Introduction
Bladder cancer is one of the most common types of
malignancy worldwide, particularly in Europe and the United States
(1). Due to serious side effects,
conventional cisplatin-based chemotherapy is not suitable for all
patients (2). Therefore, there is
an urgent need for safe and effective drugs to treat bladder
cancer. Resveratrol is an edible polyphenolic phytoalexin present
in grapes and red wine and is able to prevent numerous
age-associated diseases, including cardiovascular disease,
Alzheimer’s disease and cancer (3–5). It
has been reported that resveratrol is important in inducing the
cytotoxicity and apoptosis of bladder cancer cells (6,7).
MicroRNAs (miRNAs) are a naturally occurring class
of non-coding RNAs involved in post-transcriptional gene regulation
by binding to a target site in the 3′-untranslated region of target
mRNAs (8). Several studies have
indicated that altered microRNA expression contributes to
carcinogenesis and is implicated in cancer cell proliferation and
apoptosis. As a result, miRNAs may function as regulatory molecules
and act as tumor suppressors or oncogenes (9). Among these microRNAs, miR-21 was
highly expressed in various types of cancer, including breast, lung
and pancreatic cancer (10,11).
Functional studies have revealed that miR-21 is an oncogene, which
is important in enhancing cell proliferation, promoting cell cycle
progression and increasing anti-apoptotic activation in cancer
cells (12). In numerous cancer
cell lines, miR-21 was demonstrated to be the therapeutic target of
resveratrol (13,14). Of note, miR-21 was significantly
upregulated in advanced bladder cancer tissues and the
overexpression of miR-21 promoted the proliferation of bladder
cancer cell lines (15).
Akt (protein kinase B), a key signaling molecule in
the phosphatidylinositol 3-kinase (PI3K) pathway, is important in
the proliferation and survival of bladder cancer cells (16). Oka et al (17) reported that elevated levels of Akt
protected bladder cancer cells from apoptosis. At present, drugs
designed to specifically target Akt are being developed for
clinical use to treat human bladder cancer. The Bcl-2 protein is
also a key regulator of apoptosis and its tumorigenic potential is
supported by the finding of overexpression of Bcl-2 in various
types of tumor (18). In bladder
cancer, the inhibition of Bcl-2 expression is able to reduce cell
growth and sensitize cells to subsequent chemotherapy (19). In recent years, an increasing
number of studies have focused on the regulation of Bcl-2
expression in tumor cells. As a result, the aim of the present
study was to evaluate whether miR-21 regulatinon of the Akt/Bcl-2
signaling pathway is involved in the resveratrol-induced apoptosis
of bladder cancer cells.
Materials and methods
Chemicals and reagents
Dulbecco’s modified Eagle’s medium (DMEM) and sodium
pyruvate were purchased from Gibco-BRL (Rockville, MD, USA). Fetal
bovine serum (FBS) was purchased from Gibco-BRL (Burlington, ON,
USA). Resveratrol and dimethylthiazol-2, 5-diphenyltetrazolium
bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Human recombinant insulin-like growth factor-1 (IGF-1) was
manufactured by R&D Systems (Minneapolis, MN, USA). The Annexin
V-enhanced green fluorescent protein (EGFP)/propidium iodide (PI)
apoptosis detection kit was obtained from KeyGen (Nanjing, China).
The caspase-3 activity assay kits were obtained from Beyotime
Institute of Biotechnology (Nantong, China). Rabbit polyclonal
antibodies against phosphorylated Akt, total Akt and Bcl-2 were
purchased from Cell Signaling Technology (Beverly, MA, USA).
Polyclonal antibodies against β-actin were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The Detergent
Compatible (DC) Protein Assay kit was purchased from Bio-Rad
Laboratories (Hercules, CA, USA). The miRNeasy Mini kit, the
miScript Reverse Transcription kit and the miScript SYBR-Green PCR
kit were purchased from Qiagen (Hilden, Germany).
Cell culture
The immortalized SV-HUC-1 normal human urothelial
cell line and T24 and 5637 bladder cancer cell lines were purchased
from the Cell Bank of Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China). These cells were cultured in DMEM
supplemented with 10% FBS, 10 mM HEPES, 100 units penicillin/ml and
10 μg streptomycin/ml at 37°C in a humidified atmosphere containing
95% air and 5% CO2. Cultured cells were treated with
resveratrol [dissolved in dimethylsulfoxide (DMSO)] in complete
medium. To obtain reliable results, the final concentration of DMSO
in the culture medium was maintained at <0.1%.
