Introduction
In total, >350,000 novel bladder cancer cases are
diagnosed worldwide each year (2012), and the majority (>90%) of
these are transitional cell carcinomas (1), 70% of which are diagnosed as
superficial tumors (Ta, T1 or Tis). Despite previous refinement of
various therapeutic strategies, including surgery, intravesical
chemotherapy and combination therapy, 50–70% of those superficial
tumors are likely to recur (2). As
bladder cancer is a relatively common and potentially
life-threatening neoplasm, predicting and monitoring the condition
is usually a lifelong process using complicated methods (3). Cystoscopy is the primary diagnostic
method for bladder cancer as it is reasonably accurate, however, it
is also invasive and relatively expensive (4). Urine cytology is highly specific in
detecting bladder carcinoma, but it has a low sensitivity in
detecting low- to intermediate-grade bladder cancer (5). The lifelong follow-up of patients by
invasive cystoscopy with high costs and by cytology with low
sensitivity emphasizes the pressing requirement for a urinary
biomarker that offers a non-invasive method for the detection of
recurrence and for stratifying patients with a high-risk
profile.
Tenascin-C (TN-C), an extracellular matrix
glycoprotein, exhibits a morphoregulatory role in fetal development
and tissue remodeling (6).
Recently, accumulating evidence has indicated a supportive role for
TN-C in tumor growth, metastasis (7), tumor angiogenesis (8) and the inhibition of immune
surveillance (6). In certain tumor
types, including breast cancer, glioma and osteosarcoma, high TN-C
expression provides a poor patient survival prognosis (6). In patients with bladder cancer, a
diffuse TN-C staining pattern in the tumor stroma was shown to be
significantly associated with a worse overall patient survival rate
compared with the rate in those with moderate or negative staining,
while patients with cytoplasmic expression of TN-C had a
significantly improved overall survival rate compared with those
without (9). Most significantly,
only the expression of TN-C in invasive bladder cancer was an
independent positive prognostic factor for the overall survival
rate in the multivariate analysis (9). However, in another study using
univariate and multivariate analyses, it was reported that TN-C
expression was not an independent prognostic factor for the
recurrence and progression in patients with superficial bladder
cancer (10). Thus, the
correlation between TN-C and bladder cancer recurrence and
progression remains unclear and requires further research.
Various splicing variants or splicing domains of
TN-C have been demonstrated to exhibit specific biological
functions during tumor progression and may have different
diagnostic or predictive values (11). In breast cancer, the increased
expression of TN-C splicing variants containing the B domain is
positively associated with the invasive phenotype. Similarly, the
A1 and D domains of TN-C maybe useful for determining individual
bladder cancer biological behaviors (12), and detection of TN-C containing the
B and C domains in urine may be used as a marker for the
surveillance of bladder cancer recurrence and invasiveness
(8,9). However, since splicing domains may be
independently included in the molecule leading to >9 different
TN-C splicing variants, which may play different roles in cell
behavior, it remains difficult to analyze all the splicing variants
or splicing domains of TN-C for diagnostic or predictive values.
Additionally, one study (13) also
demonstrated that it may result in wrong prognostic impressions if
only one or certain domains are investigated due to the
comprehensive effect of all the TN-C isoforms, which may be crucial
in tumor development.
In the present study, based on the constant domains
of TN-C, the concentration of urine TN-C of bladder cancer patients
and volunteers was determined by ELISA analysis. We aimed to
demonstrate the discrepancy of urine TN-C in bladder cancer
patients vs. volunteers, and the clinical significance of this
discrepancy, including tumor grade, stage and prognosis.
Materials and methods
Urine samples
In total, 66 samples of voided urine from patients
with bladder cancer, 42 samples from healthy control volunteers and
33 sample from patients who suffered from urinary tract
inflammation without tumors who were enrolled between Nov 2008 and
Feb 2012 in the Department of Urology, First Affiliated Hospital of
Medical School, Xi’an Jiaotong University (Xi’an, China) were
collected. The patient’s ages ranged between 32 and 82 years old,
with a mean ± standard deviation (SD) of 64.7±12.4 years old and a
male/female ratio of 44/22. The healthy volunteers had ages ranging
between 35 and 75 years old, with a mean ± SD of 65.3±7.4 years old
and a male/female ratio of 29/13. All the bladder cancer urine
samples were collected following diagnosis and prior to surgery.
