Introduction
Inflammation is crucial in mammalian physiology and
is a hallmark of many human diseases, including atherosclerosis,
autoimmune disorders, diabetes, chronic infection and cancer
(1). In the presence of stimuli
such as lipopolysaccharide (LPS), activated macrophages produce
several inflammatory mediators, including nitric oxide (NO) and
prostaglandin E2 (PGE2) and pro-inflammatory
cytokines such as tumor necrosis factor (TNF)-α and interleukins
(ILs). However, vigorous production of inflammatory cytokines may
lead to septic shock and tissue damage during an infection
(2,3).
Production and release of pro-inflammatory mediators
and cytokines in response to LPS is dependent upon inducible gene
expression, which is mediated by the activation of transcription
factors including nuclear transcription factor-κB (NF-κB) and
mitogen-activated protein kinases (MAPKs) (4–8).
NF-κB regulates various genes involved in immune and acute phase
inflammatory responses. NF-κB activation, in response to
pro-inflammatory stimuli, involves the rapid phosphorylation of IκB
by the IκB kinase (IKK) signalosome complex. Free NF-κB produced by
this process is translocated to the nucleus where it binds to
κB-binding sites in the promoter regions of target genes. It then
induces the transcription of pro-signalosome mediators and
cytokines (9–11). Therefore, the NF-κB activation
pathway is a common target of anti-inflammatory drugs.
Effective herbal medicines have recently generated
renewed interest for the production of novel therapeutic strategies
to suppress pro-inflammatory mediator and cytokine production by
macrophages (12–14). Hizikia fusiforme (H.
fusiforme) is one of the most common edible brown seaweed
species of the Sargassaceae family, and is located in the littoral
zones of Korea and Japan. Findings of previous studies have
suggested that crude extracts of H. fusiforme possess a
variety of biologically active compounds, particularly
antioxidants, immuno-modulators and anticoagulants (15–18).
We have previously demonstrated that
5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone (5HHMF, Fig. 1A), isolated from H.
fusiforme extracts, potently induces apoptosis in human AGS
carcinoma cells (19). In
addition, 5HHMF and its derivative
3′-hydroxy-5,6,7,4′-tetramethoxyflavone, exhibit more potent
inhibitory effects on the growth of human leukemia and breast
cancer cells than their permethoxylated counterparts, HMF and
5,6,7,3′,4′-pentamethoxyflavone, respectively (20,21).
However, the anti-inflammatory activity of 5HHMF has not yet been
elucidated. The aim of the present study was to investigate whether
5HHMF inhibited the production and expression of inflammatory
mediators and cytokines in LPS-stimulated RAW 264.7 murine
macrophage cells. To the best of our knowledge, this is the first
study to investigate this and the results demonstrated that the
anti-inflammatory effects of 5HHMF are achieved by inhibition of
the nuclear translocation of NF-κB by preventing IκB-α
degradation.
Materials and methods
Reagents
LPS (Escherichia coli 026:B6), Griess
reagent, Tween-20, bovine serum albumin and MTT were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s
medium (DMEM), fetal bovine serum (FBS) and other tissue culture
reagents were purchased from Gibco-BRL (Grand Island, NY, USA).
Other chemicals were obtained from Sigma-Aldrich unless otherwise
indicated.
Cell culture
The RAW 264.7 cell line, which was derived from
murine macrophages, was obtained from the American Type Culture
Collection (Manassas, VA, USA). These cells were maintained in DMEM
medium, supplemented with 10% FBS, 100 U/ml penicillin and 100
μg/ml streptomycin, at 37°C in a 5% CO2 humidified air
environment. Previously purified 5HHMF (19) was used in this study. The 5HHMF was
dissolved in dimethyl sulfoxide (DMSO) to produce a 10 mg/ml stock
solution and then adjusted to final concentrations using complete
DMEM.
Cell viability assay
Cell viability was evaluated by the MTT assay. RAW
264.7 cells were seeded into 96-well plates at a density of
4×103 cells/well and maintained at 37°C for 24 h. The
cells were exposed to various concentrations of 5HHMF (0, 5, 10 and
15 μg/ml) for 1 h and stimulated with LPS (100 ng/ml). After 24 h
of incubation, the MTT (0.5 mg/ml in phosphate-buffered saline,
PBS) solution was added to each well and incubated for another 3 h.
The formazan crystals were dissolved in 200 μl DMSO and the cell
viability was determined subsequent to measuring the absorbance at
a wavelength of 540 nm with a microplate reader (Dynatech MR-7000;
Dynatech Laboratories Inc., Chantilly, VA, USA).
Measurement of NO and PGE2
production
The nitrite concentration in the medium was measured
according to the Griess reaction and the calculated concentration
was taken as an indicator of NO production. The supernatant of cell
cultures was mixed with an equal volume of Griess reagent (1%
sulfanilamide in 5% phosphoric acid and 0.1%
naphthylethylenediamine dihydrochloride in water). The optical
density at 540 nm was measured and calculated against a sodium
nitrite standard curve. The accumulated PGE2 in the
culture medium was measured using a PGE2 enzyme-linked
immunosorbent assay (ELISA) kit (Cayman Chemical, Ann Arbor, MI,
USA), according to the manufacturer’s instructions.
Measurement of pro-inflammatory cytokine
production
The inhibitory effect of 5HHMF on the production of
pro-inflammatory cytokines, TNF-α and IL-1β, from LPS-treated RAW
264.7 cells was determined using a mouse ELISA kit (R&D
Systems, Minneapolis, MN, USA), as described previously (22).
