Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide (1) and
~75–85% of lung cancers are non-small cell lung cancer (NSCLC),
which includes squamous cell carcinoma, adenocarcinoma and large
cell carcinoma. Chemotherapy agents, including cisplatin and
paclitaxel, are the main treatment measures for NSCLC, however, the
side effects of chemotherapy are usually difficult to tolerate,
particularly for elderly patients. Thus, new drugs which are safe
and effective should be developed (2). Natural dietary agents consist of
numerous bioactive compounds that have demonstrated great potential
in preventing and treating a wide variety of diseases, including
various types of cancer, the majority of which have been used as
ancient traditional medicines (3).
We consider this an interesting field worthy of exploration.
ω-3 polyunsaturated fatty acids (n-3 PUFA), in
particular the marine-derived forms eicosapentaenoic acid (EPA) and
docosahexaenoic acid (DHA), have been demonstrated to be natural,
multipotent treatments for a wide variety of diseases. In previous
decades there has been growing interest in the role of n-3 PUFA and
their potential to prevent cancer development and progression
(4,5). This was supported by a large number
of in vitro experiments demonstrating the profound
anti-tumour effects of n-3 PUFA by suppressing neoplastic
transformation, angiogenesis and tumour cell growth (6–8).
Using animal models, it has been repeatedly demonstrated that the
growth of chemically induced cancer and of human cancer xenografts
can be retarded or completely inhibited by the incorporation of n-3
PUFA in the diet (9,10). However, studies in lung cancer are
not sufficient, therefore, in the present study, we explored the
effects of DHA and EPA on the proliferation activity and apoptosis
of the human lung adenocarcinoma cell line A549.
Materials and methods
Cells and reagents
Human lung cancer A549 cells were obtained from The
Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
A549 cells were supplemented with 10% fetal bovine serum and
antibiotics (100 U/ml of penicillin and 100 μg/ml of streptomycin).
The cells were incubated in a humidified incubator under 5%
CO2 at 37°C. DHA, EPA, dimethyl sulfoxide (DMSO),
acridine orange (AO), ethidium bromide (EB) and methyl thiazolyl
tetrazolium (MTT) were obtained from Sigma-Aldrich (St. Louis, MO,
USA). Annexin V-phycoerythrin (Annexin V-PE) and
7-amino-actinomycin D (7-AAD) were obtained from Millipore
(Billerica, MA, USA).
MTT assay for the inhibition of cell
growth
Cells were seeded at a density of 8×103
cells in each well of the 96-well plates and incubated for 24 h. A
series of concentrations of DHA (40, 45, 50 and 55 μg/ml) or EPA
(45, 50, 55 and 60 μg/ml) were added to the wells for 24, 48 and 72
h. MTT (5 g/l, 20 μl/well) was added to each well and incubated at
37°C for 4 h. DMSO was then added (150 μl/well) to each well to
dissolve any crystals and the plates were agitated for 10 min.
Absorbance values at 490 nm were detected by the microplate reader
(Infinite M200; Tecan, Geneva, Switzerland). Cell growth inhibition
was calculated using the formula: Cell growth inhibition rate (%) =
[1 - A490 (experimental group)/A490 (control group)] × 100. Each
experiment was repeated three times.
Apoptosis detected by flow cytometry
Cells were seeded at 3×105 in each well
of the 6-well plates and were incubated with DHA (45 and 50 μg/ml)
or EPA (55 and 60 μg/ml) for 24 h, then cells were collected by
trypsinization and washed with PBS. Following staining with Annexin
V-PE and 7-AAD, respectively, the cells were immediately detected
using flow cytometry (Millipore).
Morphological analysis using fluorescence
microscopy
Cells were seeded at 5×104 in each well
of the 24-well plates and were incubated with DHA (45 and 50 μg/ml)
or EPA (55 and 60 μg/ml) for 24 and 48 h. The cells were then
harvested in an Eppendorf centrifuge tube, centrifuged for 5 min at
106 × g and suspended in PBS containing fluorescence dye AO/EB (AO
and EB were at the concentration of 100 mg/l in PBS) (11). The cells were prepared and placed
onto slides. Cell morphology was observed under a fluorescence
microscope (IX71; Olympus, Tokyo, Japan) and images were
captured.