Ethical approval for this study was obtained from the Ethics
Committee of the First Affiliated Hospital of Medical School, Xi’an
Jiaotong University and written informed consent was obtained from
all the patients/patient’s families.
ELISA analysis
The ELISA kits were obtained from Shanghai Westang
Biological Technology Co., Ltd. (Shanghai, China) to target the
constant domain of TN-C. The lowest concentration that may be
detected by the kit is <2 ng/ml. ELISA analysis was performed by
a blinded investigator and each sample was analyzed at least in
triplicate. Among all 66 patients, 48 were followed up for their
life status until the end of the study.
Statistical analysis
Student’s t-test was used for analyzing the
differences in the TN-C concentrations between the patients and
volunteers. A rank correlation was used for evaluating the
correlation between the urine TN-C concentration and the tumor
grade/stage and the time from bladder cancer diagnosis to
recurrence. A multivariate Cox proportional hazards model was used
to analyze the main hazard factor of survival rate among other
factors of age, relapse, gender, grade, stage and concentration of
TN-C, and the Kaplan-Meier method was used for the survival rate
analysis. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Urine concentration of TN-C in bladder
cancer patients is higher than in volunteers
All the 66 bladder cancer patients had been
diagnosed with bladder cancer for the first time and they had no
history of cancerous disease. The clinicopathological data of the
bladder cancer patients is listed in Table I. The mean concentration of the
TN-C in the voided urine was 0.0630±0.0331 vs. 0.0028±0.0026 for
the bladder cancer patients vs. the healthy control volunteers,
respectively (P<0.001). The t-test analysis indicated that
between the two groups, the concentration of TN-C in their voided
urine was markedly different, as shown in Table II and Fig. 1. The results of the present study
indicated that the bladder cancer patients had a higher
concentration of urine TN-C compared with the healthy volunteers.
However, as reported, our results also provide evidence that
inflammation contributes to the elevation of urine TN-C (Table VI).
 | Table IEpidemiology and clinicopathology of
bladder cancer patients and volunteers. |
Table I
Epidemiology and clinicopathology of
bladder cancer patients and volunteers.
| Data | Patients | Volunteers | Inflammation
patients |
|---|
| Gender, n (%) |
| Male | 44 (66.7) | 29 (69.0) | 18 (56) |
| Female | 22 (33.3) | 13 (31.0) | 15 (44) |
| Age, years |
| Range | 32–82 | 35–75 | 29–61 |
| Mean ± SD | 64.7±12.4 | 65.3±7.4 | 34.7±5.5 |
| Tumor grade, n
(%) |
| I | 22 (33.3) | - | - |
| II | 25 (37.9) | - | - |
| III | 19 (28.8) | - | - |
| Tumor stage, n
(%) |
| Ta, Tis | 11 (16.7) | - | - |
| T1 | 23 (34.8) | - | - |
| T2 | 23 (34.8) | - | - |
| T3 | 9 (13.6) | - | - |
 | Table IIUrine concentration of TN-C in bladder
cancer patients and volunteers (μg/ml). |
Table II
Urine concentration of TN-C in bladder
cancer patients and volunteers (μg/ml).
| Patients | Total number | Gender, n (M/F) | Age range, years | Age, years (mean ±
SD) | TN-C, μg/ml (mean ±
SD) |
|---|
| Bladder cancer | 66 | 44/22 | 32–82 | 64.7±12.4 | 0.0630±0.0331a |
| Volunteers | 42 | 29/13 | 35–75 | 65.3±7.4 | 0.0028±0.0026 |
 | Table VIUrine TN-C in patients with
inflammation compared with healthy volunteers and bladder cancer
patients. |
Table VI
Urine TN-C in patients with
inflammation compared with healthy volunteers and bladder cancer
patients.
