (37°C), cells were
trypsinized for ~30 sec with 10 ml of 0.05% trypsin and incubated
for 10 min in 37°C. Detached cells then were resuspended in 5 ml
ice-cold PBS and centrifuged at 400 × g (without brakes) for 10 min
in 15-ml centrifuge tubes. This step was repeated with 1 ml of
ice-cold PBS in 1.5 ml Eppendorf tubes. mRNA was extracted
immediately with RNeasy Mini kit® (Qiagen, Hilden,
Germany) in accordance with the manufacturer’s instructions.
Reverse transcription was accomplished with the RT2
First Strand kit (Qiagen) as suggested by the manufacturer.
RT2 Profiler PCR
Arrays®
The RT2 RNA QC PCR Array®
(SaBiosciences, Qiagen) was used to test for RNA quality and
inhibitors of RT-PCR analyses. For quantitative comparison of mRNA
levels, real-time PCR was performed using RT2 Profiler
PCR Arrays® Human Stress & Toxicity PathwayFinder
PCR Array® (SaBiosciences). For each condition, four
assays were carried out as independent samples. Gene expression was
related to the mean expression of β2 microglobulin (B2M)
and hypoxanthine phosphoribosyltransferase 1 (HPRT) as housekeeping
genes, since these were the two most stable of the five
housekeeping genes included in the array. Only Ct values <35
were included in the calculations.
Statistical analysis
Calculations of expression were performed with the
2−ΔΔCT method according to Pfaffl (25). For analysis the PCR Array Data
Analysis Software (Excel & Web-based) provided by SaBiosciences
was used. The cut-off was set to CT>35. The P-values are
calculated based on a Student’s t-test of the replicate
2−ΔCt values for each gene in the control and treatment
groups. Results are shown as the mean of four samples for each
condition in relation to the mean of four control samples. All
statistical analyses were performed using the statistical software
package, SPSS, 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Characterization of dust samples
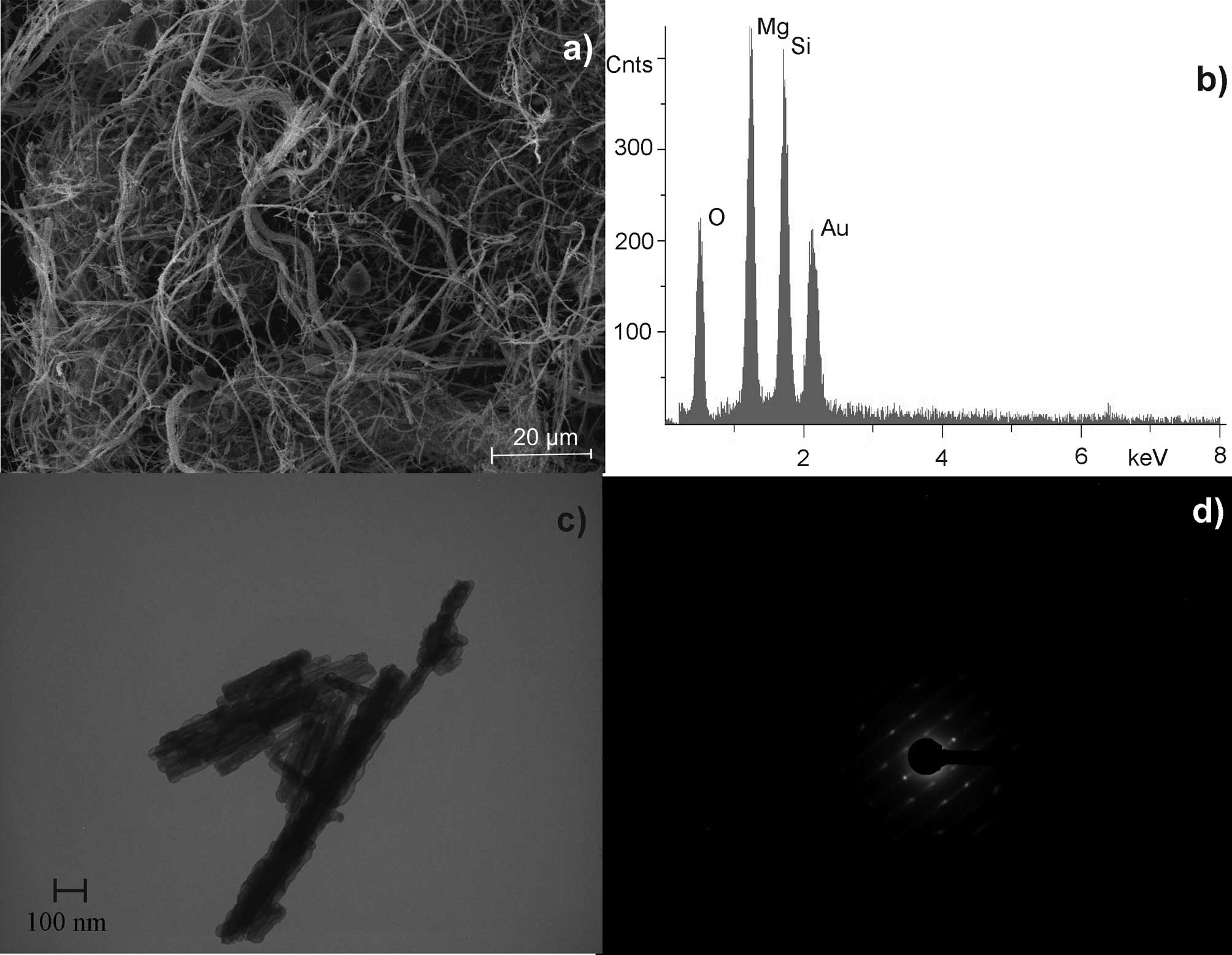
UICC crocidolite South African
(Na2(Fe32+Fe23+[(OH)2|Si8O22])
was shown to have 3,800 fibres/ml at a length of >5 μm and a
diameter of <3 μm. The length to diameter ratio was at least 3:1
(WHO fibres). Crocidolite is a rigid and rod-like fibre with
characteristic iron content (Fig.
1). Gold (Au) was detected in all EDX analyses due to the
sputtering preparation technique.
UICC chrysotile ‘A’ Rhodesian
(Mg6[(OH)8|Si4O10]) was
shown to have 200 fibres/ml at a length of >5 μm and a diameter
of <3 μm. The length to diameter ratio was at least 3:1 (WHO
fibres). Chrysotile has a curly, pliable structure with nearly
equal magnesium (Mg)/silicon (Si) distribution (Fig. 2).
Irregularly shaped crystalline titanium dioxide
aggregates (diameter, 1–3 μm) were observed (Fig 3). The micro-sized aggregates were
composed of ~20 primary particles with a diameter between 100 and
200 nm. The specific surface (BET) of titanium dioxide was 9.9
m2/g. Evaluation of the BET (for titanium dioxide and
zirconium dioxide) was performed by K.-P- Company for surface- and
solid state analysis mbH (o.f.u), Hamburg, Germany (Report B0104014
for the Federal Institute of Occupational Safety and Medicine, May
2001).
