Introduction
Flavonoids are widely distributed in plants and have
numerous functions, such as antioxidant activity in vitro
(1) and potential anticancer
activity (2). Isorhamnetin
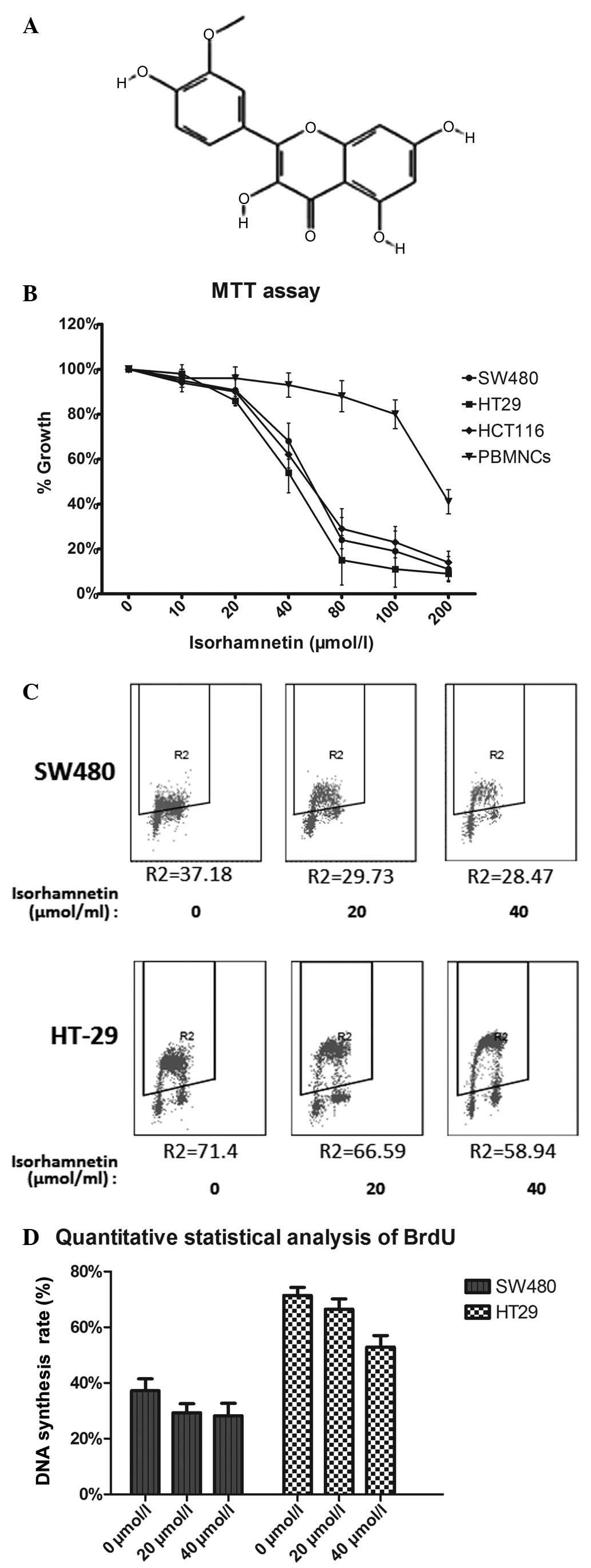
(3′-methoxy-3,4′,5,7-tetrahydroxyflavone; Fig. 1A) is a flavonoid extracted from
plants such as Persicaria thunbergii H. and Hippophae
rhamnoides L. This compound is used to treat cardiovascular
diseases and hemorrhage due to its antioxidative and metabolic
effects (3,4). Its anticancer effects have also been
reported (5–9); however, the mechanisms underlying
these effects remain unclear.
Colon cancer accounts for ~10% of all tumors and is
the most common type of cancer worldwide (10). One of the most common genetic
factors of this cancer is a PI3K mutation (11). The PIK3CA gene is mutated in ~20%
of colorectal cancers (CRCs), which activates the PI3K-Akt-mTOR
signal pathway (12). This pathway
significantly affects cell proliferation, metabolism and the stress
response, making it an important target in the treatment of
CRC.
We found that the Akt activity of colon cancer cells
can be inhibited by isorhamnetin in our pre-experiment. Thus, it
was hypothesized that isorhamnetin inhibits CRC by suppressing the
PI3K-Akt-mTOR pathway. Accordingly, the present study was performed
to validate this hypothesis.