| Patient groups | Total sample
number | TN-C, μg/ml (mean ±
SD) |
|---|
| Bladder cancer
patients | 66 | 0.0630±0.0331 |
| Inflammation
patients | 33 |
0.0891±0.0437a |
| Healthy
volunteers | 42 | 0.0028±0.0026 |
Urine concentration of TN-C has a
positive correlation with bladder cancer grade and clinical
stage
In order to illustrate the correlation between urine
TN-C and tumor grade/tumor stage, the urine TN-C concentrations for
differing grades and stages are listed, as shown in Table III. From grades 1–3, the urine
TN-C concentration (mean ± SD) was 0.0299±0.0100, 0.0592±0.0155 and
0.1061±0.0141 μg/ml, respectively (P<0.05), however, from stages
T1 to T3, the urine TN-C concentration was 0.0593±0.0379,
0.0659±0.0259 and 0.0916±0.0263 μg/ml, respectively (P<0.05).
The rank correlation analysis indicated that the coefficient
between the concentration of TN-C and bladder cancer grade was
0.9050, and this value in bladder cancer stage was 0.3080, which
indicates that the urine concentration of TN-C had a more
significantly positive correlation with the tumor grade compared
with the tumor stage; the P-value was 0.0000103 and 0.0220034,
respectively, as shown in Table
IV. These results indicate that the urine concentration of TN-C
has a positive correlation with the tumor grade and tumor stage,
particularly the former.
 | Table IIIUrine TN-C in different grades and
stages of bladder cancer. |
Table III
Urine TN-C in different grades and
stages of bladder cancer.
| Tumor
characteristics | n | Age, years (mean ±
SD) | Gender, n (M/F) | TN-C, μg/ml (mean ±
SD) |
|---|
| Stage |
| T1 | 23 | 63.7±11.7 | 17/6 | 0.0593±0.0379 |
| T2 | 23 | 65.4±12.2 | 14/9 | 0.0659±0.0259a |
| T3 | 9 | 71.2±6.1 | 6/3 |
0.0916±0.0263b |
| Ta, Tis | 11 | 60.1±16.5 | 8/3 | 0.0412±0.0251 |
| Grade |
| 1 | 22 | 65.0±12.0 | 14/8 | 0.0299±0.0100 |
| 2 | 25 | 64.4±14.1 | 17/8 |
0.0592±0.0155c |
| 3 | 19 | 64.8±10.9 | 14/5 |
0.1061±0.0141d |
 | Table IVCorrelation analysis of urine TN-C
concentration with bladder cancer grade/stage and the time from
diagnosis to recurrence. |
Table IV
Correlation analysis of urine TN-C
concentration with bladder cancer grade/stage and the time from
diagnosis to recurrence.
| Tumor
characteristics | Correlation
coefficient with urine TN-C | P-value |
|---|
| Bladder cancer
grade | 0.905 | 0.0000103 |
| Bladder cancer
stage | 0.308 | 0.0220034 |
| Time from diagnosis