Transmission electron microscope
Cells were seeded at 1×105 in each well
of the 6-well plates and incubated with DHA (50 μg/ml) or EPA (60
μg/ml) for 24 h. The cells were collected by trypsinization, washed
with PBS, fixed in 2.5% glutaraldehyde at 4°C for 2 h and then
washed again twice with PBS. The material was dehydrated in a
graded series of ethanol (50, 70, 80, 90 and 100%) and acetone for
15 min each and embedded in Epon 812. Ultrathin sections were
stained with uranyl acetate and lead acetate, followed by an
examination with a transmission electron microscope (TEM;
JEM1002CXII; Hitachi, Tokyo, Japan).
Statistical analysis
The results are expressed as the mean ± standard
deviation. SPSS 17.0 statistical software (SPSS Inc., Chicago, IL,
USA) was used to analyze the results. One-way ANOVA, Dunnett’s
t-test and Pearson’s correlation were used in the present study.
All the tests performed were two-sided. P<0.05 was considered to
indicate a statistically significant difference.
Results
n-3 PUFA inhibit the proliferation of
A549 cells
A549 cells were treated with different doses of DHA
(40, 45, 50 and 55 μg/ml) or EPA (45, 50, 55 and 60 μg/ml) for 24,
48 and 72 h. An MTT assay was used to examine the
anti-proliferative effect of DHA/EPA on A549 cells. As shown in
Fig. 1, DHA and EPA significantly
suppressed the proliferation of A549 cells, in a dose- and
time-dependent manner. The inhibitory rates of DHA and EPA on cell
growth were 97.99±1.13 and 77.99±4.43%, respectively, when treated
with high concentrations for 72 h.
 | Figure 1MTT assay for the cell growth
inhibition of A549 cells. DHA and EPA significantly suppressed the
proliferation of A549 cells, in a dose- and time-dependent manner.
Growth inhibition rates of A549 cells treated with (A) DHA (40, 45,
50 and 55 μg/ml) for 24, 48 and 72 h and (B) EPA (45, 50, 55 and 60
μg/ml) for 24, 48 and 72 h, respectively. MTT, methyl thiazolyl
tetrazolium; DHA, docosahexaenoic acid; EPA, eicosapentaenoic
acid. |
n-3 PUFA induce apoptosis in A549
cells
A549 cells were treated with different doses of DHA
(45 and 50 μg/ml) or EPA (55 and 60 μg/ml) for 24 h. Flow cytometry
was used to assay the apoptosis by Annexin V-PE/7-AAD staining.
Each concentration was measured three times. As shown in Fig. 2, DHA and EPA significantly induced
apoptosis of A549 cells, in a dose-dependent manner. The early
apoptosis rates of DHA and EPA on A549 cells were 14.68±1.81 and
14.46±1.63%, respectively, when treated with high
concentrations.
Morphological changes of A549 cells
induced by n-3 PUFA
Three types of cells can be recognized under a
fluorescence microscope: live cells (green), live apoptotic cells
(yellow) and dead cells by necrosis (red). When A549 cells were
treated with DHA (45 and 50 μg/ml) or EPA (55 and 60 μg/ml) for 24
h (Fig. 3B and C) the
morphological features of apoptotic cells, including cell surface
protuberances and nuclear fragments, were identified by AO
staining. Following 48 h, the typical apoptotic body appeared and
the late apoptotic cells were observed by EB staining (Fig. 3E and F).