Reverse transcriptase PCR (RT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). The total RNA (1
μg) obtained from the cells was reverse-transcribed using M-MLV
Reverse Transcriptase (Promega Corporation, Madison, WI, USA) to
produce cDNA. RT-generated cDNA encoding iNOS, COX-2, TNF-α, IL-1β
and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) genes was
amplified by PCR using selective primers (Table I). Subsequent to amplification,
aliquots of the PCR reaction mixture were electrophoresed on an
agarose gel.
 | Table IPrimer sequences used in the reverse
transcription PCR. |
Table I
Primer sequences used in the reverse
transcription PCR.
| Gene | Primer | Sequence | Accession
number |
|---|
| COX-2 | Sense |
CAGCAAATCCTTGCTGTTCC | XM-004028064.1 |
| Antisense |
TGGGCAAAGAATGCAAACATC | |
| iNOS | Sense |
ATGTCCGAAGCAAACATCAC | NM-010927.3 |
| Antisense |
TAATGTCCAGGAAGTAGGTG | |
| TNF-α | Sense |
ATGAGCACAGAAAGCATGATC | NM-013693.1 |
| Antisense |
TACAGGCTTGTCACTCGAATT | |
| IL-1β | Sense |
CTCGTGCTGTCGGACCCATAT | NM-008361.3 |
| Antisense |
TTGAAGACAAACCGCTTTTCCA | |
| GAPDH | Sense |
TTCACCACCATGGAGAAGGC | XR031141.1 |
| Antisense |
GGCATGGACTGTGGTCATGA | |
Western blot analysis
The cells were washed with PBS three times and lysed
with a lysis buffer (1% Triton X-100, 1% deoxycholate and 0.1%
NaN3) containing protease inhibitor cocktail tablets
(Roche Diagnostics GmbH, Mannheim, Germany). Equal quantities of
protein were separated on 10% sodium dodecyl sulfate-polyacrylamide
mini-gels and transferred to Immobilon polyvinylidene difluoride
membranes (Millipore, Milford, MA, USA). Subsequent to incubation
with the appropriate primary antibody, the membrane was hybridized
with secondary antibody conjugated to horseradish peroxidase for 1
h at room temperature. Following three washes with Tris-buffered
saline with Tween-20, immunoreactive bands were visualized using
the Enhanced Chemiluminescence Detection system (Pierce
Biotechnology Inc., Rockford, IL, USA). In a parallel experiment,
nuclear protein was prepared using nuclear extraction reagents
(Pierce Biotechnology Inc.), according to the manufacturer’s
instructions.
Confocal laser scanning microscopy
study
NF-κB p65 nuclear localization was detected by
indirect immunofluorescence assays using confocal microscopy. RAW
264.7 cells were cultured directly on glass coverslips in 6-well
plates for 24 h. Subsequent to stimulation with 100 ng/ml LPS
and/or 15 μg/ml 5HHMF, the cells were fixed with 4%
paraformaldehyde in PBS, permeabilized with 0.2% Triton X-100 in
PBS and blocked with 1.5% normal donkey serum (Sigma-Aldrich). A
polyclonal antibody against NF-κB p65 (1 μg/well) was applied for 1
h followed by a 1 h incubation with fluorescein
isothiocyanate-conjugated donkey anti-rabbit IgG (Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA). After
washing with PBS, the coverslips were mounted in Fluoromount-G™
(Southern Biotechnology Associates Inc., Birmingham, AL, USA) and
fluorescence was visualized using a Zeiss LSM 510 laser scanning
confocal device attached to an Axiovert 100 microscope using a
Plan-Apochromat ×100 Oil DIC objective (Carl Zeiss, Oberkochen,
Germany) (23).
Statistical analysis
Data are presented as the mean ± SD. Statistical
significance was determined using analysis of variance followed by
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Protective effect of 5HHMF on LPS-induced
cytotoxicity
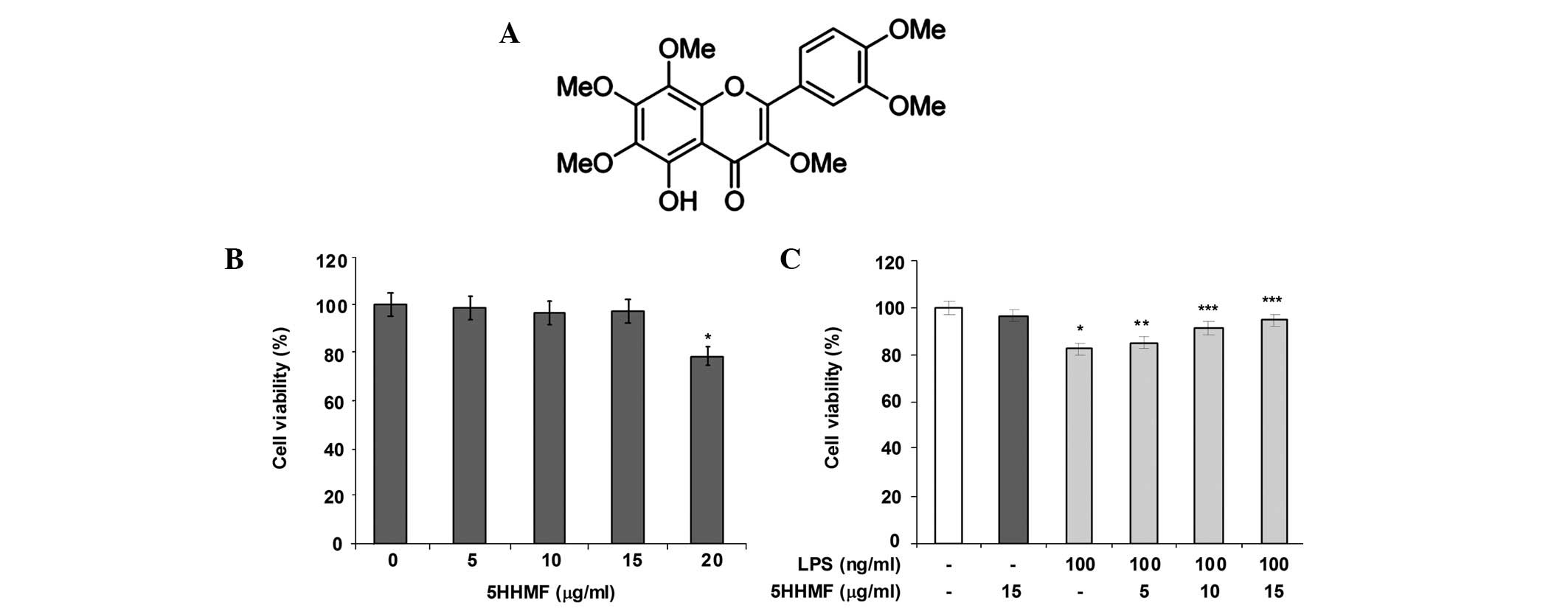
The protective effect of 5HHMF was initially
investigated based on LPS-induced cytotoxicity of RAW 264.7 cells
using an MTT assay. As shown in Fig.