Materials and methods
Reagents
Isorhamnetin was purchased from Chromadex (Irvine,
CA, USA), dissolved in dimethylsulfoxide (DMSO)and diluted to 20
mmol/l. Rapamycin and LY294002 were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA). An MTT kit was purchased from
Promoter (Wuhan, Hubei, China). Propidium iodide (PI) was obtained
from Sigma-Aldrich (St. Louis, MO, USA). The BCA Protein Assay kit,
ECL detection system and horseradish peroxidase-conjugated
secondary antibody were obtained from Pierce (Rockford, IL, USA).
Antibodies against AKT, phosphorylated (p)-AKT (ser473) and 4E-PB1
were obtained from Cell Signaling Technology, Inc.. Antibodies
against p70S6k and p-p70S6K were purchased from Epitomics, Inc.
(Burlingame, CA, USA); antibodies against p21 and Cyclin B1 were
obtained from BD Biosciences (San Jose, CA, USA); and an antibody
against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Cell lines and culture
SW480, HCT116 and HT-29 cell lines were purchased
from the Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). SW480 and HCT116 cells were
cultured in Dulbecco’s modified Eagle’s medium/high-glucose medium
with 10% fetal bovine serum (FBS; Hyclone, Waltham, MA, USA) in a
humidified atmosphere (37°C, 5% CO2). HT-29 cells were
cultured in 10% FBS/McCoy’s 5a under the same conditions. For all
experiments, the cells were grown to 90% confluency and harvested
every 2–3 days.
MTT assay
An MTT assay was performed as described previously
by Mosmann (13). MTT was
dissolved in DMSO to 5 mg/ml. The cells were digested, counted and
seeded onto 96-well culture plates at a density of 5×103
cells per well. Subsequently, the cells were incubated overnight
and the culture medium was replaced with isorhamnetin at
concentrations of 0, 10, 20, 40 and 80 μmol/l. After 3 days, 10 μl
MTT (5 mg/ml) was added to each well and cells were cultured for
another 4 h. The medium was then discarded and 150 μl DMSO was
added to the culture wells. After gently agitating for 10 min with
a table shaker (Premiere, Changzhou, China) at 40 rpm for 10 min,
the samples were placed on a microplate reader (Biotek, Winooski,
VT, USA) and the absorbance was detected at 550 nm. Data were
calculated and the growth inhibition curve was constructed.
Western blot analysis
Cells were seeded onto 6-well plates following
treatment with isorhamnetin (0, 20 and 40 μmol/l), and proteins
were harvested and collected by NP-40 lysis buffer (Beyotime,
Haimen, China). The protein concentrations were determined by a
Bicinchoninic Acid Protein Assay kit. Subsequently, 40 μg of each
protein sample was added to 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis gel. Following
electrophoresis at 100 V for 2 h, the proteins were transferred to
polyvinylidene difluoride membranes at 350 mA for 90 min. The
membranes were then blocked with 5% non-fat milk in Tris-buffered
saline and Tween 20 (TBST), incubated with primary antibody at 4°C
overnight, washed three times with TBST, incubated for 1 h at room
temperature and washed again three times. The chemical signal was
detected using an enhanced chemiluminescence detection system. The
chemical detection instrument used was FluorChem FC2 (Cell
Biosciences, Murrieta, CA, USA).
PI staining
Cells were seeded onto 6-well plates, treated with
isorhamnetin (0, 20 and 40 μmol/l) for 24 h and harvested at the
exponential phase. The samples were fixed with 75% ice-cold ethyl
alcohol overnight at −20°C. On the following day, the cells were
centrifuged at 300 × g for 5 min (Beckman, Brea, CA, USA) and added
drop wise with ethyl alcohol. The samples were washed three times
with phosphate buffered saline (PBS), resuspended in 100 μl PBS,
combined with 10 μl of 500 μg/ml PI and 5 μl of 10 mg/ml RNase A,
and then incubated for 2 h in the dark. Following this, 500 μl PBS
was added prior to flow cytometry (FCM) analysis.
Bromodeoxyuridine (BrdU) corporation
Approximately 300 ng/ml BrdU (Sigma-Aldrich) was
added to the medium, which was then incubated for 30 min prior to
harvesting. Cells were collected and fixed with 75% ice-cold ethyl
alcohol overnight at −20°C. On the following day, the samples were
washed once and treated with 0.5 ml 2 M HCl for 40 min.
Subsequently, 0.5 ml 0.1 M sodium borate (pH 8.5) was added for
neutralization, and the samples were incubated with anti-BrdU for 1
h and a secondary antibody for 30 min. After washing three times,
PI and RNase were added to the samples for another 30 min of
incubation. Finally, all samples were analyzed by FCM.