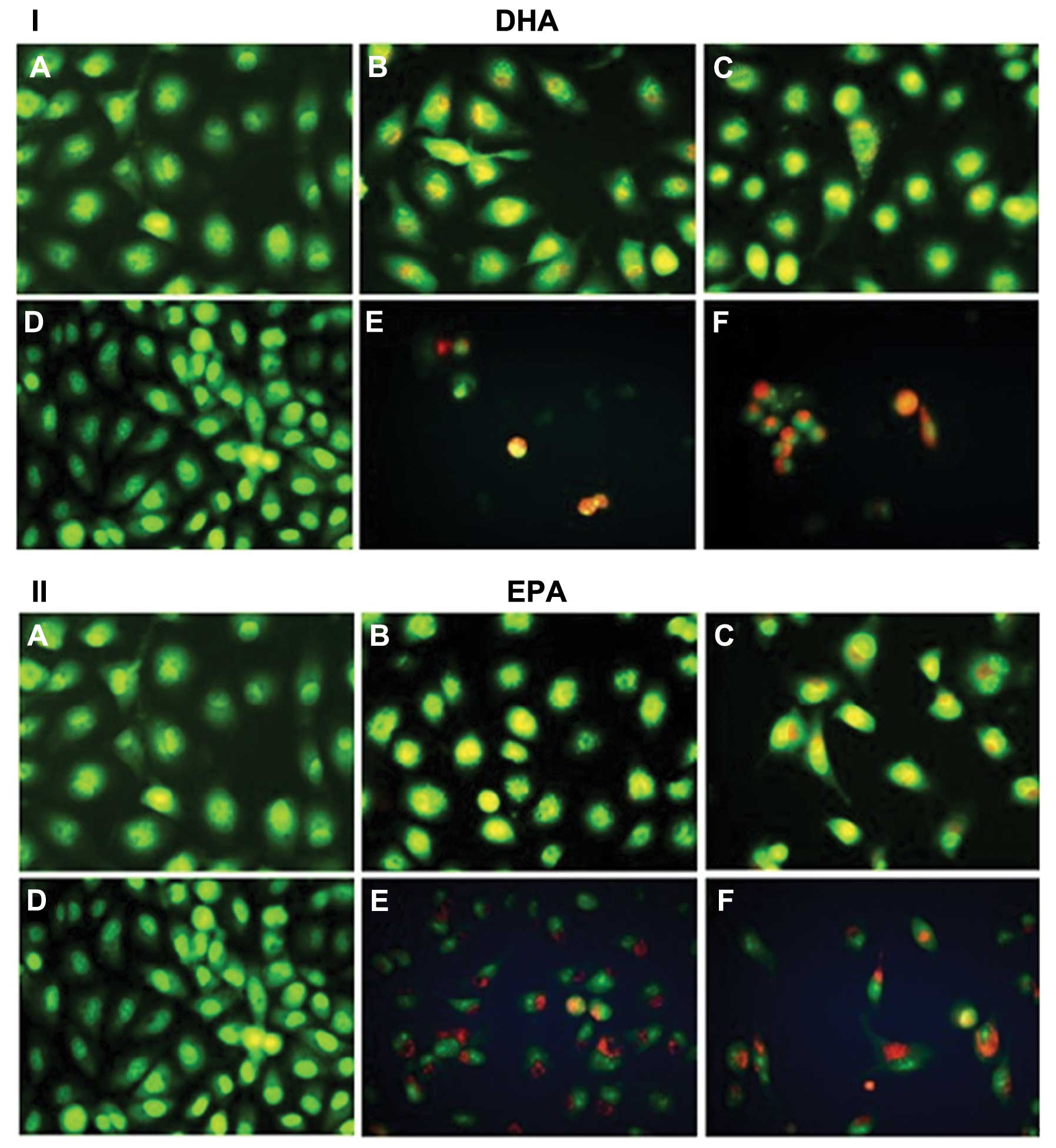 | Figure 3Morphological changes in A549 cells
following treatment with (I) DHA or (II) EPA and staining by AO/EB.