to recurrence | −0.874 | 0.0000796 |
Urine concentration of TN-C has a
negative correlation with the time from bladder cancer diagnosis to
recurrence
In order to illustrate the correlation between the
urine concentration of TN-C and the time from bladder cancer
diagnosis to recurrence, 48 bladder cancer patients were followed
up. In total, 16 of these patients exhibited cancer recurrence
(33%), and during the follow-up stage, all 16 patients only had one
recurrence. The rank correlation analysis indicated that the
coefficient between the urine concentration of TN-C and the time
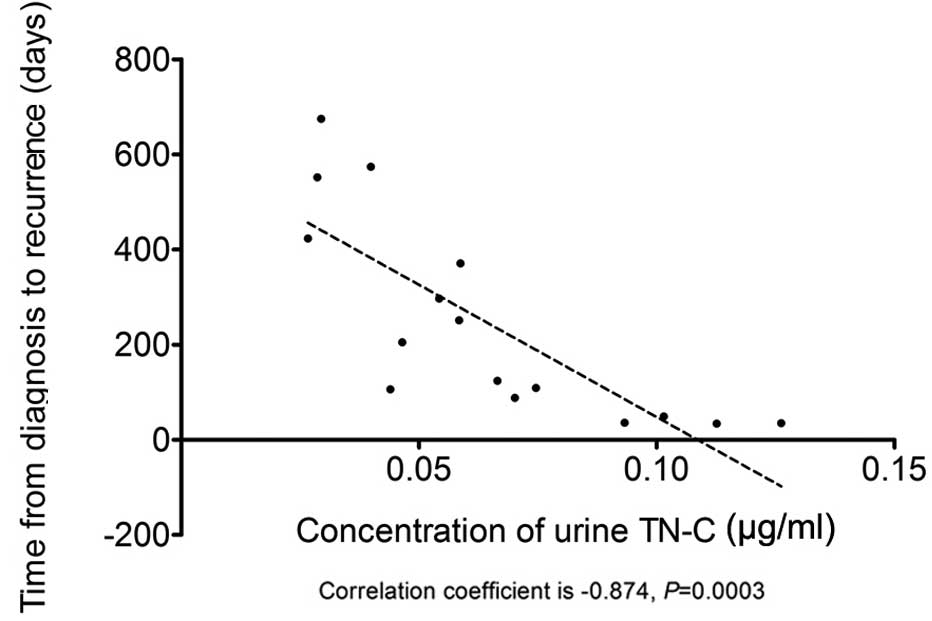
from bladder cancer diagnosis to recurrence was −0.8740 (P=0.0003),
as shown in Table IV and Fig. 2, indicating that urine TN-C
concentration has a negative correlation with the time from bladder
cancer diagnosis to recurrence.
Urine TN-C is one of the hazard factors
for the prognosis of bladder cancer patients
Among the 48 bladder cancer patients who were
followed up, 24 succumbed to bladder cancer (50%), 16 suffered
recurrence of the disease (33%) and the other 8 patients remained
alive without bladder cancer (16.7%). In the present study,
factors, including tumor grade, stage, age and gender of the
patient, recurrence and the urine concentration of TN-C, were
analyzed to evaluate the risk factors for the survival rate of the
patients with bladder cancer. The urine concentration of TN-C was
divided into four grades denoted 1, 2, 3 and 4 according to their
percentile: 1, min-X25%; 2, X25–X50%; 3, X50–X75%; and 4, 75%-max
(X represents the concentration of urine TN-C). The Cox
proportional hazards analysis revealed that among the six factors,
bladder cancer grade, recurrence and urine TN-C were the
independent risk factors for the bladder cancer patients, as shown
in Table V; the relative ratio
(RR) values were 1.7680, 0.1960 and 1.9310, respectively. The
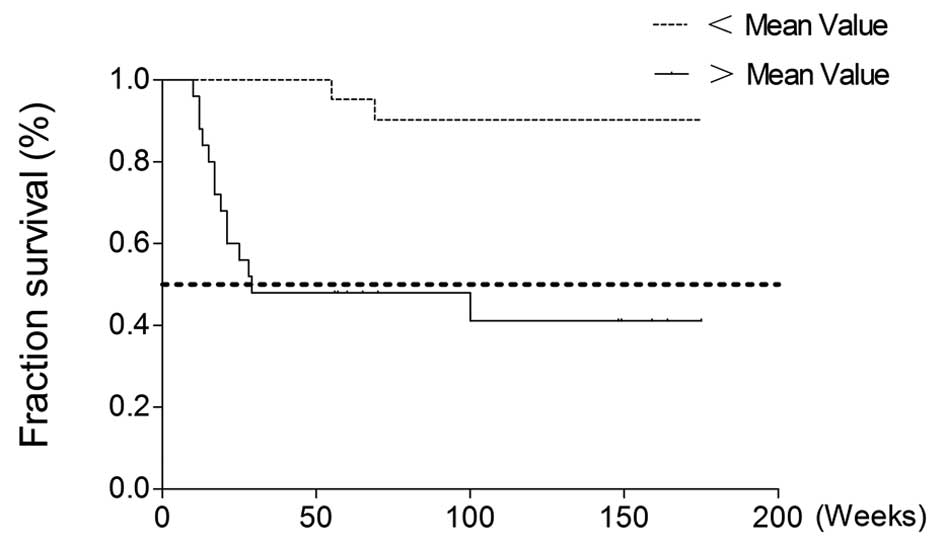
survival rate, as affected by urine TN-C concentration, was then
focused on as a single risk factor in the 48 bladder cancer
patients. The patients were divided into two groups according to
whether their urine TN-C concentration was above or below the mean
study value (0.0690 μg/ml), as shown in Fig. 3. The survival rate between the two
groups was observed to be significantly different, indicating that
urine TN-C is an independent risk factor for bladder cancer
patients.