1C, LPS (100 ng/ml) treatment significantly induced
cytotoxicity compared with that observed in the unstimulated
control cells. However, the growth of LPS-stimulated RAW 264.7
cells was significantly enhanced by 5HHMF in a dose-dependent
manner. Moreover, 5, 10 and 15 μg/ml concentrations of 5HHMF
exhibited a protective effect on LPS-stimulated cytotoxicity in RAW
264.7 cells. However, 5HHMF alone did not show any obvious
cytotoxic effect at the concentrations of 5–15 μg/ml (Fig. 1B).
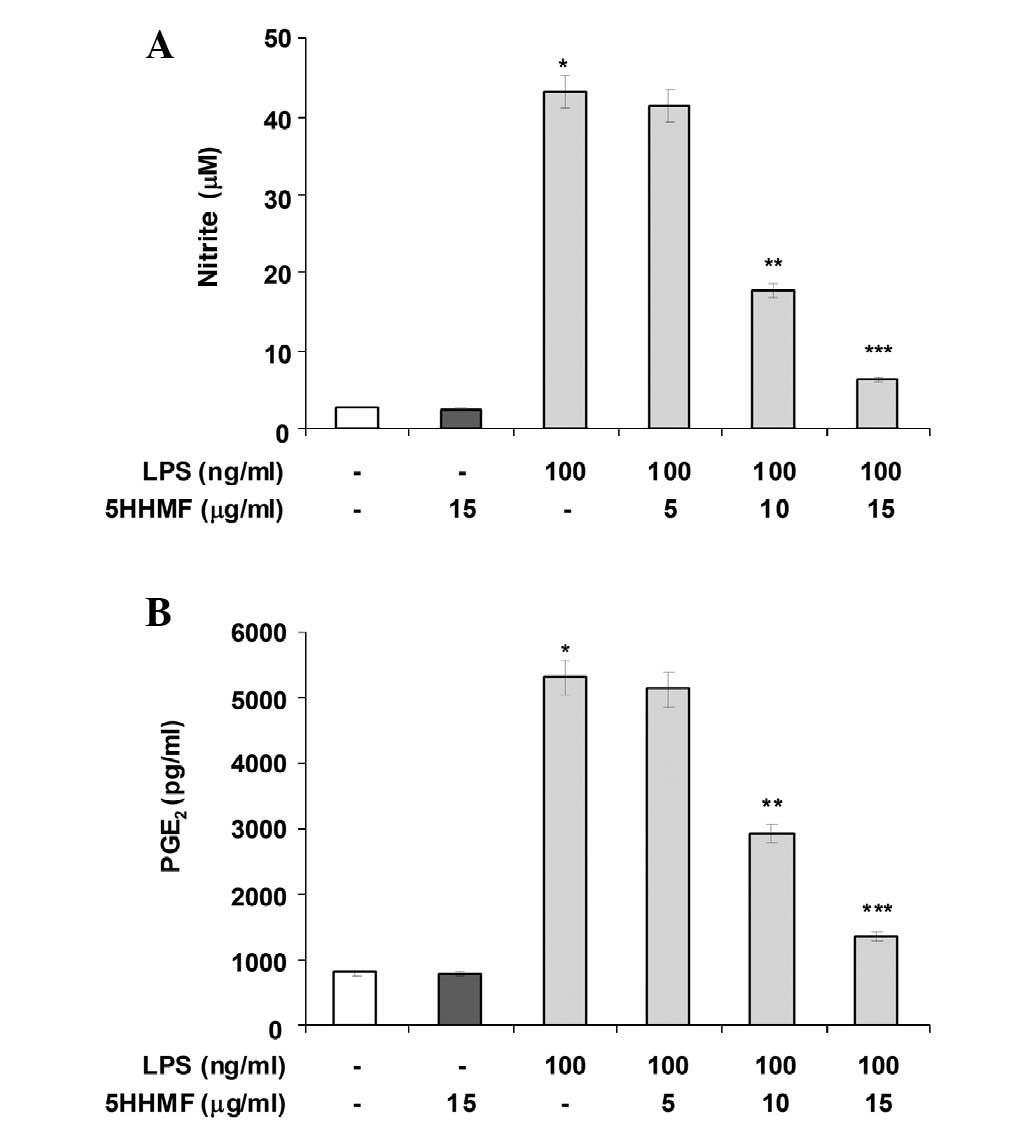
Effect of 5HHMF on NO and PGE2
production in LPS-stimulated RAW 264.7 cells
Pro-inflammatory mediators such as NO and
PGE2 are important in the inflammatory response. To
determine the level of NO production, nitrite released into the
culture medium was measured using Griess reagent. As shown in
Fig. 2A, LPS alone markedly
induced NO production in the cells compared with that in the
control. However, pre-treatment with 5HHMF (up to 15 μg/ml)
significantly repressed the levels of NO production in
LPS-stimulated RAW 264.7 cells in a concentration-dependent manner
(Fig. 2A). The effects of 5HHMF on
the production of PGE2, another important inflammatory
mediator, was also investigated in LPS-stimulated RAW 264.7 cells.