Three types of cells can be recognized under a fluorescence
microscope: live cells (green), live apoptotic cells (yellow) and
dead cells by necrosis (red). (A) Negative for 24 h, (B) treatment
with 45 μg/ml of DHA (or 55 μg/ml of EPA) for 24 h, (C) treatment
with 50 μg/ml of DHA (or 60 μg/ml of EPA) for 24 h, (D) negative
for 48 h, (E) treatment with 45 μg/ml of DHA (or 55 μg/ml of EPA)
for 48 h, and (F) treatment with 50 μg/ml of DHA (or 60 μg/ml of
EPA) for 48 h. AO, acridine orange; EB, ethidium bromide; DHA,
docosahexaenoic acid; EPA, eicosapentaenoic acid. |
The apoptotic phenomenon was also demonstrated by
transmission electron microscopy. When A549 cells were treated with
DHA (50 μg/ml; Fig. 4B) or EPA (60
μg/ml; Fig. 4C) for 24 h,
ultrastructure characteristics for the apoptotic cells included the
condensation of nuclear chromatin and the degeneration of
cytoplasmic organelles. The structure of the nuclear envelope
partly disappeared in spermatogonia. The apoptotic bodies were
observed in the cytoplasm. Furthermore, compared with the control
(Fig. 4A), the formation of
autophagosomes (double membrane structures which may have content
in them) was clearly enhanced in DHA- and EPA-treated cells
(Fig. 4D and E).
Discussion
Dietary fats have been known to be important in the
etiology of cancer. A positive association between a high intake of
fat and the incidence of breast, colon, pancreatic and prostate
cancer has been demonstrated (12). However, such an association may be
independent of the energy contents of the fats. Findings of recent
studies have demonstrated that diets rich in n-3 PUFA were
inversely correlated with the development of colorectal, prostate
and breast cancer (13–15).
Mammals, including humans, cannot synthesize either
the n-6 or the n-3 PUFA, thus, fatty acids containing these bonds
are essential fatty acids and must be obtained in the diet. The n-3
PUFA may be consumed as linolenic acid, which is contained in
various amounts in certain oils and in leafy green vegetables.
Longer chain n-3 PUFA, mainly EPA and DHA, are found in fish and
fish oils (16). While a large
body of evidence indicates that n-6 PUFA promote the growth of
tumour cells, n-3 PUFA have actually been demonstrated to inhibit
breast, colon, prostate and melanoma cell proliferation (7,8,17,18).
Supplementing the diet of tumor-bearing mice or rats with oils
containing EPA or DHA has been demonstrated to slow the growth of
various types of cancer, including lung (10,19,20),
colon (21,22), mammary (23) and prostate (9). Additionally, a number of
epidemiological studies suggest that fish consumption is
significantly inversely associated with lung cancer risk and
mortality (24–26). Furthermore, a combination of n-3
PUFA (2 g of fish oil, twice daily) and a COX-2 inhibitor
(celecoxib, 200 mg) may ameliorate the symptoms and signs
associated with systemic immune metabolic syndrome in advanced lung
cancer (27). In the present
study, the anti-proliferative effect of DHA or EPA on A549 cells
was confirmed by an MTT assay, suggesting a potential therapeutic
role of n-3 PUFA.
Apoptosis, or programmed cell death, is an essential
component of cell number regulation in colonic epithelia and a
crucial mechanism to prevent damaged or mutated cells from
surviving and dividing, and thus contributing to carcinogenesis.
The ability of n-3 PUFA to induce cancer cell apoptosis has been
documented (28–30). Dietary supplementation with EPA
resulted in a significant increase in crypt cell apoptosis in
humans with a history of colorectal adenomas (31) as well as in normal rat colonic
mucosa (32). As previously
reported, EPA and DHA have also been demonstrated to induce
apoptosis in the human lung cancer cell line A549, in the present
study.
Autophagy is induced as a survival response to
either growth factor or nutrient deprivation and it is also an
important mechanism of tumor cell death. The autophagic process is
characterized by the sequestration of bulk cytoplasm and organelles
in double or multimembrane autophagic vesicles and their subsequent
degradation by lysosomes (28). It
has also been reported that DHA induces autophagy through
p53-mediated AMPK/mTOR signaling and promotes apoptosis in human
cancer cells harboring wild-type p53 (33). We revealed that DHA or EPA
treatment induced the formation of autophagosomes in A549 cells,
which confirmed that autophagy was associated with their
anti-cancer mechanisms.