Statistical analysis
All statistical analyses were conducted using SPSS,
version 12.0 (SPSS Inc., Chicago, IL, USA). The values are
presented as the mean ± SD. Student’s t-test was used to determine
the statistical significance of the differences between the
treatment group and the control group. The data were statistically
analyzed by analysis of variance and P<0.05 was considered to
indicate a statistically significant difference.
Results
Isorhamnetin suppresses the proliferation
of CRC cells
The effect of isorhamnetin on the proliferation of
CRC cells was determined by an MTT assay. The three cell lines were
treated with isorhamnetin at five concentrations (0, 10, 20, 40 and
80 μmol/l) for three days. Peripheral blood mononuclear cells
(PBMNCs) were added to verify the cytotoxicity of isorhamnetin. The
results shown in Fig. 1B indicated
that the inhibition effect was dose dependent. The IC50
values of isorhamnetin were as follows: 56.24±1.25 μmol/l for SW480
cells, 54.87±2.13 μmol/l for HCT116 cells and 43.85±3.45 μmol/l for
HT-29 cells. The IC50 value of isorhamnetin in PBMNCs
was 170 μmol/l. This result showed that the IC50 value
of isorhamnetin in PBMNCs was considerably higher than that of the
three CRC cell types. Thus, it is possible to eliminate the
cytotoxicity of isorhamnetin to non-tumor cells. The effect of
isorhamnetin on HCT116 cells has previously been demonstrated
(8), therefore the remaining two
cell lines were selected for subsequent experiments.
A BrdU assay was directly used to detect cell
proliferation, as shown in Fig. 1C and
D. The results were similar to those of the MTT assay. At 40
μmol/l isorhamnetin, the average inhibition ratio was 10±4%
(P=0.002) in the SW480 cell line and 12±3.6% (P=0.001) in the HT-29
cell line.
Isorhamnetin induces G2/M growth arrest
in human CRC cells
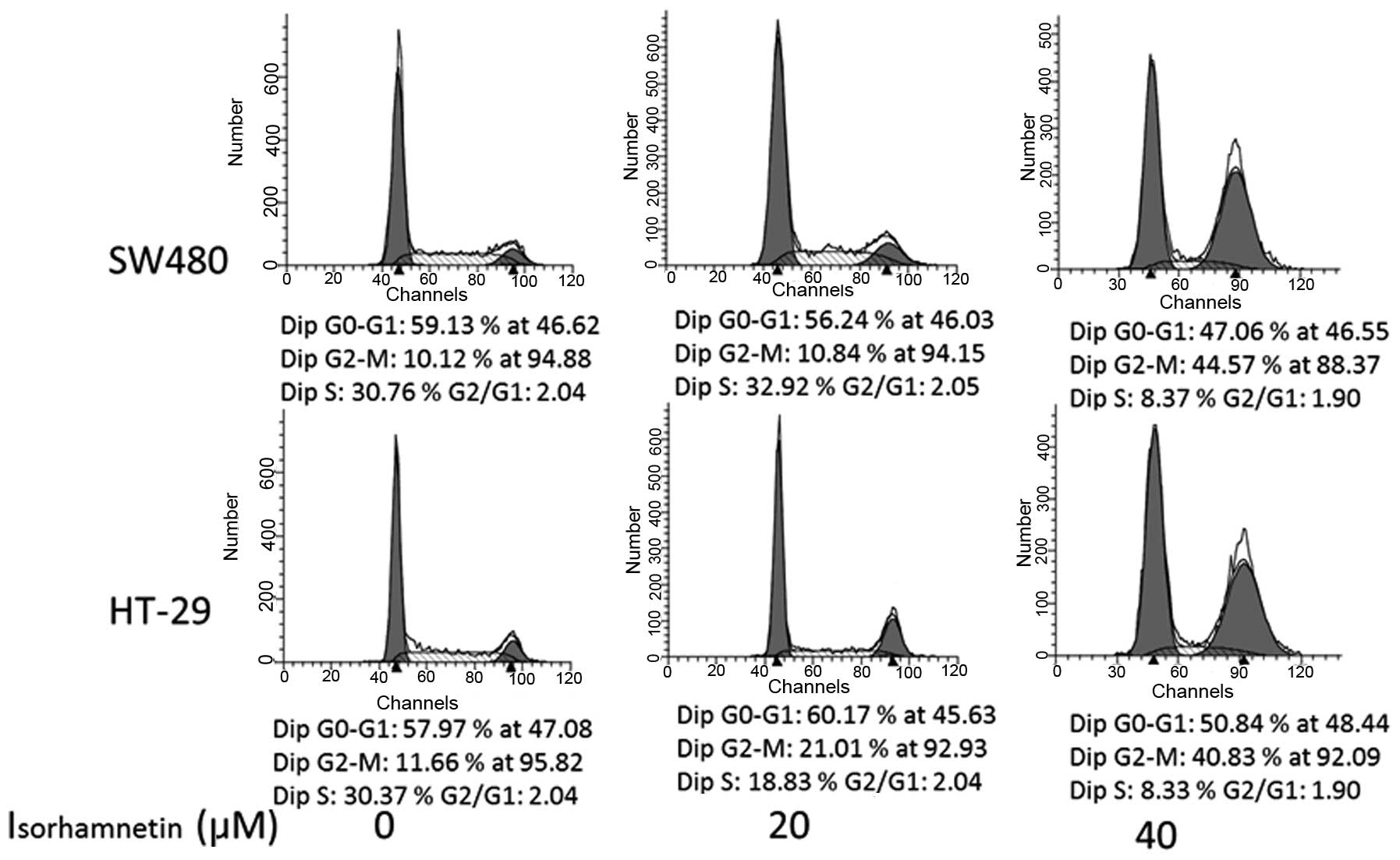
PI staining was conducted to further investigate