As shown in Fig. 2B, treatment of
RAW 264.7 cells with LPS resulted in a marked increase in
PGE2 release compared with that in the untreated control
after 24 h of exposure to LPS. However, 5HHMF inhibited
LPS-mediated PGE2 production in a
concentration-dependent manner at the concentrations tested. These
results suggest that pretreatment with 5HHMF results in significant
suppression of the expression of LPS-mediated pro-inflammatory
mediators.
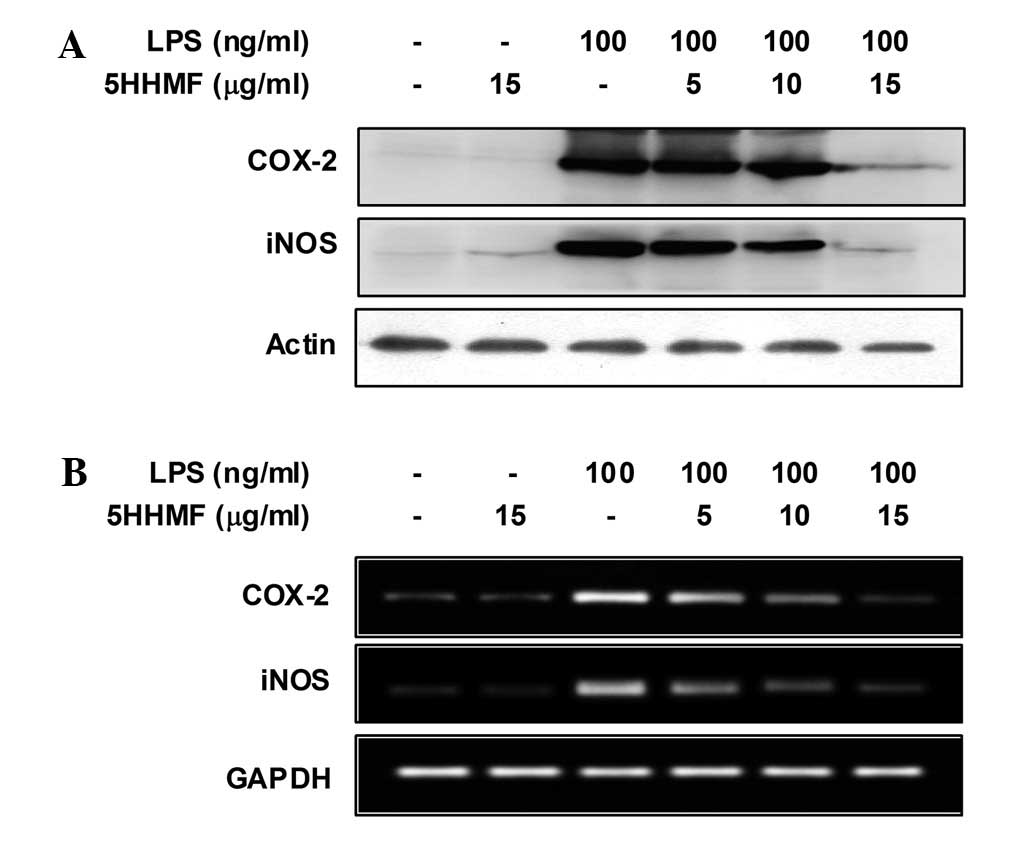
Effect of 5HHMF on LPS-stimulated iNOS
and COX-2 expression
To elucidate the mechanism involved in the
inhibition of NO and PGE2 by 5HHMF in LPS-stimulated RAW
264.7 cells, the effect of 5HHMF on iNOS and COX-2 protein and gene
expression levels was investigated by western blot and RT-PCR
analyses (Fig. 3). iNOS and COX-2
protein and mRNA expression in unstimulated RAW 264.7 cells was
marginally detectable. However, iNOS and COX-2 expression increased
markedly in response to LPS, and 5HHMF significantly inhibited the
iNOS and COX-2 proteins in a dose-dependent manner (Fig. 3A). Under the same conditions, the
levels of iNOS and COX-2 mRNA expression were correlated with their
protein levels (Fig. 3B). These
results indicated that reduced expression of iNOS and COX-2 by
5HHMF was responsible for inhibiting NO and PGE2
production.