For zirconium dioxide, an aggregate diameter of 1–2
μm was determined. The crystalline aggregates were composed of ~50
primary particles with a diameter of ~100 nm (Fig. 4). The specific surface (BET) of
zirconium dioxide was 5.9 m2/g.
Hematite was found to be a spherical formed,
nano-sized material. Agglomerates of 0.2–2 μm were formed by 50–500
primary particles with a diameter of ~20 nm. Additionally, smaller
aggregates (<100 nm) were detected by electron microscopy
(Fig. 5). The usually observed
integration of water within the crystal lattice, caused by the
production (precipitation) process of hematite, was excluded by TG
(26–28).
After a 48-h exposure to the described fibres and
particles, relative mRNA expression of 84 genes were determined
four times. The average and standard deviation of the Ct values of
each gene are shown in Table
I.
 | Table IRelative mRNA expression of 84 genes
after incubation of fibrous and granular dust in bronchial
epithelial cells (BEAS-2B). |
Table I
Relative mRNA expression of 84 genes
after incubation of fibrous and granular dust in bronchial
epithelial cells (BEAS-2B).
| | | Control group | Crocidolite | Chrysotile |
ZrO2 |
TiO2 | Hematite |
|---|
| | |
|
|
|
|
|
|
|---|
| Pathway | Symbol | Refseq | AVG Ct | ± SD | AVG Ct | ± SD | AVG Ct | ± SD | AVG Ct | ± SD | AVG Ct | ± SD | AVG Ct | ± SD |
|---|
| Apoptosis
signalling | ANXA5 | NM_001154 | 20.94 | 0.18 | 21.15 | 0.28 | 20.96 | 0.49 | 21.26 | 0.92 | 21.33 | 0.58 | 21.04 | 0.31 |
| BAX | NM_004324 | 23.24 | 0.18 | 23.52 | 0.11 | 23.45 | 0.51 | 23.83 | 0.63 | 23.76 | 0.41 | 23.59 | 0.41 |
| BCL2L1 | NM_138578 | 26.37 | 0.33 | 26.29 | 0.21 | 26.55 | 0.29 | 26.85 | 0.16 | 27 | 0.33 | 27.41 | 1.36 |
| CASP1 | NM_033292 | 25.53 | 0.24 | 25.75 | 0.19 | 25.66 | 0.45 | 25.59 | 0.46 | 25.94 | 0.62 | 25.64 | 0.35 |
| CASP10 | NM_001230 | 30.54 | 0.34 | 30.76 | 0.39 | 30.61 | 0.54 | 31.16 | 0.72 | 30.92 | 0.52 | 30.86 | 0.60 |
| CASP8 | NM_001228 | 29.59 | 0.18 | 29.36 | 0.40 | 29.34 | 0.58 | 29.84 | 0.47 | 29.67 | 0.80 | 29.22 | 0.53 |
| FASLG | NM_000639 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 |
| NFKBIA | NM_020529 | 23.25 | 0.27 | 23.57 | 0.27 | 23.52 | 0.43 | 23.28 | 0.54 | 23.67 | 0.56 | 23.03 | 0.33 |
| TNF | NM_000594 | 35 | 0.01 | 35 | 0.00 | 34.99 | 0.03 | 34.76 | 0.26 | 35 | 0.00 | 34.70 | 0.60 |
| TNFRSF1A | NM_001065 | 23.27 | 0.28 | 23.56 | 0.25 | 23.33 | 0.38 | 23.38 | 0.60 | 24.02 | 0.61 | 23.33 | 0.38 |
| TNFSF10 | NM_003810 | 27.03 | 0.24 | 27.70 | 0.39 | 27.58 | 0.30 | 27.81 | 1.02 | 28.21 | 0.59 | 27.59 | 0.52 |
| DNA damage and
repair | ATM | NM_000051 | 29.32 | 0.09 | 29.36 | 0.36 | 29.45 | 0.34 | 29.68 | 0.25 | 29.75 | 0.47 | 29.69 | 0.89 |
| CHEK2 | NM_007194 | 26.51 | 0.27 | 27.03 | 0.28 | 27.02 | 0.53 | 27.04 | 0.36 | 27.36 | 0.49 | 26.99 | 0.54 |
| DDB1 | NM_001923 | 24.18 | 0.21 | 24.27 | 0.27 | 24.59 | 0.37 | 24.81 | 0.28 | 24.71 | 0.40 | 24.75 | 0.84 |
| ERCC1 | NM_001983 | 24.71 | 0.36 | 24.80 | 0.19 | 24.82 | 0.56 | 25.33 | 0.72 | 25.23 | 0.41 | 25.04 | 1.00 |
| ERCC3 | NM_000122 | 26.25 | 0.40 | 26.37 | 0.26 | 26.43 | 0.46 | 26.88 | 0.65 | 26.72 | 0.43 | 26.58 | 1.03 |
| RAD23A | NM_005053 | 24.74 | 0.34 | 25.04 | 0.23 | 25.19 | 0.47 | 25.63 | 1.11 | 25.39 | 0.34 | 24.90 | 0.68 |
| RAD50 | NM_005732 | 28.39 | 0.56 | 28.66 | 0.39 | 28.93 | 0.51 | 29.54 | 0.71 | 29.10 | 0.71 | 29.07 | 1.36 |
| UGT1A4 | NM_007120 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 |
| UNG | NM_003362 | 24.48 | 0.25 | 24.74 | 0.14 | 24.80 | 0.26 | 24.70 | 0.35 | 25.07 | 0.37 | 24.68 | 0.21 |
| XRCC1 | NM_006297 | 24.90 | 0.38 | 25.29 | 0.21 | 25.38 | 0.45 | 25.52 | 0.62 | 25.70 | 0.35 | 25.19 | 0.54 |
| XRCC2 | NM_005431 | 26.83 | 0.20 | 27.43 | 0.75 | 27.16 | 0.43 | 27.63 | 0.56 | 27.55 | 0.26 | 27.23 | 0.69 |
| Growth arrest and
senescence | CDKN1A | NM_000389 | 21.32 | 0.20 | 21.80 | 0.15 | 21.79 | 0.61 | 21.62 | 0.47 | 22.22 | 0.41 | 21.61 | 0.13 |
| DDIT3 | NM_004083 | 24.77 | 0.10 | 25.05 | 0.23 | 25.04 | 0.51 | 25.18 | 0.63 | 25.08 | 0.36 | 24.68 | 0.36 |
| GADD45A | NM_001924 | 26.80 | 0.40 | 26.90 | 0.28 | 26.99 | 0.55 | 27.29 | 0.71 | 27.44 | 0.53 | 26.85 | 0.68 |
| GDF15 | NM_004864 | 22.93 | 0.10 | 23.08 | 0.09 | 23.21 | 0.38 | 23.41 | 0.59 | 23.56 | 0.52 | 23.01 | 0.17 |