whether isorhamnetin affected the cell cycle. Following treatment
with isorhamnatin at three concentrations (0, 20 and 40 μmol/l),
the average proportions of the G2/M phase in each group were
respectively as follows (Fig. 2):
11.6, 21 and 44.57% in SW480 cells; and 10, 10.9 and 40.83% in
HT-29 cells. The G2/M phase of HT-29cells increased by 9.5% at 20
μmol/l (P=0.003) and 32.8% (P=0.004) at 40 μmol/l. The increase in
SW480 cells was 30.8% at 40 μmol/l (P=0.0067), but no obvious
change was observed at 20 μmol/l (P=0.65). The result was similar
in HCT116 cells (data not shown). These data suggested that
isorhamnetin is able to induce cell cycle arrest at the G2/M phase
in a dose-dependent manner.
Isorhamnetin inhibits the phosphorylation
of Akt in CRC cells
Similar to other flavonoids, isorhamnetin may affect
the receptor-tyrosine signaling pathway. Yang et al
(14) reported that the
phosphorylation of Akt may be suppressed by chrysoeriol, a type of
flavonoid. It was hypothesized that isorhamnetin also affects the
phosphorylation level of Akt in CRC cells and, therefore, in the
present study possible changes were detected by western blot
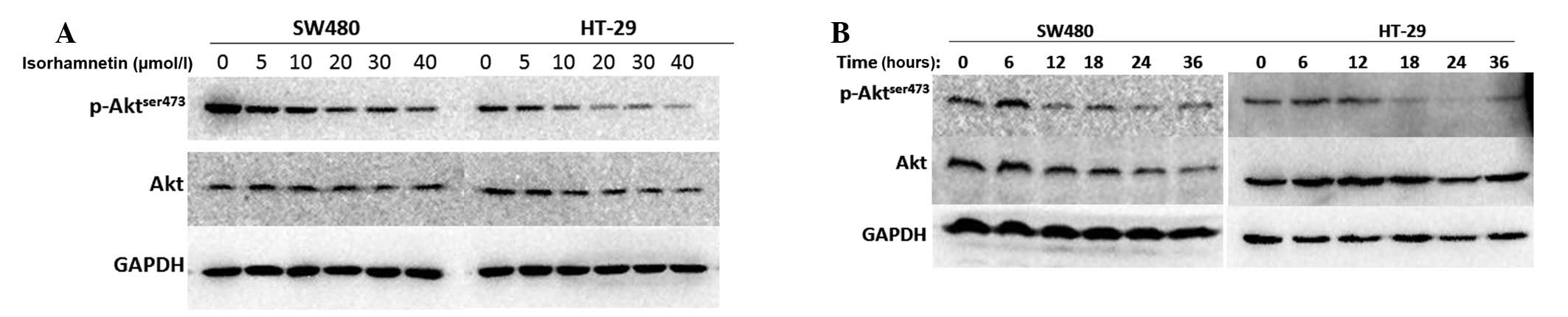
analysis. As shown in Fig. 3, the
two cell lines in the dose gradient group were treated with
isorhamnetin for 24 h at different concentrations. The results
showed that phospho-Akt (ser473) was suppressed at 20 μmol/l
isorhamnetin and the change was significant at 40 μmol/l
isorhamnetin. All cells in the time gradient group were treated
with the same concentration (20 μmol/l) at six time points, 0, 6,
12, 18, 24 and 36 h. It was identified that the phosphorylation
level of Akt (ser473) was suppressed after 12 h treatment with
isorhamnetin and the change was significant after 24 h. Thus,
isorhamnetin downgraded the phosphorylation level of Akt (ser473)
in a dose- and time-dependent manner.