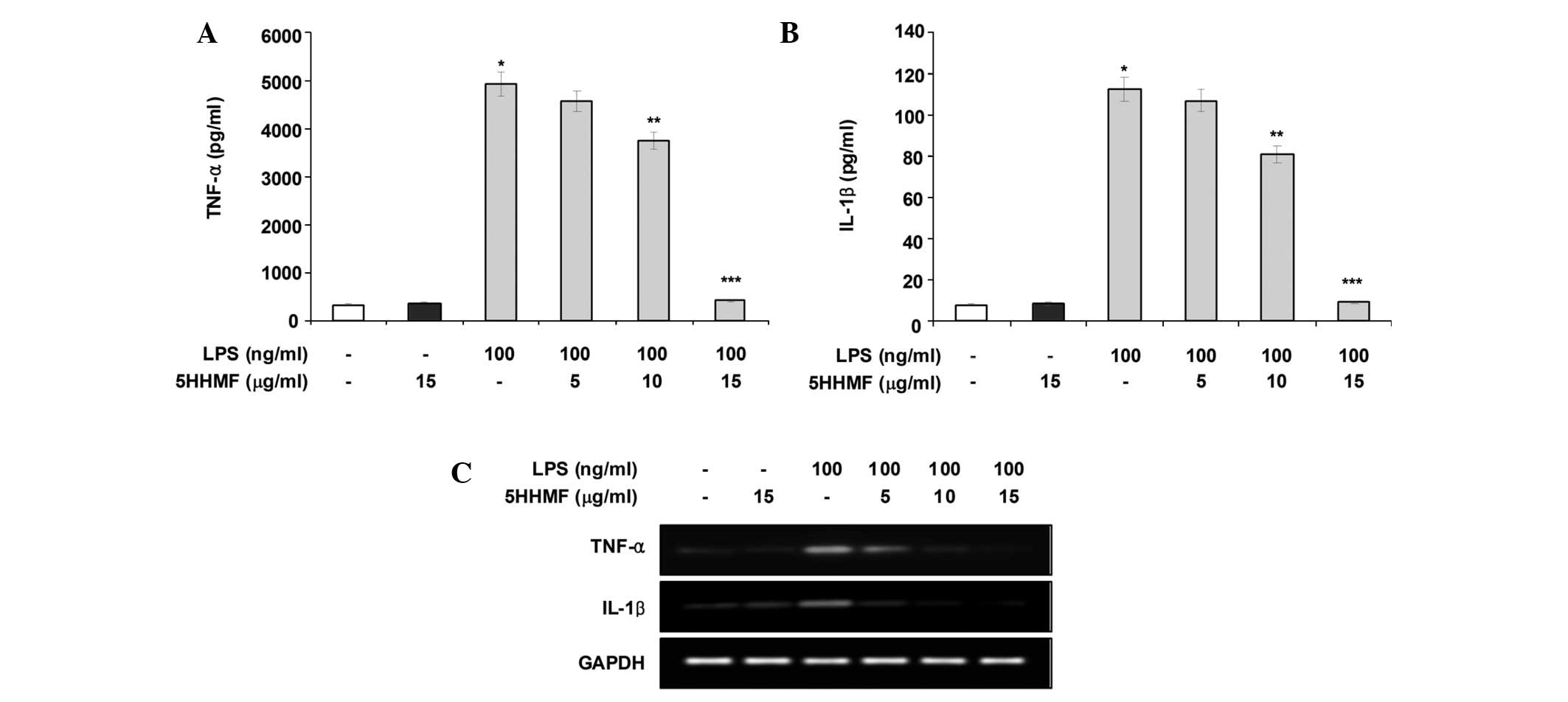
Effect of 5HHMF on TNF-α and IL-1β
production and expression in LPS-stimulated RAW 264.7 cells
As 5HHMF potently inhibited the pro-inflammatory
mediators NO and PGE2, its effects on LPS-stimulated
pro-inflammatory cytokines, such as TNF-α and IL-1β, were
investigated by an enzyme immunoassay and RT-PCR analysis. As shown
in Fig. 4A and B, TNF-α and IL-1β
levels increased significantly in the culture media of
LPS-stimulated RAW 264.7 cells. However, pre-treatment with 5HHMF
significantly decreased the release of these pro-inflammatory
cytokines in a concentration-dependent manner. In addition, the
TNF-α and IL-1β mRNA levels induced by LPS decreased significantly
in a concentration-dependent manner following 5HHMF treatment
(Fig. 4C). These results suggested
that 5HHMF is effective in suppressing pro-inflammatory cytokine
production by altering TNF-α and IL-1β transcription levels in
activated RAW 264.7 cells.
Inhibition of NF-κB activation by 5HHMF
in LPS-stimulated RAW 264.7 cells
Previous studies have suggested that NF-κB is an
important transcription factor that regulates iNOS, COX-2 and
inflammatory cytokine expression (24,25).
A number of the predominant mechanisms involving the activation of
NF-κB include the phosphorylation of IKK and degradation of IκB-α,
which allow the release of free NF-κB and its translocation into
the nucleus (26). To investigate
whether 5HHMF regulates the NF-κB pathway, we investigated whether
5HHMF prevented the translocation of the NF-κB p65 subunit to the
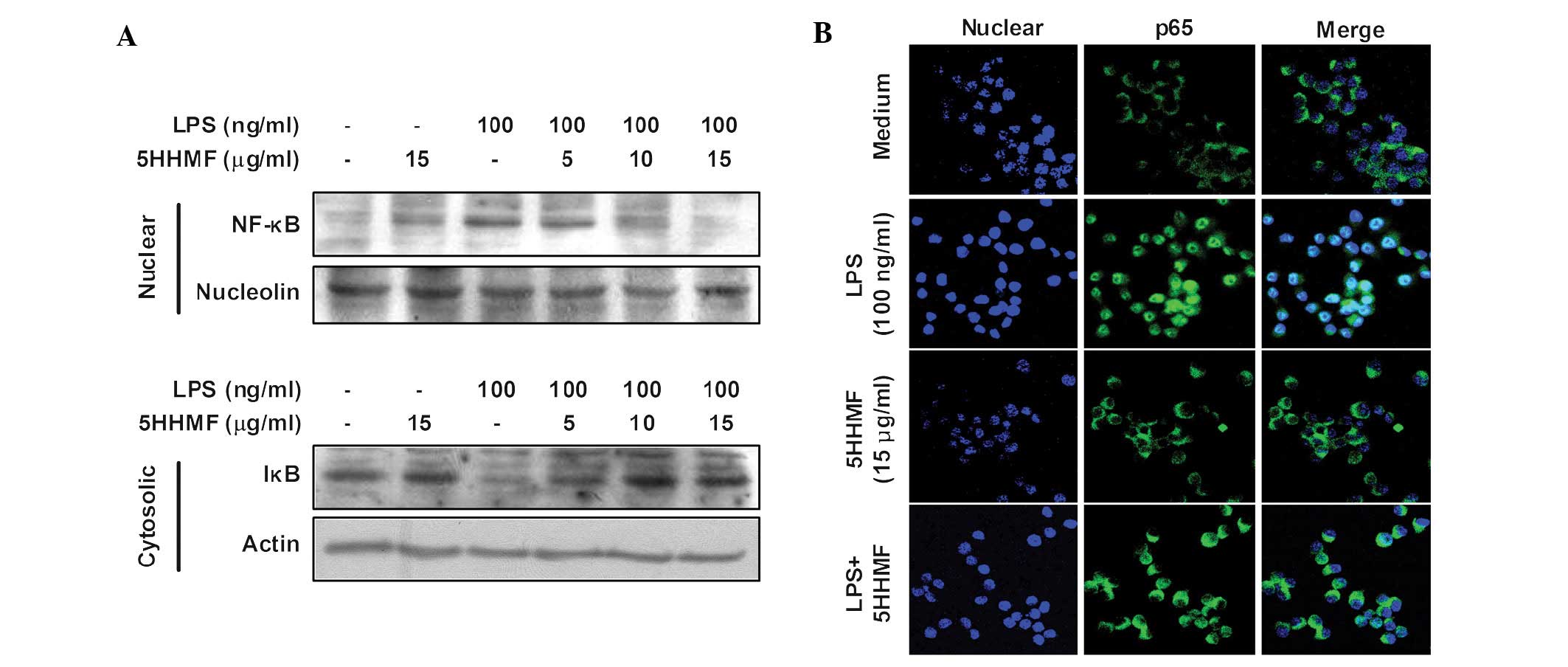
nucleus. Western blot analysis showed that the quantity of NF-κB
p65 in the nucleus increased markedly following exposure to LPS
alone; however, the LPS-induced p65 level in the nuclear fractions
decreased following 5HHMF pre-treatment. In addition, western blot
analysis was used to investigate whether 5HHMF blocked
LPS-stimulated degradation of IκB-α. As shown in Fig. 5A, IκB-α was markedly degraded 15
min after LPS treatment. This LPS-induced IκB-α degradation was
significantly reversed by 5HHMF. Furthermore, the translocation of
NF-κB to the nucleus in RAW 264.7 cells was analyzed using
immunofluorescence staining and confocal microscopy to clearly
understand the effect of 5HHMF on NF-κB p65 nuclear translocation
(Fig. 5B). The confocal images
revealed that NF-κB p65 was normally sequestered in the cytoplasm
(Fig. 5B, middle panel), and that
nuclear accumulation of NF-κB p65 was markedly induced following
the stimulation of RAW 264.7 cells with LPS (Fig. 5B, LPS panel). The LPS-induced
translocation of NF-κB p65 was completely eliminated subsequent to
pre-treating the cells with 5HHMF (Fig. 5B, LPS+5HHMF panel). Nuclear
translocation of NF-κB p65 was not induced in the cells following