| IGFBP6 | NM_002178 | 23.26 | 0.21 | 23.72 | 0.14 | 23.55 | 0.60 | 23.97 | 0.78 | 23.94 | 0.46 | 23.74 | 0.77 |
| MDM2 | NM_002392 | 22.95 | 0.23 | 23.40 | 0.25 | 23.49 | 0.48 | 23.53 | 0.38 | 23.92 | 0.26 | 23.73 | 0.82 |
| TP53 | NM_000546 | 23.63 | 0.40 | 24.03 | 0.19 | 24.03 | 0.40 | 24.69 | 1.18 | 24.49 | 0.33 | 24.24 | 0.98 |
| Heat shock | DNAJA1 | NM_001539 | 22.66 | 0.30 | 23.08 | 0.28 | 23.06 | 0.39 | 23.25 | 0.64 | 23.06 | 0.61 | 22.84 | 0.56 |
| DNAJB4 | NM_007034 | 25.19 | 0.37 | 25.41 | 0.32 | 25.61 | 0.49 | 25.83 | 0.32 | 25.85 | 0.38 | 25.63 | 0.84 |
| HSF1 | NM_005526 | 23.21 | 0.28 | 23.62 | 0.26 | 23.48 | 0.50 | 23.73 | 0.74 | 23.66 | 0.71 | 22.89 | 0.27 |
| HSPA1A | NM_005345 | 22 | 0.20 | 22.27 | 0.24 | 22.17 | 0.47 | 22.29 | 0.46 | 22.53 | 0.65 | 21.95 | 0.34 |
| HSPA1L | NM_005527 | 30.30 | 0.37 | 30.63 | 0.15 | 30.51 | 0.46 | 31.09 | 0.71 | 30.60 | 0.51 | 30.45 | 0.51 |
| HSPA2 | NM_021979 | 25.13 | 0.13 | 25.48 | 0.24 | 25.49 | 0.32 | 25.58 | 0.47 | 26.09 | 0.50 | 25.57 | 0.09 |
| HSPA4 | NM_002154 | 25.53 | 0.35 | 25.92 | 0.19 | 25.92 | 0.48 | 26.15 | 0.24 | 26.19 | 0.39 | 25.94 | 0.62 |
| HSPA5 | NM_005347 | 26.45 | 0.18 | 26.39 | 0.30 | 26.36 | 0.37 | 26.50 | 0.22 | 26.24 | 0.47 | 25.93 | 0.21 |
| HSPA6 | NM_002155 | 34.11 | 0.37 | 34.13 | 0.47 | 34.25 | 0.25 | 34.31 | 0.60 | 34.17 | 0.58 | 33.75 | 0.47 |
| HSPA8 | NM_006597 | 21.29 | 0.30 | 21.70 | 0.23 | 21.70 | 0.57 | 21.97 | 0.44 | 21.87 | 0.48 | 21.69 | 0.63 |
| HSPB1 | NM_001540 | 20.63 | 0.31 | 21.23 | 0.30 | 21.15 | 0.57 | 21.27 | 0.61 | 21.32 | 0.62 | 20.69 | 0.28 |
| HSP90AA2 | NM_001040141 | 27.19 | 0.37 | 27.58 | 0.34 | 27.35 | 0.58 | 27.51 | 0.56 | 27.37 | 0.87 | 26.63 | 0.24 |
| HSP90AB1 | NM_007355 | 20.68 | 0.37 | 22.64 | 2.85 | 21.25 | 0.61 | 21.18 | 0.49 | 21.35 | 0.58 | 20.77 | 0.39 |
| HSPD1 | NM_002156 | 22.56 | 0.40 | 22.93 | 0.27 | 22.73 | 0.55 | 23.14 | 0.67 | 23.20 | 0.51 | 22.61 | 0.49 |
| HSPE1 | NM_002157 | 21.75 | 0.24 | 22.05 | 0.44 | 21.88 | 0.44 | 22.26 | 0.44 | 22.21 | 0.63 | 21.64 | 0.25 |
| HSPH1 | NM_006644 | 25.70 | 0.40 | 26.12 | 0.44 | 25.89 | 0.59 | 26.31 | 0.57 | 25.96 | 0.79 | 25.54 | 0.26 |
| Inflammation | CCL21 | NM_002989 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 |
| CCL3 | NM_002983 | 33.94 | 0.25 | 33.55 | 0.54 | 32.94 | 0.58 | 34.04 | 0.50 | 33.27 | 0.33 | 33.34 | 1.12 |
| CCL4 | NM_002984 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 |
| CSF2 | NM_000758 | 30.44 | 0.22 | 30.46 | 0.26 | 30.46 | 0.39 | 30.74 | 0.99 | 30.79 | 0.68 | 30.15 | 0.35 |
| CXCL10 | NM_001565 | 34.55 | 0.55 | 35 | 0.00 | 34.69 | 0.42 | 35 | 0.00 | 34.96 | 0.08 | 35 | 0.00 |
| IL18 | NM_001562 | 24.38 | 0.22 | 24.89 | 0.27 | 24.93 | 0.77 | 25.16 | 0.87 | 25.18 | 0.39 | 24.58 | 0.36 |
| IL1A | NM_000575 | 28.48 | 0.25 | 28.70 | 0.18 | 28.75 | 0.53 | 29.04 | 0.55 | 29.23 | 0.34 | 28.77 | 0.76 |
| IL1B | NM_000576 | 27.65 | 0.29 | 27.83 | 0.28 | 27.95 | 0.56 | 28.35 | 0.75 | 28.51 | 0.41 | 27.84 | 0.45 |
| IL6 | NM_000600 | 26.89 | 0.31 | 26.73 | 0.25 | 26.88 | 0.46 | 27.37 | 0.66 | 27.43 | 0.53 | 26.62 | 0.39 |
| LTA | NM_000595 | 34.47 | 0.50 | 34.62 | 0.51 | 34.28 | 0.83 | 34.75 | 0.43 | 34.77 | 0.14 | 34.26 | 0.12 |
| MIF | NM_002415 | 18.60 | 0.03 | 18.95 | 0.31 | 18.83 | 0.45 | 18.94 | 0.42 | 19.24 | 0.47 | 18.61 | 0.35 |
| NFKB1 | NM_003998 | 23.72 | 0.28 | 24.17 | 0.35 | 23.97 | 0.28 | 23.97 | 0.49 | 24.51 | 0.56 | 23.75 | 0.28 |
| NOS2 | NM_000625 | 33 | 0.43 | 33.20 | 0.21 | 32.93 | 0.50 | 33.23 | 0.92 | 33.20 | 0.76 | 32.88 | 0.14 |
| SERPINE1 | NM_000602 | 25.42 | 0.18 | 25.53 | 0.29 | 25.56 | 0.39 | 25.80 | 0.32 | 25.77 | 0.35 | 25.22 | 0.58 |
| Oxidative or
metabolic stress | CAT | NM_001752 | 25.52 | 0.24 | 25.48 | 0.12 | 25.52 | 0.43 | 25.80 | 0.52 | 26.03 | 0.49 | 25.90 | 0.55 |
| CRYAB | NM_001885 | 29.21 | 0.43 | 29.88 | 0.53 | 29.75 | 0.83 | 29.80 | 1.10 | 30.21 | 1.05 | 29.32 | 0.22 |
| CYP1A1 | NM_000499 | 33.09 | 0.38 | 33.30 | 0.35 | 33.20 | 0.49 | 33.14 | 0.56 | 33.31 | 0.51 | 32.97 | 0.19 |
| CYP2E1 | NM_000773 | 33.96 | 0.30 | 34 | 0.48 | 34.13 | 0.53 | 34.09 | 0.79 | 34.22 | 0.44 | 34.16 | 0.63 |
| CYP7A1 | NM_000780 | 35 | 0.00 | 34.92 | 0.16 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 |
| EPHX2 | NM_001979 | 33.73 | 0.44 | 33.76 | 0.46 | 33.78 | 1.11 | 34.41 | 0.59 | 33.65 | 0.60 | 33 | 0.25 |