Numerous mechanisms have been suggested for the
suppression of tumor cell growth by n-3 PUFA and new mechanisms are
frequently reported as we gain additional knowledge regarding the
regulation of gene expression by fatty acids. It has been recently
documented that fish oil-derived fatty acids have anti-inflammatory
or anti-proliferative activity through the reduction of COX-2
expression as well as the suppression of the formation of the
proinflammatory lipid mediator prostaglandin E2 (34). It has been reported that DHA
inhibits eicosanoid synthesis from arachidonic acid (AA), EPA is a
better substrate for COX than AA and EPA competes more successfully
than AA for COX activity (35).
When activated, the transcription factor, nuclear factor-κB
(NF-κB), inhibits programmed cell death or apoptosis. The n-3 PUFA
can restore functional apoptosis by downregulating NF-κB (36), which in turn downregulates COX-2
expression. Furthermore, n-3 PUFA decrease the activation of
oncogenic transcription factors Ras, transcription factor AP1
(37) and protein kinase C
(38). It is likely that the
suppression of tumor cell growth by n-3 PUFA is due to the
combination of these mechanisms rather than to a single, unique
activity.
In conclusion, typical n-3 PUFA, including DHA and
EPA inhibit the proliferation of A549 cells and induce cell
apoptosis and autophagy in a dose- and time-dependent manner. This
may provide new safe and effective options for the treatment of
lung cancer in the future.
Acknowledgements
This study was supported by a grant from the
Zhejiang Provincial Natural Science Foundation of China (Grant no.
64212006). We would like to thank the Zhejiang Provincial Key
Laboratory of Gastroenterology for providing the experimental
facilities, instruments and guidance.
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andrews J, Yeh P, Pao W and Horn L:
Molecular predictors of response to chemotherapy in non-small cell
lung cancer. Cancer J. 17:104–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chatterjee S and Bhattacharjee B: Use of
natural molecules as anti-angiogenic inhibitors for vascular
endothelial growth factor receptor. Bioinformation. 8:1249–1254.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hull MA: Omega-3 polyunsaturated fatty
acids. Best Pract Res Clin Gastroenterol. 25:547–554. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murphy RA, Mourtzakis M and Mazurak VC:
n-3 polyunsaturated fatty acids: the potential role for
supplementation in cancer. Curr Opin Clin Nutr Metab Care.
15:246–251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blanckaert V, Ulmann L, Mimouni V, Antol
J, Brancquart L and Chénais B: Docosahexaenoic acid intake
decreases proliferation, increases apoptosis and decreases the
invasive potential of the human breast carcinoma cell line
MDA-MB-231. Int J Oncol. 36:737–742. 2010. View Article : Google Scholar
|
|
7
|
Hawcroft G, Volpato M, Marston G, Ingram
N, Perry SL, Cockbain AJ, Race AD, Munarini A, Belluzzi A, Loadman
PM, Coletta PL and Hull MA: The omega-3 polyunsaturated fatty acid
eicosapentaenoic acid inhibits mouse MC-26 colorectal cancer cell
liver metastasis via inhibition of PGE2-dependent cell motility. Br
J Pharmacol. 166:1724–1737. 2012. View Article : Google Scholar
|
|
8
|
Zajdel A, Wilczok A, Chodurek E, Gruchlik
A and Dzierzewicz Z: Polyunsaturated fatty acids inhibit melanoma
cell growth in vitro. Acta Pol Pharm. 70:365–369. 2013.PubMed/NCBI
|
|
9
|
Akinsete JA, Ion G, Witte TR and Hardman
WE: Consumption of high ω-3 fatty acid diet suppressed prostate
tumorigenesis in C3(1) Tag mice. Carcinogenesis. 33:140–148.
2012.
|
|
10
|
Yam D, Peled A and Shinitzky M:
Suppression of tumor growth and metastasis by dietary fish oil
combined with vitamins E and C and cisplatin. Cancer Chemother
Pharmacol. 47:34–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Xu T, Lei WW, Liu DM, Li YJ, Xuan
RJ and Ma JJ: Cadmium-induced oxidative stress and apoptotic
changes in the testis of freshwater crab, Sinopotamon henanense.