pre-treatment with 5HHMF alone, in the absence of LPS stimulation
(Fig. 5B, 5HHMF panel). Thus,
these results demonstrated that the anti-inflammatory effect of
5HHMF in LPS-stimulated RAW 264.7 cells involves the NF-κB
pathway.
Discussion
The results of ther present study have demonstrated
that 5HHMF exhibits pharmacological and biological activities via
significant inhibitory effects on the production of the LPS-induced
pro-inflammatory mediators, such as iNOS and COX-2, as well as
cytokines (including TNF-α and IL-1β) in activated RAW 264.7 cells.
These effects were accompanied by the downregulation of NF-κB
activation.
Macrophages produce NO and pro-inflammatory
cytokines in response to bacterial LPS. This NO production is
controlled by selective pharmacological inhibition of distinct NO
synthase isoforms (27,28). iNOS is one of three key enzymes
that generate NO from arginine. NO is pivotal in numerous body
functions; however, its overproduction in macrophages, in
particular, leads to cytotoxicity, inflammation and autoimmune
disorders (6). Therefore, NO
inhibitors are essential for preventing inflammatory diseases.
PGE2 is considered an important mediator in the
processes of inflammation produced by COX-2 (29). As a result, a detailed
understanding of the intracellular mechanisms of the expression of
inflammatory mediators and the effects of inhibiting those
inflammatory mediators is important to identify therapeutic
strategies for inflammatory diseases. In the present study, 5HHMF
significantly suppressed LPS-stimulated NO and PGE2
production in RAW 264.7 cells in a concentration-dependent manner,
which appeared to be due to the transcriptional suppression of
COX-2 and iNOS. The results of the present study also indicated
that 5HHMF suppressed the production of the pro-inflammatory
cytokines TNF-α and IL-1β. These cytokines are key in the induction
of inflammation in macrophages (28,30).
TNF-α exhibits its pro-inflammatory activity by regulating several
intercellular and vascular cell adhesion molecules, which results
in the recruitment of leukocytes to sites of inflammation (31). IL-1β is also a key pro-inflammatory
cytokine that is released from immune responding cells when
stimulated by LPS (32). Thus,
inhibition of cytokine production or function may be considered a
key mechanism in RAW 264.7 cells. Treatment with 5HHMF prior to LPS
stimulation significantly attenuated the production of cytokines in
RAW 264.7 cells. Therefore, the inhibitory effect of 5HHMF on
inflammatory mediator expression aided in the identification of one
of the mechanisms responsible for its anti-inflammatory action and
suggests that 5HHMF is a potential therapeutic agent for treating
LPS-induced sepsis syndrome.