| FMO1 | NM_002021 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 | 35 | 0.00 |
| FMO5 | NM_001461 | 30.80 | 0.37 | 31.13 | 0.33 | 31.26 | 0.57 | 31.56 | 0.46 | 31.86 | 0.54 | 31.55 | 0.72 |
| GPX1 | NM_000581 | 19.95 | 0.20 | 20.54 | 0.34 | 20.44 | 0.58 | 20.70 | 0.81 | 20.76 | 0.51 | 19.90 | 0.42 |
| GSR | NM_000637 | 28.92 | 0.54 | 28.85 | 0.37 | 29.13 | 0.36 | 29.15 | 0.12 | 28.92 | 0.43 | 28.89 | 0.95 |
| GSTM3 | NM_000849 | 28.58 | 0.41 | 29.26 | 0.53 | 29.31 | 0.66 | 29.31 | 0.37 | 29.50 | 0.61 | 29.01 | 0.52 |
| HMOX1 | NM_002133 | 23.13 | 0.23 | 23.60 | 0.39 | 23.63 | 0.59 | 23.80 | 0.75 | 23.40 | 0.64 | 22.63 | 0.46 |
| MT2A | NM_005953 | 17.82 | 0.15 | 18.12 | 0.39 | 18.03 | 0.24 | 18.01 | 0.56 | 18.26 | 0.53 | 17.71 | 0.27 |
| POR | NM_000941 | 27.09 | 0.42 | 27.38 | 0.32 | 27.29 | 0.64 | 28.10 | 0.87 | 27.80 | 0.34 | 27.36 | 0.95 |
| PRDX1 | NM_002574 | 25.53 | 0.11 | 25.83 | 0.38 | 25.68 | 0.42 | 25.94 | 0.41 | 26.04 | 0.54 | 25.66 | 0.44 |
| PRDX2 | NM_005809 | 23.35 | 0.29 | 23.80 | 0.26 | 23.64 | 0.54 | 24.03 | 0.81 | 24.07 | 0.19 | 23.67 | 0.75 |
| PTGS1 | NM_000962 | 30.79 | 0.30 | 31.16 | 0.34 | 31.17 | 0.52 | 30.95 | 0.63 | 31.46 | 0.52 | 30.85 | 0.31 |
| SOD1 | NM_000454 | 20.90 | 0.25 | 21.32 | 0.25 | 21.35 | 0.21 | 21.10 | 0.29 | 21.62 | 0.38 | 21.01 | 0.24 |
| SOD2 | NM_000636 | 22.73 | 0.45 | 23 | 0.18 | 23.07 | 0.44 | 23.10 | 0.32 | 23.49 | 0.38 | 23.02 | 0.59 |
| Proliferation and
carcinogenesis | CCNC | NM_005190 | 24.85 | 0.42 | 24.74 | 0.20 | 24.83 | 0.35 | 24.72 | 0.34 | 24.93 | 0.63 | 25.24 | 0.97 |
| CCND1 | NM_053056 | 29.24 | 0.24 | 29.24 | 0.33 | 28.98 | 0.36 | 29.68 | 0.52 | 28.82 | 0.66 | 28.97 | 0.88 |
| CCNG1 | NM_004060 | 22.22 | 0.16 | 22.59 | 0.38 | 22.57 | 0.49 | 22.63 | 0.47 | 22.87 | 0.65 | 22.53 | 0.29 |
| E2F1 | NM_005225 | 26.93 | 0.44 | 27.20 | 0.32 | 27.42 | 0.46 | 27.56 | 0.23 | 27.84 | 0.23 | 27.90 | 1.36 |
| EGR1 | NM_001964 | 25.11 | 0.63 | 25.33 | 0.37 | 25.46 | 0.60 | 25.69 | 0.29 | 26.20 | 0.53 | 25.54 | 0.98 |
| PCNA | NM_182649 | 20.89 | 0.24 | 21.30 | 0.40 | 21.14 | 0.54 | 21.44 | 0.85 | 21.50 | 0.50 | 21.12 | 0.60 |
| HSK | B2M | NM_004048 | 19.20 | 0.40 | 19.90 | 0.23 | 19.97 | 0.54 | 19.87 | 0.62 | 20.10 | 0.63 | 19.54 | 0.50 |
| HPRT1 | NM_000194 | 23.14 | 0.25 | 23.73 | 0.25 | 23.68 | 0.43 | 23.69 | 0.48 | 23.86 | 0.61 | 23.47 | 0.30 |
Direct genotoxicity
Molecules of the ‘DNA damage and repair’ pathway
were assigned to direct genotoxicity. In BEAS-2B cells, UGT1A4 mRNA
expression could not be detected. mRNA expression of the ‘DNA
damage and repair’ pathway was induced mainly by crocidolite,
followed by chrysotile. Zirconium dioxide significantly upregulated
UNG (1.31; P=0.043) but downregulated RAD50 (−1.45; P=0.038), while
neither titanium dioxide nor hematite altered mRNA expression of
‘DNA damage and repair’ pathway signalling molecules (Table II).
 | Table IIComparing mRNA expression (95% CI) of
DNA damage and repair molecules. |
Table II
Comparing mRNA expression (95% CI) of
DNA damage and repair molecules.
| DNA damage and
repair | Crocidolite fold
change (95% CI) | Chrysotile fold
change (95% CI) | Titanium dioxide
fold change (95% CI) | Zirconium dioxide
fold change (95% CI) | Hematite fold
change (95% CI) |
|---|
| ATM | 1.53a (1.07–1.98) | - | - | - | - |
| CHEK2 | - | - | - | - | - |
| DDB1 | 1.48a (1.19–1.76) | - | - | - | - |
| ERCC1 | 1.47a (1.18–1.76) | 1.46a (1.10–1.83) | - | - | - |
| ERCC3 | 1.44a (1.21–1.68) | 1.39a (1.14–1.64) | - | - | - |
| RAD23A | 1.27a (1.06–1.48) | - | - | - | - |
| RAD50 | 1.29b (1.03–1.57) | - | - | −1.45c [−1.19-(−1.85)] | - |
| UGT1A4 | - | - | - | - | - |
| UNG | 1.31a (1.13–1.49) | - | - | 1.31a (1.04–1.58).... | - |
| XRCC1 | 1.20a (1.07–1.34) | - | - | - | - |
| XRCC2 | - | 1.26a (1.04–1.47) | - | - | - |
Indirect genotoxicity
Molecules of the ‘oxidative or metabolic stress’,
‘growth arrest and senescence’ as well as ‘inflammation’ pathway
were assigned to indirect genotoxicity. In BEAS-2B cells, Cyp7A1,
FMO1, CCL21, CCL4, CXCL10 and LTA mRNA expression could not be
detected. The majority of changes in the signalling molecule mRNA
expression of the ‘oxidative or metabolic stress’ pathway were due
to crocidolite. Notably, changes in signalling molecule expression
were comparable for chrysotile, zirconium dioxide, titanium dioxide
and hematite (Table III).