PLoS One. 6:e278532011. View Article : Google Scholar
|
|
12
|
Kroenke CH, Kwan ML, Sweeney C, Castillo A
and Caan BJ: High- and low-fat dairy intake, recurrence, and
mortality after breast cancer diagnosis. J Natl Cancer Inst.
105:616–623. 2013. View Article : Google Scholar
|
|
13
|
Shen XJ, Zhou JD, Dong JY, Ding WQ and Wu
JC: Dietary intake of n-3 fatty acids and colorectal cancer risk: a
meta-analysis of data from 489 000 individuals. Br J Nutr.
108:1550–1556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Apte SA, Cavazos DA, Whelan KA and
Degraffenried LA: A low dietary ratio of omega-6 to omega-3 Fatty
acids may delay progression of prostate cancer. Nutr Cancer.
65:556–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Signori C, El-Bayoumy K, Russo J, Thompson
HJ, Richie JP, Hartman TJ and Manni A: Chemoprevention of breast
cancer by fish oil in preclinical models: trials and tribulations.
Cancer Res. 71:6091–6096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karapanagiotidis IT, Bell MV, Little DC
and Yakupitiyage A: Replacement of dietary fish oils by
alpha-linolenic acid-rich oils lowers omega 3 content in tilapia
flesh. Lipids. 42:547–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu Z, Wu J, Wang S, Suburu J, Chen H,
Thomas MJ, Shi L, Edwards IJ, Berquin IM and Chen YQ:
Polyunsaturated fatty acids affect the localization and signaling
of PIP3/AKT in prostate cancer cells. Carcinogenesis. 34:1968–1975.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao W, Ma Z, Rasenick MM, Yeh S and Yu J:
N-3 poly-unsaturated fatty acids shift estrogen signaling to
inhibit human breast cancer cell growth. PLoS One. 7:e528382012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mernitz H, Lian F, Smith DE, Meydani SN
and Wang XD: Fish oil supplementation inhibits NNK-induced lung
carcinogenesis in the A/J mouse. Nutr Cancer. 61:663–669. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mannini A, Kerstin N, Calorini L, Mugnai G
and Ruggieri S: An enhanced apoptosis and a reduced angiogenesis
are associated with the inhibition of lung colonisation in animals
fed an n-3 polyunsaturated fatty acid-rich diet injected with a
highly metastatic murine melanoma line. Br J Nutr. 101:688–693.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Algamas-Dimantov A, Davidovsky D, Ben-Ari
J, Kang JX, Peri I, Hertz R, Bar-Tana J and Schwartz B:
Amelioration of diabesity-induced colorectal ontogenesis by omega-3
fatty acids in mice. J Lipid Res. 53:1056–1070. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bathen TF, Holmgren K, Lundemo AG,
Hjelstuen MH, Krokan HE, Gribbestad IS and Schønberg SA: Omega-3
fatty acids suppress growth of SW620 human colon cancer xenografts
in nude mice. Anticancer Res. 28:3717–3723. 2008.PubMed/NCBI
|
|
23
|
Yee LD, Young DC, Rosol TJ, Vanbuskirk AM
and Clinton SK: Dietary (n-3) polyunsaturated fatty acids inhibit
HER-2/neu-induced breast cancer in mice independently of the
PPARgamma ligand rosiglitazone. J Nutr. 135:983–988.
2005.PubMed/NCBI
|
|
24
|
Takezaki T, Hirose K, Inoue M, Hamajima N,
Yatabe Y, Mitsudomi T, Sugiura T, Kuroishi T and Tajima K: Dietary
factors and lung cancer risk in Japanese: with special reference to
fish consumption and adenocarcinomas. Br J Cancer. 84:1199–1206.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Temme EH and Kesteloot H: Fish
consumption is inversely associated with male lung cancer mortality
in countries with high levels of cigarette smoking or animal fat
consumption. Int J Epidemiol. 29:615–621. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Veierød MB, Laake P and Thelle DS: Dietary
fat intake and risk of lung cancer: A prospective study of 51, 452
Norwegian men and women. Eur J Cancer Prev. 6:540–549.