Isorhamnetin affects the phosphorylation
of p70S6K and 4E-BP1 protein
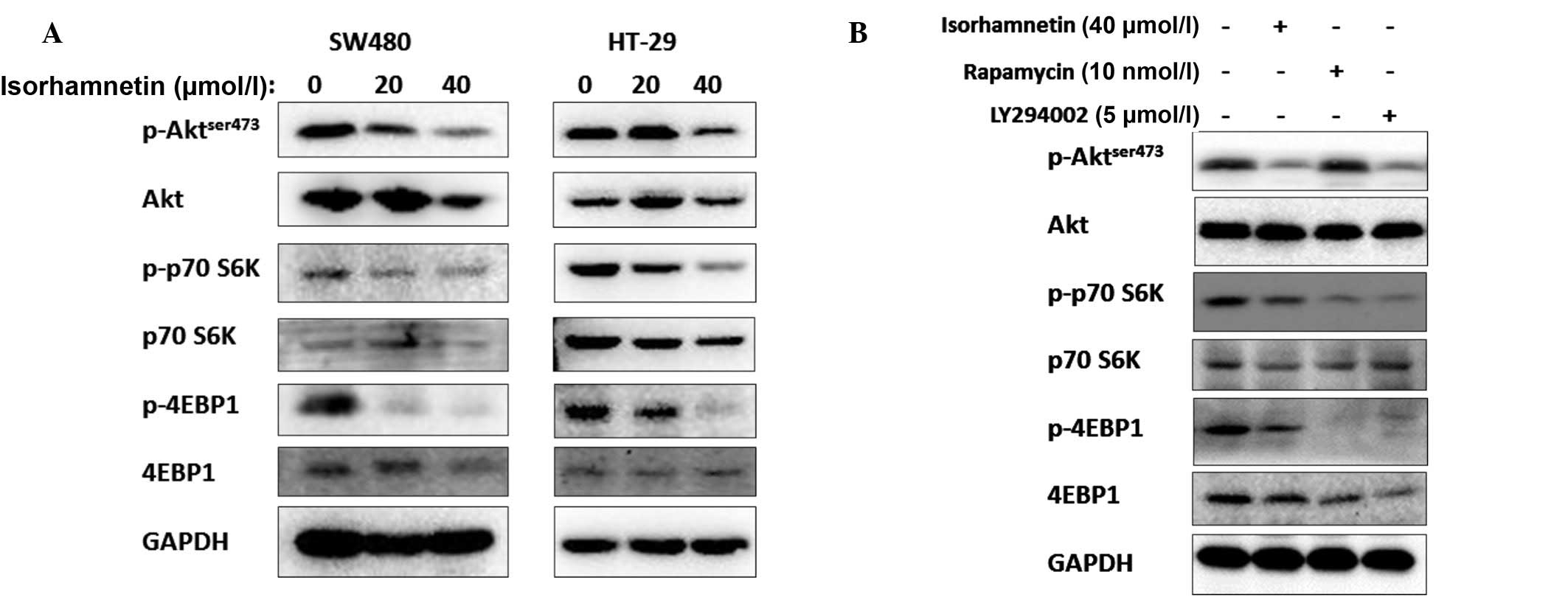
Isorhamnetin degraded the phosphorylation level of
Akt; thus, on the following day it was determined whether
isorhamnetin affected the other Akt-related proteins, particularly
those downstream of Akt. Certain PI3K-AKT-mTOR-related proteins
were detected and it was identified that the phosphorylation levels
of the two predominant mTOR target proteins, p70S6K and 4E-BP1,
were inhibited following treatment with isorhamnetin. The results
are shown in Fig. 4. In contrast
to other PI3K inhibitors, isorhamnetin did not induce feedback
activation of Akt, as is the case with rapamycin. The inhibition
effect of isorhamnetin on p70S6K and 4E-BP1 was similar to
(although marginally lower than) that of LY294002. Thus, it was
inferred that isorhmantin inhibited the PI3K-Akt-mTOR pathway.
Moreover, the phosphorylation levels of GSK3β protein were not
changed (data not shown).