 | Table IIIComparing mRNA expression (95% CI) of
oxidative and metabolic stress molecules. |
Table III
Comparing mRNA expression (95% CI) of
oxidative and metabolic stress molecules.
| Oxidative or
metabolic stress | Crocidolite fold
change (95% CI) | Chrysotile fold
change (95% CI) | Titanium dioxide
fold change (95% CI) | Zirconium dioxide
fold change (95% CI) | Hematite fold
change (95% CI) |
|---|
| CAT | 1.62a (1.25–1.99) | 1.58a (1.15–2.01) | - | - | - |
| CRYAB | - | - | - | - | - |
| CYP1A1 | 1.35a (1.07–1.63) | - | - | 1.47a (1.18–1.76) | 1.37b (1.01–1.72) |
| CY | - | - | - | - | - |
| CYP7A1 | - | - | - | - | - |
| EPHX2 | 1.53a (1.01–1.72) | - | - | 2.09a (1.12–3.05) | |
| FMO1 | - | - | - | - | - |
| FMO5 | - | - | - | - | - |
| GPX1 | - | - | - | - | - |
| GSR | 1.64a (1.11–2.18) | 1.37a (1.04–1.69) | 1.76a (1.02–2.50) | - | - |
| GSTM3 | - | - | - | - | - |
| HMOX1 | - | - | - | 1.46a (1.19–1.73) | 1.79a (1.56–2.01) |
| MT2A | 1.28b (1.02–1.54) | - | 1.30a (1.09–1.50) | 1.34a (1.15–1.53) | 1.37a (1.12–1.61) |
| POR | 1.28a (1.13–1.43) | - | - | - | - |
| PRDX1 | 1.28b (1.01–1.55) | 1.42a (1.09–1.76) | 1.23b (1.01–1.46) | - | - |
| PRDX2 | 1.29a (1.03–1.54) | - | - | - | |
| PTGS1 | - | - | - | 1.37a (1.16–1.58) | 1.21a (1.04–1.39) |
| SOD1 | - | - | - | - | - |
| SOD2 | - | - | - | - | - |
Both fibres showed a moderate increase in signalling
molecule expression of the ‘growth arrest and senescence’ pathway,
while titanium dioxide only induced DDIT3 (1.4, P=0.048). There was
no significant change in mRNA expression due to zirconium dioxide
and hematite (Table IV).
 | Table IVComparing mRNA expression (95% CI) of
DNA growth arrest and senescence molecules. |
Table IV
Comparing mRNA expression (95% CI) of
DNA growth arrest and senescence molecules.
| Growth arrest and
senescence | Crocidolite fold
change (95% CI) | Chrysotile fold
change (95% CI) | Titanium dioxide
fold change (95% CI) | Zirconium dioxide
fold change (95% CI) | Hematite fold
change (95% CI) |
|---|
| CDKN1A |
| DDIT3 | 1.30a (1.02–1.57) | 1.30a (1.02–1.57) | 1.40a (1.02–1.57) | - | - |
| GADD45A | 1.46a (1.11–1.80) | - | - | - | - |
| GDF15 | 1.41a (1.13–1.69) | - | - | - | - |
| IGFBP6 | - | 1.30a (1.13–1.69) | - | - | - |
| MDM2 | - | - | - | - | - |
| TP53 | 1.19a (1.02–1.35) | - | - | - | - |
Molecules belonging to the ‘inflammation’ pathway
were induced mainly by crocidolite. Chrysotile and hematite
provoked a comparable moderate increase in gene expression.
Titanium dioxide distinctly induced CCL3 (2.79, P=0.007), while
there were no expression changes due to zirconium dioxide (Table V).
 | Table VComparing mRNA expression (95% CI) of
inflammatory molecules. |
Table V
Comparing mRNA expression (95% CI) of
inflammatory molecules.
| Inflammation | Crocidolite fold
change (95% CI) | Chrysotile fold
change (95% CI) | Titanium dioxide
fold change (95% CI) | Zirconium dioxide
fold change (95% CI) | Hematite fold
change (95% CI) |
|---|
| CCL21 | | | | | |
| CCL3 | 2.05a (1.34–2.77) | 3.16a (1.47–4.85) | 2.79a (1.63–3.94) | - | - |
| CCL4 | - | - | - | - | - |
| CSF2 | 1.54a (1.28–1.80) | - | - | - | 1.54a (1.20–1.88) |
| CXCL10 | - | - | - | - | - |
| IL18 | - | - | - | - | - |
| IL1A | 1.34a (1.09–1.59) | - | - | - | - |
| IL1B | 1.39a (1.30–1.47) | - | - | - | - |
| IL6 | 1.76a (1.48–2.04) | 1.59a (1.24–1.95) | - | - | 1.53a (1.34–1.72) |
| LTA | - | - | - | - | - |
| MIF | - | 1.35a (1.34–1.72) | - | - | - |
| NFKB1 | - | - | - | - | - |
| NOS2 | - | - | - | - | - |
| SERPINE1 | 1.45a (1.25–1.65) | 1.42a (1.14–1.70) | - | - | 1.45a (1.14–1.70) |
Initiation and promotion of
carcinogenesis
Molecules of the ‘proliferation and carcinogenesis’
pathway were assigned to initiation and promotion of
carcinogenesis. The only gene of this pathway, which was induced
was Cyclin D1 (CCND1). Cyclin D expression was induced by
crocidolite (1.57, P=0.004), chrysotile (1.89, P=0.019) and
titanium dioxide (2.36, P=0.007) (Table VI).
 | Table VIComparing mRNA expression (95% CI) of
DNA proliferation and carcinogenesis molecules. |
Table VI
Comparing mRNA expression (95% CI) of
DNA proliferation and carcinogenesis molecules.
| Proliferation and
carcinogenesis | Crocidolite fold
change (95% CI) | Chrysotile fold
change (95% CI) | Titanium dioxide
fold change (95% CI) | Zirconium dioxide
fold change (95% CI) | Hematite fold
change (95% CI) |
|---|
| CCNC | | | | | |
| CCND1 | 1.57a (1.28–1.87) | 1.89a (1.33–2.44) | 2.36a (1.33–2.44) | - | - |
| CCNG1 | - | - | - | - | - |
| E2F1 | - | - | - | - | - |
| EGR1 | - | - | - | - | - |
| PCNA | - | - | - | - | - |
Acute toxicity and/or genotoxicity
Molecules of the ‘heat shock’ and ‘apoptosis’
pathways were assigned to acute toxicity and/or genotoxicity. Of
all investigated pathways, the greatest changes were found within
these two pathways. Crocidolite, titanium dioxide and hematite
provoked the most changes in mRNA expression of signalling
molecules of the ‘heat shock’ pathway, while crocidolite and
zirconium dioxide provoked the most changes in mRNA expression of
signalling molecules of the ‘apoptosis pathway’. Chrysotile showed
a moderate increase of ‘heat shock’ genes and only a moderate
increase of ‘apoptosis’ genes (Tables VII and VIII).