 | Table VResults of the Cox proportional
hazards analysis for the main risk factor for bladder cancer
patients [−2ln(L)=126.86]. |
Table V
Results of the Cox proportional
hazards analysis for the main risk factor for bladder cancer
patients [−2ln(L)=126.86].
| | | | | | | 95% CI for RR |
|---|
| | | | | | |
|
|---|
| Variable | df | b | SE(b) | Wald
χ2 | P-value | RR | Upper | Lower |
|---|
| Relapse | 1 | −1.630 | 0.536 | 9.243 | 0.002 | 0.196 | 0.068 | 0.560 |
| TN-C | 1 | 0.658 | 0.198 | 11.068 | 0.001 | 1.931 | 1.311 | 2.846 |
| Grade | 1 | 0.570 | 0.281 | 4.114 | 0.043 | 1.768 | 1.019 | 3.067 |
Discussion
The most difficult obstacle to the therapeutics of
bladder cancer is the high rate of tumor recurrence and the absence
of specific manifestations to monitor using cystoscopy following
surgery. Due to the limitations of invasive and expensive
cystoscopy (15), numerous urine
marker-based tests for bladder cancer have been developed and
tested in different populations in the last decades (16), including cytology, ImmunoCyt™,
fluorescence in situ hybridization, proteomics,
microsatellite analysis and biomarkers, including BTAstat, BTAtrak,
NMP22 (17), FDP, Engrailed-2
(18), Apo-I (19) and UPK3A (15). However, all these biomarkers have
their own limitations and must be combined with other biomarkers in
order to monitor bladder cancer (20). Currently, no single urine marker
guides us in monitoring bladder cancer perfectly, and a set of
novel markers are required.
TN-C is a main component of the extracellular matrix
in solid tumor tissues (21), and
is a molecule of ~300 kDa as an intact monomer and up to 1,800 kDa
when assembled into a hexamer. The multi-domain molecule consists
of an N-terminal assembly domain, followed by 14.5 EGF-like
repeats, 8 constant and ≤9 alternatively spliced fibronectin type
III repeats and a C-terminal fibrinogen-like globular domain
(6); this special mRNA and protein
structure is the reason for its complicated and multiple functions,
which involve adhesion/anti-adhesion, epithelial-mesenchymal
transition, proliferation and cell cycle control (6). A growing body of evidence has
indicated the vital role of TN-C in breast (7), pancreatic (22), prostate (23,24)
and lung cancers (25) as well as
squamous cell carcinoma of the head and neck (26). In bladder cancer, TN-C has been
reported to contribute to migration and invasion (9,27);
this function depends on its isoforms, which are produced by
divergent splicing of the alternative domains, and those isoforms
may also be regarded as prognostic markers of bladder cancer
(12,28). In the present study, ELISA kits
were used to target the constant splicing domains in order to
detect all the isoforms of TN-C in the urine samples. To the best
of our knowledge, this is the first study focused on all the
isoforms of urine TN-C and the correlation between urine TN-C and
bladder cancer grade/stage. The urine TN-C concentration was
revealed to be positively associated with the tumor stage and
histological grade, which is consistent with a previous study
performed in bladder cancer tissues (28). Additionally, the rank correlation
analysis also indicated that the urine TN-C concentration had a
negative correlation with the time from bladder cancer diagnosis to
recurrence, indicating that a higher urine TN-C concentration may
be a predictor for bladder cancer recurrence. Furthermore, the Cox
proportional hazards analysis revealed that urine TN-C may be
regarded as an independent risk factor for the prognosis of bladder
cancer, and the Kaplan-Meier analysis indicated that urine TN-C, as
a single risk factor, is vital in the survival rate of bladder
cancer patients.