Isorhamnetin increases the level of
Cyclin B1 proteins
To determine whether the G2/M arrest induced by
isorhamnetin in CRC cells was due to the changes in cell
cycle-related proteins, western blot analysis was performed to
assess the expression of these proteins, including Cyclin B1,
Cyclin D1 and p21. As shown in Fig.
5, the expression levels of Cyclin D1 were not changed in HT-29
cells but increased in SW480 cells; those of p21 were not changed
in HT-29 cells but decreased in SW480 cells; and the expression
levels of Cyclin B1 increased in the two cell types. The Cyclin
B1-CDK1 complex was correlated with the G2-mitosis phase, i.e., the
increase in Cyclin B1 expression levels prolonged the G2/M phase.
Therefore, the isorhamnetin-induced cell cycle arrest was
associated with the increase in Cyclin B1 expression levels. Cyclin
D1 and p21 expression levels did not change similarly in the two
types of cells; thus, it was not possible to confirm the effect of
isorhamnetin on these two cell cycle proteins.
Discussion
The PI3K-Akt-mTOR signaling pathway is important
throughout the life of a cell, including during proliferation,
glucose metabolism and for survival (15,16).
In normal cells, this pathway benefits growth and metabolism, and
its function is controlled by other regulatory proteins. One
negative regulatory protein is phosphatase and tensin homolog
(PTEN), which is known to be an antioncogene. The PTEN gene is
usually inactivated due to mutation, deletion or epigenetic
silencing. By contrast, the PI3K-Akt-mTOR pathway is frequently
activated in tumorgenesis. Thus, this signaling pathway is widely
recognized as a key regulator of cancer cells and is an important
target of anticancer drugs (17,18).
Numerous small-molecule inhibitors that inhibit the aberrant
PI3K-Akt-mTOR pathway have been developed (19,20).
However, a number of unidentified inhibitors may be toxic.
Rapamycin was the first mTORC1 inhibitor approved by the Food and
Drug Administration (21). The
number of studies in this field are increasing, but no satisfactory
inhibitor that may be used to cure cancer has been identified. The
possible reasons for this include incomplete inhibition and
secondary activation. Rapamycin inhibits mTORC1 but simultaneously
triggers a negative feedback mechanism that activates an upstream
bypass (22). This bypass
persistently activates the p-S6K1 protein, which is downstream of
mTORC1 and leads to treatment failure.
Flavonoids are a group of natural plant secondary
metabolites or yellow pigments, with a structure similar to that of
flavones. They are most commonly known for their antioxidant
activity in vitro. Flavonoids are able to inhibit tumor
invasion, and their antiproliferative effects are associated with
their structure (23). However,
the potential mechanisms underlying their anticancer activity are
unclear. Isorhamnetin is a dietary flavonoid found in several
fruits, such as apples, pears and blackberries. It is also a
predominant plasma metabolite of quercetin. A previous study has
shown that quercetin induces cell cycle arrest at G0/G1 in SK-Br3
breast carcinoma cells (24).
Additionally, quercetin blocks SW480 cells at the G2/M phase by
suppressing Cyclin D1 and survivin expression. Similar results have
been obtained after treating cells with structurally related
analogs of quercetin (25,26). Isorhamnetin has a structure similar
to other flavonoids; it is an intermediate 3′O-methylated
metabolite of quercetin. Its antiproliferative effect has been
confirmed in skin cancer cells, esophageal squamous carcinoma cells
and hepatocellular carcinoma cells (6,8,9).
Jaramillo et al (5)
investigated the effect of isorhamnetin on the HCT116 cell line. In
the present study, the suppressive effect was also identified in
three CRC cell lines, and isorhamnetin was observed to inhibit the
PI3K-Akt-mTOR pathway and affect cell cycle-related proteins.
Accordingly, the inhibition ratio of isorhmnetin was
investigated by an MTT assay. The results showed that isorhamnetin
suppressed the growth of the three CRC cell lines. The
IC50 value after 72 h was ~50 μmol/l and growth was
slow, as shown by the BrdU assay. Cell cycle analysis confirmed
that isorhamnetin degraded the G1 phase and induced G2/M phase
arrest. The findings of the present study were consistent with
those of a previous study showing that isorhamnetin inhibited
proliferation and induced cell cycle arrest in human HCT116 cells
(27).