1997.PubMed/NCBI
|
|
27
|
Cerchietti LC, Navigante AH and Castro MA:
Effects of eicosapentaenoic and docosahexaenoic n-3 fatty acids
from fish oil and preferential Cox-2 inhibition on systemic
syndromes in patients with advanced lung cancer. Nutr Cancer.
59:14–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fukui M, Kang KS, Okada K and Zhu BT: EPA,
an omega-3 fatty acid, induces apoptosis in human pancreatic cancer
cells: role of ROS accumulation, caspase-8 activation, and
autophagy induction. J Cell Biochem. 114:192–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Serini S, Fasano E, Piccioni E, Monego G,
Cittadini AR, Celleno L, Ranelletti FO and Calviello G: DHA induces
apoptosis and differentiation in human melanoma cells in vitro:
involvement of HuR-mediated COX-2 mRNA stabilization and β-catenin
nuclear translocation. Carcinogenesis. 33:164–173. 2012.PubMed/NCBI
|
|
30
|
Sun H, Hu Y, Gu Z, Owens RT, Chen YQ and
Edwards IJ: Omega-3 fatty acids induce apoptosis in human breast
cancer cells and mouse mammary tissue through syndecan-1 inhibition
of the MEK-Erk pathway. Carcinogenesis. 32:1518–1524. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Courtney ED, Matthews S, Finlayson C, Di
Pierro D, Belluzzi A, Roda E, Kang JY and Leicester RJ:
Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and
increases apoptosis in normal colonic mucosa in subjects with a
history of colorectal adenomas. Int J Colorectal Dis. 22:765–776.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Calviello G, Palozza P, Maggiano N,
Piccioni E, Franceschelli P, Frattucci A, Di Nicuolo F and Bartoli
GM: Cell proliferation, differentiation, and apoptosis are modified
by n-3 polyunsaturated fatty acids in normal colonic mucosa.
Lipids. 34:599–604. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rovito D, Giordano C, Vizza D, et al:
Omega-3 PUFA ethanolamides DHEA and EPEA induce autophagy through
PPARγ activation in MCF-7 breast cancer cells. Cell Physiol.
228:1314–1322. 2013.PubMed/NCBI
|
|
34
|
Gravaghi C, La Perle KM, Ogrodwski P, Kang
JX, Quimby F, Lipkin M and Lamprecht SA: Cox-2 expression, PGE(2)
and cytokines production are inhibited by endogenously synthesized
n-3 PUFAs in inflamed colon of fat-1 mice. J Nutr Biochem.
22:360–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Serini S, Fasano E, Piccioni E, Cittadini
AR and Calviello G: Differential anti-cancer effects of purified
EPA and DHA and possible mechanisms involved. Curr Med Chem.
18:4065–4075. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Siriwardhana N, Kalupahana NS, Fletcher S,
Xin W, Claycombe KJ, Quignard-Boulange A, Zhao L, Saxton AM and
Moustaid-Moussa N: n-3 and n-6 polyunsaturated fatty acids
differentially regulate adipose angiotensinogen and other
inflammatory adipokines in part via NF-κB-dependent mechanisms. J
Nutr Biochem. 23:1661–1667. 2012.PubMed/NCBI
|
|
37
|
Liu G, Bibus DM, Bode AM, Ma WY, Holman RT
and Dong Z: Omega 3 but not omega 6 fatty acids inhibit AP-1
activity and cell transformation in JB6 cells. Proc Natl Acad Sci
USA. 98:7510–7515. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Judé S, Martel E, Vincent F, et al:
Dietary long-chain n-3 fatty acids modify blood and cardiac
phospholipids and reduce protein kinase-C-delta and protein
kinase-C-epsilon translocation. Br J Nutr. 98:1143–1151.
2007.PubMed/NCBI
|


