The present study mainly focused on the total
isoforms of TN-C containing the constant domain, but not the
specific alternative domain, which is different from the studies
previously reported (29). Based
on numerous investigations, the superiority of the present study
may be attributed to the following points: The exact numbers of
functional isoforms that contribute to the progression of bladder
cancer remain unclear. The functional isoforms of TN-C may be far
beyond the numbers observed in this study. Therefore, targeting one
or several specific isoforms of urine TN-C may lead to an
underestimation of the actual correlation between TN-C and cancer
progression. Furthermore, the concentration of urine TN-C is
measured at the nanogram level. Detection of the specific isoforms
of TN-C magnifies the random error on this level, since it is too
low to be detected perfectly. Detection targeting to the isoform
containing the constant domain may provide more accurate results
due to its higher concentration compared with any specific
isoforms. Additionally, the specificity of the antibodies targeting
specific isoforms may also result in false-positive or
false-negative errors.
There is another point that is worth noting. The
expression of TN-C may be affected by numerous factors (6), including wound healing and
inflammation, and the data in Table
VI also indicated that the urine TN-C of the patients with
infection of the urinary tract was significantly elevated.
Therefore, a diagnosis of inflammation/infection must first be
excluded when using the urine TN-C concentration for predicting the
prognosis of the bladder cancer patients.
In conclusion, the present study indicated that the
voided urine concentration of TN-C in bladder cancer patients is
significantly elevated and correlated with the progression of the
tumor grade and stage, and that elevated urine TN-C concentration
may be an independent life-threatening factor for bladder cancer
patients. The present study thus provides a potentially useful
prognostic marker for patients with bladder cancer and indicates
that TN-C concentration merits further investigation.
Acknowledgements
This study was partly supported by the National
Natural Science Foundation of China (grant no. 81101936).
References
|
1
|
Griffiths TR: Action on Bladder Cancer:
Current perspectives in bladder cancer management. Int J Clin
Pract. 67:435–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al Hussain TO and Akhtar M: Molecular
basis of urinary bladder cancer. Adv Anat Pathol. 20:53–60.
2013.
|
|
4
|
Kausch I and Böhle A: Bladder cancer. II
Molecular aspects and diagnosis. Eur Urol. 39:498–506.
2001.PubMed/NCBI
|
|
5
|
Lokeshwar VB, Schroeder GL, Selzer MG, et
al: Bladder tumor markers for monitoring recurrence and screening
comparison of hyaluronic acid-hyaluronidase and BTA-Stat tests.
Cancer. 95:61–72. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orend G and Chiquet-Ehrismann R:
Tenascin-C induced signaling in cancer. Cancer Lett. 244:143–163.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oskarsson T, Acharyya S, Zhang XH, et al:
Breast cancer cells produce tenascin C as a metastatic niche
component to colonize the lungs. Nat Med. 17:867–874. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berndt A, Köllner R, Richter P, et al: A
comparative analysis of oncofetal fibronectin and tenascin-C
incorporation in tumour vessels using human recombinant SIP format
antibodies. Histochem Cell Biol. 133:467–475. 2010. View Article : Google Scholar
|
|
9
|
Brunner A, Mayerl C, Tzankov A, et al:
Prognostic significance of tenascin-C expression in superficial and
invasive bladder cancer. J Clin Pathol. 57:927–931. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ioachim E, Michael M, Stavropoulos NE,
Kitsiou E, Salmas M and Malamou-Mitsi V: A clinicopathological
study of the expression of extracellular matrix components in
urothelial carcinoma. BJU Int. 95:655–659. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hancox RA, Allen MD, Holliday DL, et al:
Tumour-associated tenascin-C isoforms promote breast cancer cell
invasion and growth by matrix metalloproteinase-dependent and
independent mechanisms. Breast Cancer Res. 11:R242009. View Article : Google Scholar
|
|
12
|
Wunderlich H, Berndt A, Anger K, et al: A1
and D domain of tenascin C - New prognostic marker in bladder
cancer. J Urology. 179:3152008. View Article : Google Scholar
|
|
13
|
Fischer D, Brown-Lüdi M, Schulthess T and
Chiquet-Ehrismann R: Concerted action of tenascin-C domains in cell
adhesion, anti-adhesion and promotion of neurite outgrowth. J Cel
Sci. 110:1513–1522
|
|
14
|
Babjuk M, Oosterlinck W, Sylvester R, et
al: EAU guidelines on non-muscle-invasive urothelial carcinoma of
the bladder. Eur Urol. 54:303–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goodison S, Rosser CJ and Urquidi V:
Bladder cancer detection and monitoring: assessment of urine- and
blood-based marker tests. Mol Diagn Ther. 17:71–84. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Rhijn BW, van der Poel HG and van der
Kwast TH: Urine markers for bladder cancer surveillance: a
systematic review. Eur Urol. 47:736–748. 2005.