Previous studies have shown that isorhamnetin
affected the phosphorylation level of Akt in JB6 and A431 skin
cancer cells (6). Thus, western
blot analysis was conducted on the cell lines in this study to
detect the phosphorylation of Akt after different time periods and
with different doses of isorhamnetin. The results showed that the
phosphorylation of Akt was inhibited with 20 μmol/l isorhamnetin
after 12 h and the effective time point and dose indicated that
this compound was low-dose isorhamnetin-dependent and had a rapid
onset. Akt is the key factor downstream of PI3K, to a certain
extent, it could reflect whether isorhamnetin inhibited the entire
downstream pathway. The predominant downstream factors of
PI3K-Akt-mTOR, p70S6K and 4E-BP1 were also detected. In SW480 and
HT-29 cells, isorhamnetin suppressed AKT and downregulated the
phosphorylation levels of p70S6K and 4E-BP1. Thus, it was proposed
that isorhamnetin may suppress the growth of CRC cells by
inhibiting the PI3K-Akt-mTOR-4E-BP1/p70S6K signaling pathway. In
addition, rapamycin induces the feedback activation of Akt
signaling through an IGF-1R-dependent mechanism (22) and LY294002 is a synthetic PI3K
inhibitor based on quercetin, which inhibits a broad range of
protein kinases, but has been identified to be toxic (28). This key PI3K signaling pathway is
highly activated in the majority of malignant types of cancer, and
contributes to malignant transformation, proliferation and
metastasis of tumor cells (29,30).
The expression pattern of cell cycle-related
proteins was analyzed further. The cell cycle checkpoint is a
monitoring mechanism that ensures the faithful replication of cells
(31). Cyclin B1 is produced at
the late S phase and degraded at the meta-mitosis phase. The Cyclin
B1-CDK1 complex aids in G2/M transition, resulting in entry of the
cells into mitosis. The G2/M transition phase is an important
checkpoint of the cell cycle and a target for the inhibition of
cell proliferation. A previous study has demonstrated that Cyclin
B1 is enriched when cells are arrested at the G2/M checkpoint
(32). When SW480 and HT-29 cells
were treated with 40 μmol/l isorhamnetin, the expression of Cyclin
B1 increased compared with that in the controls. Cyclin D1, which
controls the G1/S checkpoint, did not change when the cells were
treated with chrysoeriol. These results suggested that isorhamnetin
predominantly induced G2/M cell cycle arrest, which may be
attributed to the increase in Cyclin B1 expression levels.
All of the aforementioned biological activities were
exerted in vitro by isorhamnetin at small concentrations;
however, the values may not coincide with in vivo results.
In conclusion, cancer is a proliferative disease characterized by
an imbalance between oncogenes and antioncogenes. Cancer cells
often grow rapidly, escape from the cell-cycle checkpoints and
cause abnormal activation of certain signaling pathways. In the
present study, isorhamnetin significantly inhibited the
proliferation of CRC cells, delayed the G2/M cell cycle phase and
inhibited the PI3K-Akt-mTOR pathway. Thus, isorhamnetin may
potentially serve as an agent for CRC therapy as evidenced by
preliminary data. However, additional studies are required to
explain the role of isorhamnetin as a PI3K-Akt-mTOR pathway
inhibitor.
References
|
1
|
Murtaza I, Adhami VM, Hafeez BB, Saleem M
and Mukhtar H: Fisetin, a natural flavonoid, targets chemoresistant
human pancreatic cancer AsPC-1 cells through DR3-mediated
inhibition of NF-κB. Int J Cancer. 125:2465–2473. 2009.PubMed/NCBI
|
|
2
|
Lin Y, Shi R, Wang X and Shen HM:
Luteolin, a flavonoid with potential for cancer prevention and
therapy. Curr Cancer Drug Targets. 8:634–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Devi VG, Rooban BN, Sasikala V,
Sahasranamam V and Abraham A: Isorhamnetin-3-glucoside alleviates
oxidative stress and opacification in selenite cataract in vitro.
Toxicol In Vitro. 24:1662–1669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee J, Jung E, Kim S, et al: Isorhamnetin
represses adipogenesis in 3T3-L1 cells. Obesity (Silver Spring).
17:226–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jaramillo S, Lopez S, Varela LM, et al:
The flavonol isorhamnetin exhibits cytotoxic effects on human colon
cancer cells. J Agric Food Chem. Oct 5–2010.(Epub ahead of
print).
|
|
6
|
Kim JE, Lee DE, Lee KW, et al:
Isorhamnetin suppresses skin cancer through direct inhibition of
MEK1 and PI3-K. Cancer Prev Res (Phila). 4:582–591. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong CS, Kim YA, Kim MM, et al: Flavonoid
glycosides isolated from Salicornia herbacea inhibit matrix
metalloproteinase in HT1080 cells. Toxicol In Vitro. 22:1742–1748.