 | Table VIIComparing mRNA expression (95% CI) of
heat shock molecules. |
Table VII
Comparing mRNA expression (95% CI) of
heat shock molecules.
| Heat shock | Crocidolite fold
change (95% CI) | Chrysotile fold
change (95% CI) | Titanium dioxide
fold change (95% CI) | Zirconium dioxide
fold change (95% CI) | Hematite fold
change (95% CI) |
|---|
| DNAJA1 | 1.17a (1.03–1.31) | - | - | - | - |
| DNAJB4 | - | - | - | - | - |
| HSF1 | - | 1.31a (1.05–1.56) | 1.29a (1.14–1.44) | - | 1.57a (1.33–1.81) |
| HSPA1A | 1.30a (1.10–1.51) | - | 1.22a (1.05–1.38) | 1.25a (1.06–1.44) | - |
| HSPA1L | 1.25a (1.05–1.46) | 1.36a (1.13–1.60) | 1.43a (1.21–1.64) | - | - |
| HSPA2 | - | - | - | - | - |
| HSPA4 | - | - | - | - | - |
| HSPA5 | 1.63a (1.21–2.04) | 1.67a ( 1.07–2.27) | 2.02a (1.37–2.67) | - | 1.81a (1.25–2.36 ) |
| HSPA6 | - | - | 1.68b (0.83–2.53) | - | - |
| HSPA8 | 1.19a (1.09–1.28) | 1.19b (1.02–1.35) | - | - | - |
| HSPB1 | - | - | - | - | 1.22a (1.09–1.34) |
| HSP90AA2 | 1.20a (1.03–1.37) | - | 1.54a (1.10–1.98) | - | 1.86a (1.44, 2.28) |
| HSP90AB1 | - | - | - | - | 1.19a (1.05–1.32) |
| HSPD1 | - | 1.40a (1.18–1.62) | - | - | 1.23a (1.03–1.43) |
| HSPE1 | - | - | - | - | 1.36a (1.02–1.69) |
| HSPH1 | - | - | 1.47a (1.12–1.82) | - | 1.41a (1.15–1.68) |
 | Table VIIIComparing mRNA expression (95% CI) of
apoptosis molecules. |
Table VIII
Comparing mRNA expression (95% CI) of
apoptosis molecules.
| Apoptosis | Crocidolite fold
change (95% CI) | Chrysotile fold
change (95% CI) | Titanium dioxide
fold change (95% CI) | Zirconium dioxide
fold change (95% CI) | Hematite fold
change (95% CI) |
|---|
| ANXA5 | - | 1.55b (1.01–2.08) | - | - | - |
| BAX | 1.29a (1.05–1.53) | 1.36b (1.00–1.72) | - | - | - |
| BCL2L1 | 1.66a (1.30–2.03) | - | - | - | - |
| CASP1 | 1.35b (1.11–1.58) | 1.44a (1.07–1.81) | 1.32a (1.02–1.61).... | 1.46a (1.21–1.70) | - |
| CASP10 | - | - | - | - | - |
| CASP8 | 1.84a (1.22–2.45) | 1.87b (1.01–2.73) | - | - | - |
| FASLG | - | - | - | - | - |
| NFKBIA | - | - | 1.31a (1.06–1.57).... | 1.50a (1.21–1.78) | 1.47a (1.15–1.79) |
| TNF | - | - | - | - | - |
| TNFRSF1A | - | - | - | 1.42a (1.02–1.69) | - |
| TNFSF10 | - | - | −1.29c [−1.11–(−1.45)] | - | - |
A comparison of the pathways induced by crocidolite,
chrysotile, titanium dioxide, zirconium dioxide and hematite is
provided in Table IX.
 | Table IXComparison of induced signalling
pathways by investigated particles. |
Table IX
Comparison of induced signalling
pathways by investigated particles.
| Direct
genotoxicity | Indirect
genotoxicity | Initiation and
promotion of carcinogenesis | Acute toxicity
and/or genotoxicity |
|---|
|
|
|
|
|
|---|
| DNA damage and
repair | Oxidative or
metabolic stress | Growth arrest and
senescence | Inflammation | Proliferation and
carcinogenesis | Heat shock | Apoptosis |
|---|
| Crocidolite | XX | XX | X | XX | (X) | XX | XX |
| Chrysotile | X | X | X | X | (X) | X | (X) |
| TiO2
Anastas | - | X | (X) | (X) | (X) | XX | X |
|
ZrO2 | (X) | X | - | - | - | (X) | XX |
| Hematite | - | X | - | X | - | XX | (X) |
Discussion
In this study, we compared the ability of two
different fibres (crocidolite and chrysotile) and three different
sized particles (titanium dioxide, zirconium dioxide and hematite)
to induce the mRNA expression of signalling molecules involved in
diverse pathways. We characterized the toxicologically relevant
chemical and physical properties of the fibres and particles to
ensure the comparability of the present results. UICC crocidolite
South African and UICC chrysotile ‘A’ are asbestos fibres, and
their cytotoxic and genotoxic potential is well studied. The
selected bio-persistent dust particles, titanium dioxide (100–200
nm) and zirconium dioxide (50–100 nm), were of the same origin as
formerly used in vivo(29).
After intratracheal installation, both particles induced lung
tumours in female SPF Wistar rats (29). Hematite (20 nm), the smallest of
all particles, was investigated, to observe whether the obtained
reaction may be provoked by the iron content.
Asbestos fibres caused the most relevant changes in
gene expression of all tested pathways. This finding is in
accordance with the general knowledge that crocidolite as well as
chrysotile are asbestos fibres with a high cytotoxic and genotoxic
effect (2,20,21,30,31).
A literature search, including in vitro analysis, animal
experiments and epidemiological studies, confirmed that all fibre
types show comparable harmful effects (32). Chrysotile is, due to its higher
solubility, less bio-persistent than the crocidolite (33). Since our study determines the early
effects (48 h) of fibres and particles, the 5-year clearance rate
is of minor relevance to our results. In accordance with our study,
it appears that chrysotile and crocidolite develop their
genotoxicity due to direct and indirect (inflammatory driven)
molecular mechanisms (18,19,34–36).
The iron content appears to not to be of major
relevance for the observed induction of direct genotoxicity or the
initiation and promotion of carcinogenesis, since these pathways
are not induced by hematite (Fe 70%) but by zirconium dioxide (Fe
0%). In a study by Schürkes et al, the iron content appeared
not to be relevant for the induction of 8-hydroxydeoxyguanosine
(8-OHdG), since fibres with different iron amounts (0.025–20%)
revealed comparable results (35).
In the present study, nano-sized hematite and titanium dioxide
showed an inflammatory and oxidative stress response and a high
increase in gene expression attributed to the ‘heat shock’ pathway.
These findings are in accordance with the results of Park et
al, where single intratracheal instillation of iron
nanoparticles (NP) in mice elevated the expression of many genes
related to inflammation or tissue damage, such as heat shock
proteins (37). Additionally,
significant generation of ROS was described for titanium dioxide-NP
and hematite (9,24,38).
None of the investigated genes of the ‘DNA damage and repair’
pathway were induced by hematite or titanium dioxide in our study.