Bacterial pathogens such as LPS stimulate the
transcription of genes involved in the inflammatory and immune
responses, including the NF-κB pathway (33). NF-κB is activated by
phosphorylation, ubiquitination and subsequent proteolytic
degradation of NF-κB-bound IκB via activated IκB kinase (34). The excreted NF-κB transcription
factor then translocates to the nucleus and binds to NF-κB motifs
in the promoters of target genes such as those encoding iNOS, COX-2
and cytokines to promote transcription (35). The results of the present study
suggest that 5HHMF significantly inhibits the LPS-stimulated
nuclear translocation of p65 in RAW 264.7 macrophages. Thus, the
potential inhibition of cytokine production by 5HHMF is in
accordance with the inhibition of NF-κB-dependent cytokines and
reduced inflammation. Although inhibiting NF-κB activation has been
proposed as a therapeutic approach for sepsis, NF-κB is an
essential component of normal host defenses and blocking the
regulatory actions of NF-κB may result in severe immunosuppression
(36).
In conclusion, the results of the present study have
demonstrated that 5HHMF treatment results in a decrease of
pro-inflammatory mediators following LPS stimulation in RAW 264.7
cells. 5HHMF also significantly inhibited the release of TNF-α and
IL-1β and decreased their mRNA expression levels in a
dose-dependent manner. In addition, the anti-inflammatory
properties of 5HHMF were mediated by the downregulation of NF-κB
activation. Therefore, 5HHMF is a potential therapeutic agent for
patients with, or at risk of, septic shock and other inflammatory
diseases.
Acknowledgements
This study was supported by the Technology
Development Program for Agriculture and Forestry (grant no.
610003-03-1-SB110), Ministry for Food, Agriculture, Forestry and
Fisheries, Republic of Korea.
References
|
1
|
Andreasen AS, Krabbe KS, Krogh-Madsen R,
Taudorf S, Pedersen BK and Møller K: Human endotoxemia as a model
of systemic inflammation. Curr Med Chem. 15:1697–1705. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aderem A: Role of Toll-like receptors in
inflammatory response in macrophages. Crit Care Med. 29(Suppl 7):
S16–S18. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ritchlin CT, Haas-Smith SA, Li P, Hicks DG
and Schwarz EM: Mechanisms of TNF-alpha- and RANKL-mediated
osteoclastogenesis and bone resorption in psoriatic arthritis. J
Clin Invest. 111:821–831. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown MD and Sacks DB: Compartmentalised
MAPK pathways. Handb Exp Pharmacol. 186:205–235. 2008. View Article : Google Scholar
|
|
5
|
Ci X, Song Y, Zeng F, Zhang X, Li H, Wang
X, Cui J and Deng X: Ceftiofur impairs pro-inflammatory cytokine
secretion through the inhibition of the activation of NF-κB and
MAPK. Biochem Biophys Res Commun. 372:73–77. 2008.PubMed/NCBI
|
|
6
|
Liu RH and Hotchkiss JH: Potential
genotoxicity of chronically elevated nitric oxide: a review. Mutat
Res. 339:73–89. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siebenlist U, Franzoso G and Brown K:
Structure, regulation and function of NF-kappa B. Annu Rev Cell
Biol. 10:405–455. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Li H, Feng H, Xiong H, Zhang L,
Song Y, Yu L and Deng X: Valnemulin downregulates nitric oxide,
prostaglandin E2, and cytokine production via inhibition of
NF-kappaB and MAPK activity. Int Immunopharmacol. 9:810–816. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Edwards MR, Bartlett NW, Clarke D, Birrell
M, Belvisi M and Johnston SL: Targeting the NF-kappaB pathway in
asthma and chronic obstructive pulmonary disease. Pharmacol Ther.
121:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghosh S and Hayden MS: New regulators of
NF-kappaB in inflammation. Nat Rev Immunol. 8:837–848. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wong ET and Tergaonkar V: Roles of
NF-kappaB in health and disease: mechanisms and therapeutic
potential. Clin Sci (Lond). 116:451–465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barak V, Kalickman I, Halperin T,
Birkenfeld S and Ginsburg I: PADMA-28, a Tibetan herbal preparation
is an inhibitor of inflammatory cytokine production. Eur Cytokine
Netw. 15:203–209. 2004.PubMed/NCBI
|
|
13
|
Chi YS, Lim H, Park H and Kim HP: Effects
of wogonin, a plant flavone from Scutellaria radix, on skin
inflammation: in vivo regulation of inflammation-associated gene
expression. Biochem Pharmacol. 66:1271–1278. 2003.PubMed/NCBI
|
|
14
|
Genovese MC: Biologic therapies in
clinical development for the treatment of rheumatoid arthritis. J
Clin Rheumatol. 11:S45–S54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim KI, Seo HD, Lee HS, Jo HY and Yang HC:
Studies on the blood anticoagulant polysaccharide isolated from hot
water extracts of Hizikia fusiforme. Korean J Food Sci Nutr.
27:1204–1210. 1998.
|
|
16
|
Nagai T and Yukimoto T: Preparation and
functional properties of beverages made from sea algae. Food Chem.
81:327–332. 2003. View Article : Google Scholar
|
|
17
|
Okai Y, Okai KH, Ishizaka S, Ohtani K,
Yuasa IS and Yamashita U: Possible immunodulating activities in
extract of edible brown alga Hizikia fusiforme (Hiziki). J
Food Agricul. 76:56–62. 1998. View Article : Google Scholar
|
|
18
|
Yan X, Chuda Y, Suzuki M and Nagata T:
Fucoxanthin as the major antioxidant in Hijikia fusiformis, a
common edible seaweed. Biosci Biotechnol Biochem. 63:605–607. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim MJ, Lee HH, Seo MJ, Kang BW, Park JU,
Kim KS, Kim KY, Joo WH, Choi YH, Cho YS and Jeong YK:
Identification of 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone from
Hizikia fusiforme involved in the induction of the apoptosis
mediators in human AGS carcinoma cells. J Microbiol Biotechnol.