2008.PubMed/NCBI
|
|
8
|
Ma G, Yang C, Qu Y, Wei H, Zhang T and
Zhang N: The flavonoid component isorhamnetin in vitro inhibits
proliferation and induces apoptosis in Eca-109 cells. Chem Biol
Interact. 167:153–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teng BS, Lu YH, Wang ZT, Tao XY and Wei
DZ: In vitro anti-tumor activity of isorhamnetin isolated from
Hippophae rhamnoides L. against BEL-7402 cells. Pharmacol
Res. 54:186–194. 2006.PubMed/NCBI
|
|
10
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sartore-Bianchi A, Martini M, Molinari F,
et al: PIK3CA mutations in colorectal cancer are associated with
clinical resistance to EGFR-targeted monoclonal antibodies. Cancer
Res. 69:1851–1857. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Samuels Y and Velculescu VE: Oncogenic
mutations of PIK3CA in human cancers. Cell Cycle. 3:1221–1224.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Y, Zhou X, Xiao M, et al: Discovery
of chrysoeriol, a PI3K-AKT-mTOR pathway inhibitor with potent
antitumor activity against human multiple myeloma cells in vitro. J
Huazhong Univ Sci Technolog Med Sci. 30:734–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jacinto E, Loewith R, Schmidt A, et al:
Mammalian TOR complex 2 controls the actin cytoskeleton and is
rapamycin insensitive. Nat Cell Biol. 6:1122–1128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morgensztern D and McLeod HL:
PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer
Drugs. 16:797–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yap TA, Garrett MD, Walton MI, Raynaud F,
de Bono JS and Workman P: Targeting the PI3K-AKT-mTOR pathway:
progress, pitfalls, and promises. Curr Opin Pharmacol. 8:393–412.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tokunaga E, Oki E, Egashira A, et al:
Deregulation of the Akt pathway in human cancer. Curr Cancer Drug
Targets. 8:27–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng JQ, Lindsley CW, Cheng GZ, Yang H
and Nicosia SV: The Akt/PKB pathway: molecular target for cancer
drug discovery. Oncogene. 24:7482–7492. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hidalgo M and Rowinsky EK: The
rapamycin-sensitive signal transduction pathway as a target for
cancer therapy. Oncogene. 19:6680–6686. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang WY, Cai YZ and Zhang Y: Natural
phenolic compounds from medicinal herbs and dietary plants:
potential use for cancer prevention. Nutr Cancer. 62:1–20. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeong JH, An JY, Kwon YT, Rhee JG and Lee
YJ: Effects of low dose quercetin: cancer cell-specific inhibition
of cell cycle progression. J Cell Biochem. 106:73–82. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shan BE, Wang MX and Li RQ: Quercetin
inhibit human SW480 colon cancer growth in association with
inhibition of cyclin D1 and survivin expression through
Wnt/β-catenin signaling pathway. Cancer Invest. 27:604–612.
2009.PubMed/NCBI
|
|
25
|
Wang W, VanAlstyne PC, Irons KA, Chen S,
Stewart JW and Birt DF: Individual and interactive effects of
apigenin analogs on G2/M cell-cycle arrest in human colon carcinoma
cell lines. Nutr Cancer. 48:106–114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wan X, Harkavy B, Shen N, Grohar P and
Helman LJ: Rapamycin induces feedback activation of Akt signaling
through an IGF-1R-dependent mechanism. Oncogene. 26:1932–1940.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hyun J, Shin SY, So KM, Lee YH and Lim Y:
Isoflavones inhibit the clonogenicity of human colon cancer cells.
Bioorg Med Chem Lett. 22:2664–2669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abdul-Ghani R, Serra V, Gyorffy B, et al:
The PI3K inhibitor LY294002 blocks drug export from resistant colon
carcinoma cells overexpressing MRP1. Oncogene. 25:1743–1752. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hoeffer CA and Klann E: mTOR signaling: at
the crossroads of plasticity, memory and disease. Trends Neurosci.
33:67–75. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bjornsti MA and Houghton PJ: The TOR
pathway: a target for cancer therapy. Nat Rev Cancer. 4:335–348.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nasmyth K: Viewpoint: putting the cell
cycle in order. Science. 274:1643–1645. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cappelletti V, Fioravanti L, Miodini P and
Di Fronzo G: Genistein blocks breast cancer cells in the G(2)M
phase of the cell cycle. J Cell Biochem. 79:594–600. 2000.
View Article : Google Scholar : PubMed/NCBI
|