Nanoparticles of hematite but not those of titanium dioxide induced
significant DNA-breakage, measured by the Comet-assay in IMR-90
cells. DNA-damage and cytotoxic effects by hematite in BEAS-2B
cells were not observed until a concentration of 50
μg/cm2 was used (9). On
the contrary to ultrafine titanium dioxide, there were no
significant alterations in micronuclei induction by fine titanium
dioxide observed in Syrian hamster embryo cells (23). Incorporation into human lung cells
was described for fine and ultrafine titanium dioxide as well as
for hematite (24,39).
Notably, Cyclin D1 which, as a regulatory subunit of
CDK4 or CDK6, promotes cell cycle progression through G1-phase is
significantly upregulated by titanium dioxide (relative expression
2.36) correspondingly with chrysotile (relative expression 1.89)
and crocidolite (relative expression 1.57). The deregulation of
cyclin D1 plays an important role in tumorigenesis and has
frequently been linked to various types of human cancer (40).
Zirconium dioxide with particle sizes between 50 and
100 nm induced molecules attributed to the ‘oxidative or metabolic
stress’ pathway, which suggests an indirect genotoxicity. We also
found a high increase of apoptotic molecules. Zirconium dioxide
induced UNG, which eliminates uracil from DNA molecules by cleaving
the N-glycosylic bond and initiates the base-excision repair (BER)
pathway. Uracil appearing in DNA, for example as a result of
cytosine deamination, is potentially mutagenic and deleterious for
cell regulation (41).
In particular, properties such as size, geometry,
chemical composition and surface behaviour of particles play
important roles in interaction with cells and modify their
pathogenicity. Many published studies are missing detailed
information on properties and the concentration of the particles
used, which makes it difficult to compare results.
Our study and recent reports in the literature
demonstrate that gene expression profiling in human lung epithelial
cells can be an important tool for analyzing the pathogenicity of
potentially harmful fibres and particles (42–44).
Gene expression profiling, for example in response to asbestos, is
valuable to define early molecular effects as demonstrated in
diverse human cells, such as normal human bronchial epithelial
cells (NHEC) (45), human lung
adenocarcinoma cells (A549) (46,47),
SV40-transformed human bronchial epithelial cells (BEAS-2B) and
SV40-immortalized pleural mesothelial cells (MET5A) (47). Changes in gene expression are also
valuable to determine the pathogenicity pathway of asbestos fibres,
as demonstrated in the human mesothelial (LP9/TERT-1) cell line
(42).
In further studies, new particles can be screened to
complete the toxicological knowledge on the molecular effects and
to assess potentially hazardous risks. Altogether, analysis of gene
expression profiles may play an important role in the early
detection of fibres or potential hazards of particles to human
health.
Acknowledgements
This study was supported by the E.W. Baader-Stiftung
supervised by the German Stiftungszentrum, Barkhovenallee 1, 45239
Essen, Germany, Az. T007/20368/2010/sm.
References
|
1
|
DFG. List of MAK and BAT Values 2011.
Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim: 2011
|
|
2
|
Mossman BT, Bignon J, Corn M, Seaton A and
Gee JB: Asbestos: scientific developments and implications for
public policy. Science. 247:294–301. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamp DW: Asbestos-induced lung diseases:
an update. Transl Res. 153:143–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shukla A, MacPherson MB, Hillegass J,
Ramos-Nino ME, Alexeeva V, Vacek PM, Bond JP, Pass HI, Steele C and
Mossman BT: Alterations in gene expression in human mesothelial
cells correlate with mineral pathogenicity. Am J Respir Cell Mol
Biol. 41:114–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lemaire I and Ouellet S: Distinctive
profile of alveolar macrophage-derived cytokine release induced by
fibrogenic and nonfibrogenic mineral dusts. J Toxicol Environ
Health. 47:465–478. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robledo R and Mossman B: Cellular and
molecular mechanisms of asbestos-induced fibrosis. J Cell Physiol.
180:158–166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mossman BT and Churg A: Mechanisms in the
pathogenesis of asbestosis and silicosis. Am J Respir Crit Care
Med. 157:1666–1680. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kamp DW and Weitzman SA: The molecular
basis of asbestos induced lung injury. Thorax. 54:638–652. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhattacharya K, Davoren M, Boertz J,
Schins RP, Hoffmann E and Dopp E: Titanium dioxide nanoparticles
induce oxidative stress and DNA-adduct formation but not
DNA-breakage in human lung cells. Part Fibre Toxicol. 6:172009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hext PM, Tomenson JA and Thompson P:
Titanium dioxide: inhalation toxicology and epidemiology. Ann Occup
Hyg. 49:461–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bernard BK, Osheroff MR, Hofmann A and
Mennear JH: Toxicology and carcinogenesis studies of dietary
titanium dioxide-coated mica in male and female Fischer 344 rats. J
Toxicol Environ Health. 29:417–429. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hart GA and Hesterberg TW: In vitro
toxicity of respirable-size particles of diatomaceous earth and
crystalline silica compared with asbestos and titanium dioxide. J
Occup Environ Med. 40:29–42. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gurr JR, Wang AS, Chen CH and Jan KY:
Ultrafine titanium dioxide particles in the absence of
photoactivation can induce oxidative damage to human bronchial
epithelial cells. Toxicology. 213:66–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang SJ, Kim BM, Lee YJ and Chung HW:
Titanium dioxide nanoparticles trigger p53-mediated damage response
in peripheral blood lymphocytes. Environ Mol Mutagen. 49:399–405.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
DaNa: Zirconium dioxide. http://nanopartikel.info/cms/lang/en/Wissensbasis/Zirkoniumdioxid.
Accessed October 8, 2013
|
|
16
|
NanoCare Project Partners. NanoCare.
Health Related Aspects of Nanomaterials. Final Scientific Report.
Kuhlbusch TAJ, Krug HF and Nau K: 1st edition. DECHEMA eV (in
cooperation with the NanoCare Project Consortium); Frankfurt am
Main: 2009
|
|
17
|
Schneider J, Walter D, Brückel B and
Rödelsperger K: Primary particles and their agglomerate formation
as modifying risk factors of nonfibrous nanosized dust. J Toxicol
Environ Health A. 76:131–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dopp E, Schuler M, Schiffmann D and
Eastmond DA: Induction of micronuclei, hyperdiploidy and
chromosomal breakage affecting the centric/pericentric regions of
chromosomes 1 and 9 in human amniotic fluid cells after treatment
with asbestos and ceramic fibers. Mutat Res. 377:77–87. 1997.
View Article : Google Scholar
|
|
19
|
Burmeister B, Schwerdtle T, Poser I,
Hoffmann E, Hartwig A, Müller WU, Rettenmeier AW, Seemayer NH and
Dopp E: Effects of asbestos on initiation of DNA damage, induction
of DNA-strand breaks, P53-expression and apoptosis in primary,
SV40-transformed and malignant human mesothelial cells. Mutat Res.