|
|
17
|
Jeong S, Park Y, Cho Y, Kim YR and Kim HS:
Diagnostic values of urine CYFRA21–1, NMP22, UBC, and FDP for the
detection of bladder cancer. Clin Chim Acta. 414:93–100. 2012.
|
|
18
|
Morgan R, Bryan RT, Javed S, et al:
Expression of Engrailed-2 (EN2) protein in bladder cancer and its
potential utility as a urinary diagnostic biomarker. Eur J Cancer.
Feb 21–2013.(Epub ahead of print).
|
|
19
|
Lei T, Zhao X, Jin S, Meng Q, Zhou H and
Zhang M: Discovery of potential bladder cancer biomarkers by
comparative urine proteomics and analysis. Clin Genitourin Cancer.
11:56–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vrooman OP and Witjes JA: Urinary markers
in bladder cancer. Eur Urol. 53:909–916. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vargová V, Pytliak M and Mechírová V:
Matrix metalloproteinases. EXS. 103:1–33. 2012.
|
|
22
|
Paron I, Berchtold S, Vörös J, et al:
Tenascin-C enhances pancreatic cancer cell growth and motility and
affects cell adhesion through activation of the integrin pathway.
PLoS One. 6:e216842011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xue Y, Li J, Latijnhouwers MA, et al:
Expression of periglandular tenascin-C and basement membrane
laminin in normal prostate, benign prostatic hyperplasia and
prostate carcinoma. Br J Urol. 81:844–851. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Katenkamp K, Berndt A, Hindermann W, et
al: mRNA expression and protein distribution of the unspliced
tenascin-C isoform in prostatic adenocarcinoma. J Pathol.
203:771–779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parekh K, Ramachandran S, Cooper J, Bigner
D, Patterson A and Mohanakumar T: Tenascin-C, over expressed in
lung cancer down regulates effector functions of tumor infiltrating
lymphocytes. Lung Cancer. 47:17–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pauli C, Stieber P, Schmitt UM,
Andratschke M, Hoffmann K and Wollenberg B: The significance of
Tenascin-C serum level as tumor marker in squamous cell carcinoma
of the head and neck. Anticancer Res. 22:3093–3097. 2002.PubMed/NCBI
|
|
27
|
Booth C, Harnden P, Selby PJ and Southgate
J: Towards defining roles and relationships for tenascin-C and
TGFbeta-1 in the normal and neoplastic urinary bladder. J Pathol.
198:359–368. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Berndt A, Anger K, Richter P, et al:
Differential expression of tenascin-C splicing domains in
urothelial carcinomas of the urinary bladder. J Cancer Res Clin
Oncol. 132:537–546. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Richter P, Tost M, Franz M, et al: B and C
domain containing tenascin-C: urinary markers for invasiveness of
urothelial carcinoma of the urinary bladder? J Cancer Res Clin
Oncol. 135:1351–1358. 2009. View Article : Google Scholar : PubMed/NCBI
|