22:1665–1672. 2012.
|
|
20
|
Pan MH, Lai YS, Lai CS, Wang YJ, Li S, Lo
CY, Dushenkov S and Ho CT:
5-Hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone induces apoptosis
through reactive oxygen species production, growth arrest and DNA
damage-inducible gene 153 expression, and caspase activation in
human leukemia cells. J Agric Food Chem. 55:5081–5091. 2007.
|
|
21
|
Sergeev IN, Li S, Colby J, Ho CT and
Dushenkov S: Polymethoxylated flavones induce
Ca2+-mediated apoptosis in breast cancer cells. Life
Sci. 80:245–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bae DS, Kim YH, Pan CH, Nho CW, Samdan J,
Yansan J and Lee JK: Protopine reduces the inflammatory activity of
lipopolysaccharide-stimulated murine macrophages. BMB Rep.
5:108–113. 2012.PubMed/NCBI
|
|
23
|
Lee YH, Jeon SH, Kim SH, Kim C, Lee SJ,
Koh D, Lim Y, Ha K and Shin SY: A new synthetic chalcone
derivative, 2-hydroxy-3′,5,5′-trimethoxychalcone (DK-139),
suppresses the Toll-like receptor 4-mediated inflammatory response
through inhibition of the Akt/NF-κB pathway in BV2 microglial
cells. Exp Mol Med. 44:369–377. 2012.PubMed/NCBI
|
|
24
|
Guo LY, Hung TM, Bae KH, Shin EM, Zhou HY,
Hong YN, Kang SS, Kim HP and Kim YS: Anti-inflammatory effects of
schisandrin isolated from the fruit of Schisandra chinensis
Baill. Eur J Pharmacol. 591:293–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Southan GJ and Szabó C: Selective
pharmacological inhibition of distinct nitric oxide synthase
isoforms. Biochem Pharmacol. 51:383–394. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Plummer SM, Holloway KA, Manson MM, Munks
RJ, Kaptein A, Farrow S and Howells L: Inhibition of
cyclo-oxygenase 2 expression in colon cells by the chemopreventive
agent curcumin involves inhibition of NF-kappaB activation via the
NIK/IKK signalling complex. Oncogene. 18:6013–6020. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sarkar D, Saha P, Gamre S, Bhattacharjee
S, Hariharan C, Ganguly S, Sen R, Mandal G, Chattopadhyay S,
Majumdar S and Chatterjee M: Anti-inflammatory effect of
allylpyrocatechol in LPS-induced macrophages is mediated by
suppression of iNOS and COX-2 via the NF-kappaB pathway. Int
Immunopharmacol. 8:1264–1271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tao JY, Zheng GH, Zhao L, Wu JG, Zhang XY,
Zhang SL, Huang ZJ, Xiong FL and Li CM: Anti-inflammatory effects
of ethyl acetate fraction from Melilotus suaveolens Ledeb on
LPS-stimulated RAW 264.7 cells. J Ethnopharmacol. 123:97–105. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van Q, Nayak BN, Reimer M, Jones PJ,
Fulcher RG and Rempel CB: Anti-inflammatory effect of Inonotus
obliquus, Polygala senega L, and Viburnum
trilobum in a cell screening assay. J Ethnopharmacol.
125:487–493. 2009.
|
|
30
|
Yun KJ, Kim JY, Kim JB, Lee KW, Jeong SY,
Park HJ, Jung HJ, Cho YW, Yun K and Lee KT: Inhibition of
LPS-induced NO and PGE2 production by asiatic acid via NF-kappa B
inactivation in RAW 264.7 macrophages: possible involvement of the
IKK and MAPK pathways. Int Immunopharmacol. 8:431–441. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aggarwal BB and Natarajan K: Tumor
necrosis factors: developments during the last decade. Eur Cytokine
Netw. 7:93–124. 1996.PubMed/NCBI
|
|
32
|
Lee HS, Ryu DS, Lee GS and Lee DS:
Anti-inflammatory effects of dichloromethane fraction from
Orostachys japonicus in RAW 264.7 cells: suppression of
NF-kappaB activation and MAPK signaling. J Ethnopharmacol.
140:271–276. 2012.PubMed/NCBI
|
|
33
|
Kim YG, Ohta T, Takahashi T, Kushiro A,
Nomoto K, Yokokura T, Okada N and Danbara H: Probiotic
Lactobacillus casei activates innate immunity via NF-kappaB
and p38 MAP kinase signaling pathways. Microbes Infect. 8:994–1005.
2006.
|
|
34
|
Rajapakse N, Kim MM, Mendis E and Kim SK:
Inhibition of inducible nitric oxide synthase and cyclooxygenase-2
in lipopolysaccharide-stimulated RAW264.7 cells by
carboxybutyrylated glucosamine takes place via down-regulation of
mitogen-activated protein kinase-mediated nuclear factor-kappaB
signaling. Immunology. 123:348–357. 2008.
|
|
35
|
Lee SJ, Bai SK, Lee KS, Namkoong S, Na HJ,
Ha KS, Han JA, Yim SV, Chang K, Kwon YG, et al: Astaxanthin
inhibits nitric oxide production and inflammatory gene expression
by suppressing IκB kinase-dependent NF-kappaB activation. Mol
Cells. 16:97–105. 2003.PubMed/NCBI
|
|
36
|
Abraham E: Nuclear factor-kappaB and its
role in sepsis-associated organ failure. J Infect Dis. 187(Suppl
2): S364–S369. 2003. View
Article : Google Scholar : PubMed/NCBI
|