558:81–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dopp E, Yadav S, Ansari FA, Bhattacharya
K, von Recklinghausen U, Rauen U, Rödelsperger K, Shokouhi B, Geh S
and Rahman Q: ROS-mediated genotoxicity of asbestos-cement in
mammalian lung cells in vitro. Part Fibre Toxicol. 2:92005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Poser I, Rahman Q, Lohani M, Yadav S,
Becker HH, Weiss DG, Schiffmann D and Dopp E: Modulation of
genotoxic effects in asbestos-exposed primary human mesothelial
cells by radical scavengers, metal chelators and a glutathione
precursor. Mutat Res. 559:19–27. 2004. View Article : Google Scholar
|
|
22
|
Bhattacharya K: Comparative analysis of
fine and nanoparticles for cellular uptake, oxidative stress and
genomic damage in human lung cells (unpublished PhD thesis).
University of Duisburg-Essen; 2009
|
|
23
|
Rahman Q, Lohani M, Dopp E, Pemsel H,
Jonas L, Weiss DG and Schiffmann D: Evidence that ultrafine
titanium dioxide induces micronuclei and apoptosis in Syrian
hamster embryo fibroblasts. Environ Health Perspect. 110:797–800.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bhattacharya K, Hoffmann E, Schins RF, et
al: Comparison of micro- and nanoscale Fe+3-containing
(Hematite) particles for their toxicological properties in human
lung cells in vitro. Toxicol Sci. 126:173–182. 2012.PubMed/NCBI
|
|
25
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Buxbaum G and Paff G: Industrial Inorganic
Pigments. 3rd edition. Wiley-VCH Verlag GmbH & Co. KGaA;
Weinheim: 2005, View Article : Google Scholar
|
|
27
|
Buxbaum G and Printzen H: Ullmann’s
Encyclopedia of Industrial Chemistry. A20. 5th edition. VCH
Verlagsgesellschaft mbH; Weinheim: pp. 2971992
|
|
28
|
Walter D: Characterization of synthetic
hydrous hematite pigments. Thermochimica Acta. 445:195–199. 2006.
View Article : Google Scholar
|
|
29
|
Pott F and Roller M: Carcinogenicity study
with nineteen granular dusts in rats. Eur J Oncol. 10:249–281.
2005.PubMed/NCBI
|
|
30
|
Jones JSP, Smith PG, Pooley FD, et al:
Biological effects of mineral fibres. IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans WHO. International
Agency for Research on Cancer; Lyon: pp. 637–653. 1980
|
|
31
|
WHO, IARC. IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans Overall Evaluations of
Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42.
(Supplement 7)IARC; Lyon: 1987, http://monographs.iarc.fr/ENG/Monographs/suppl7/suppl7.pdf.
Accessed October 8, 2013
|
|
32
|
Baur X, Schneider J, Woitowitz HJ and
Velasco Garrido M: Do advers health effects of chrysotile and
amphibole asbestos differ? Pneumologie. 66:497–506. 2012.(In
German).
|
|
33
|
Bernstein DM, Rogers R and Smith P: The
biopersistence of Canadian chrysotile asbestos following
inhalation. Inhal Toxicol. 15:1247–1274. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dopp E, Poser I and Papp T: Interphase
fish analysis of cell cycle genes in asbestos-treated human
mesothelial cells (HMC), SV40-transformed HMC (MeT-5A) and
mesothelioma cells (COLO). Cell Mol Biol (Noisy-le-grand).
48:OL271–OL277. 2002.PubMed/NCBI
|
|
35
|
Schürkes C, Brock W, Abel J and Unfried K:
Induction of 8-hydroxydeoxyguanosine by man made vitreous fibres
and crocidolite asbestos administered intraperitoneally in rats.
Mutat Res. 553:59–65. 2004.PubMed/NCBI
|
|
36
|
Ruosaari S, Hienonen-Kempas T, Puustinen
A, Sarhadi VK, Hollmén J, Knuutila S, Saharinen J, Wikman H and
Anttila S: Pathways affected by asbestos exposure in normal and
tumour tissue of lung cancer patients. BMC Med Genomics. 1:552008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park EJ, Kim H, Kim Y, Yi J, Choi K and
Park K: Inflammatory responses may be induced by a single
intratracheal instillation of iron nanoparticles in mice.
Toxicology. 275:65–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Könczöl M, Ebeling S, Goldenberg E, Treude
F, Gminski R, Gieré R, Grobéty B, Rothen-Rutishauser B, Merfort I
and Mersch-Sundermann V: Cytotoxicity and genotoxicity of
size-fractionated iron oxide (magnetite) in A549 human lung
epithelial cells: role of ROS, JNK, and NF-kappaB. Chem Res
Toxicol. 24:1460–1475. 2011.PubMed/NCBI
|
|
39
|
Singh S, Shi T, Duffin R, Albrecht C, van
Berlo D, Höhr D, Fubini B, Martra G, Fenoglio I, Borm PJ and Schins
RP: Endocytosis, oxidative stress and IL-8 expression in human lung
epithelial cells upon treatment with fine and ultrafine
TiO2: role of the specific surface area and of surface
methylation of the particles. Toxicol Appl Pharmacol. 222:141–151.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Witzel II, Koh LF and Perkins ND:
Regulation of cyclin D1 gene expression. Biochem Soc Trans.
38:217–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zharkov DO, Mechetin GV and Nevinsky GA:
Uracil-DNA glycosylase: structural, thermodynamic and kinetic
aspects of lesion search and recognition. Mutat Res. 685:11–20.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hillegass JM, Shukla A, MacPherson MB,
Bond JP, Steele C and Mossman BT: Utilization of gene profiling and
proteomics to determine mineral pathogenicity in a human
mesothelial cell line (LP9/TERT-1). J Toxicol Environ Health A.
73:423–436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Perkins TN, Shukla A, Peeters PM,
Steinbacher JL, Landry CC, Lathrop SA, Steele C, Reynaert NL,
Wouters EF and Mossman BT: Differences in gene expression and
cytokine production by crystalline vs. amorphous silica in human
lung epithelial cells. Part Fibre Toxicol. 9:62012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang YC, Karoly ED, Dailey LA, Schmitt
MT, Silbajoris R, Graff DW and Devlin RB: Comparison of gene
expression profiles induced by coarse, fine, and ultrafine
particulate matter. J Toxicol Environ Health A. 74:296–312. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Belitskaya-Levy I, Hajjou M, Su WC, Yie
TA, Tchou-Wong KM, Tang MS, Goldberg JD and Rom WN: Gene profiling
of normal human bronchial epithelial cells in response to asbestos
and benzo(a)pyrene diol epoxide (BPDE). J Environ Pathol Toxicol
Oncol. 26:281–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hevel JM, Olson-Buelow LC, Ganesan B,
Stevens JR, Hardman JP and Aust AE: Novel functional view of the
crocidolite asbestos-treated A549 human lung epithelial
transcriptome reveals an intricate network of pathways with
opposing functions. BMC Genomics. 9:3762008. View Article : Google Scholar
|
|
47
|
Nymark P, Lindholm PM, Korpela MV, Lahti
L, Ruosaari S, Kaski S, Hollmén J, Anttila S, Kinnula VL and
Knuutila S: Gene expression profiles in asbestos-exposed epithelial
and mesothelial lung cell lines. BMC Genomics. 8:622007. View Article : Google Scholar : PubMed/NCBI
|