3-(4, 5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) assay
Cell viability was determined using MTT assays.
Briefly, the cells were seeded in 96-well dishes at
1×104 cells per well, and treated with different
concentrations of resveratrol (0, 10, 30 and 50 μmol/l) for 48 h.
Then each well was supplemented with 10 μl MTT and incubated for 4
h at 37°C. The medium was then removed and the resultant MTT
formazan was solubilized in 150 μl DMSO. The optical density was
measured at 490 nm using a microplate ELISA reader (Bio-Rad). The
experiment was repeated three times and each experiment had six
replicate wells.
Flow cytometric analysis
Flow cytometric analysis is based on the
translocation of phosphatidylserine from the inner leaflet of the
plasma membrane to the cell surface in early apoptotic cells
(15). Briefly, cells were
resuspended in a binding buffer. Next, Annexin V-EGFP and PI were
added and the solution was incubated at room temperature for 15 min
in the dark, followed by an assay on FACScan (Becton-Dickinson,
Franklin Lakes, NJ, USA). The percentage of apoptosis was computed
using Cell-Quest software (Becton-Dickinson).
Caspase-3 activity assays
Caspase-3 activity was analyzed using the caspase-3
activity assay kit according to the manufacturer’s instructions.
Cells were lysed and total cellular protein extracts were
quantified using a protein-assay kit. Next, an equal quantity of
total protein extract was incubated at 37°C overnight with
Ac-IETD-pNA for the caspase-3 assay. The release of pNA was
estimated by determining the absorbance at 405 nm on a microplate
ELISA reader (Bio-Rad). The relative activity of caspase-3 was
calculated as follows: Caspase-3 activity = (mean experimental
absorbance/mean control absorbance) × 100.
Quantitative PCR (qPCR) analysis of miRNA
expression
Mature miRNAs of cultured SV-HUC-1, T24 and 5637
cells were isolated utilizing the miRNeasy Mini kit and
reverse-transcribed with the miScript Reverse Transcription kit in
accordance with the manufacturer’s instructions. cDNA was subjected
to quantitative PCR using an miScript SYBR-Green PCR kit.
miRNA-specific quantitative PCR was performed using 3 ng cDNA per
reaction. The relative quantities of miRNAs were calculated by
calibration with U6 small nuclear RNA. All primers for miRNAs were
purchased from Qiagen. Analysis and fold change were determined
using the comparative threshold cycle (Ct) method. The change in
miRNA expression was calculated as the fold-change, i.e. relative
to the control.
Western blot analysis
T24 and 5637 cells were lysed with ice-cold lysis
buffer containing: 50 mmol/l Tris-HCl (pH 7.4) 1% NP-40, 150 mmol/l
NaCl, 1 mmol/l EDTA, 1 mmol/l phenylmethylsulfonyl fluoride and
complete proteinase inhibitor mixture (one tablet per 10 ml; Roche
Molecular Biochemicals, Indianapolis, IN, USA). The protein
concentration in the cell lysate was quantified using the DC
protein assay kit (Bio-Rad). Following protein content
determination using a DC Protein Assay kit, western blot analysis
was performed.
Transfection procedures
miR-21 was knocked down or overexpressed by
transfection with an miRNA inhibitor or an miRNA mimic. The miR-21
mimic (5′-AACAUCAGUCUGAUAAGCUAUU-3′), miR-21 inhibitor
(5′-UCAACAUCAGUCUGAUAAGCUA-3′) and negative control (NC;
5′-CAGUACUUUUGUGUAGUACAA-3′) were synthesized by Ribobio (Ribobio
Co., Ltd, Guangzhou, Guangdong, China). All the oligonucleotides
were transfected at a final concentration of 100 nM. T24 and 5637
cells were transfected with the miR-21 inhibitor or mimic using
siPort Neo-FX (Ambion, Austin, TX, USA) according to the
manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed with statistical
analysis software SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
Statistical analyses were performed using either an analysis of
variance or Student’s t-test. Data are expressed as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Resveratrol induces cytotoxicity and
apoptosis in bladder cancer cells
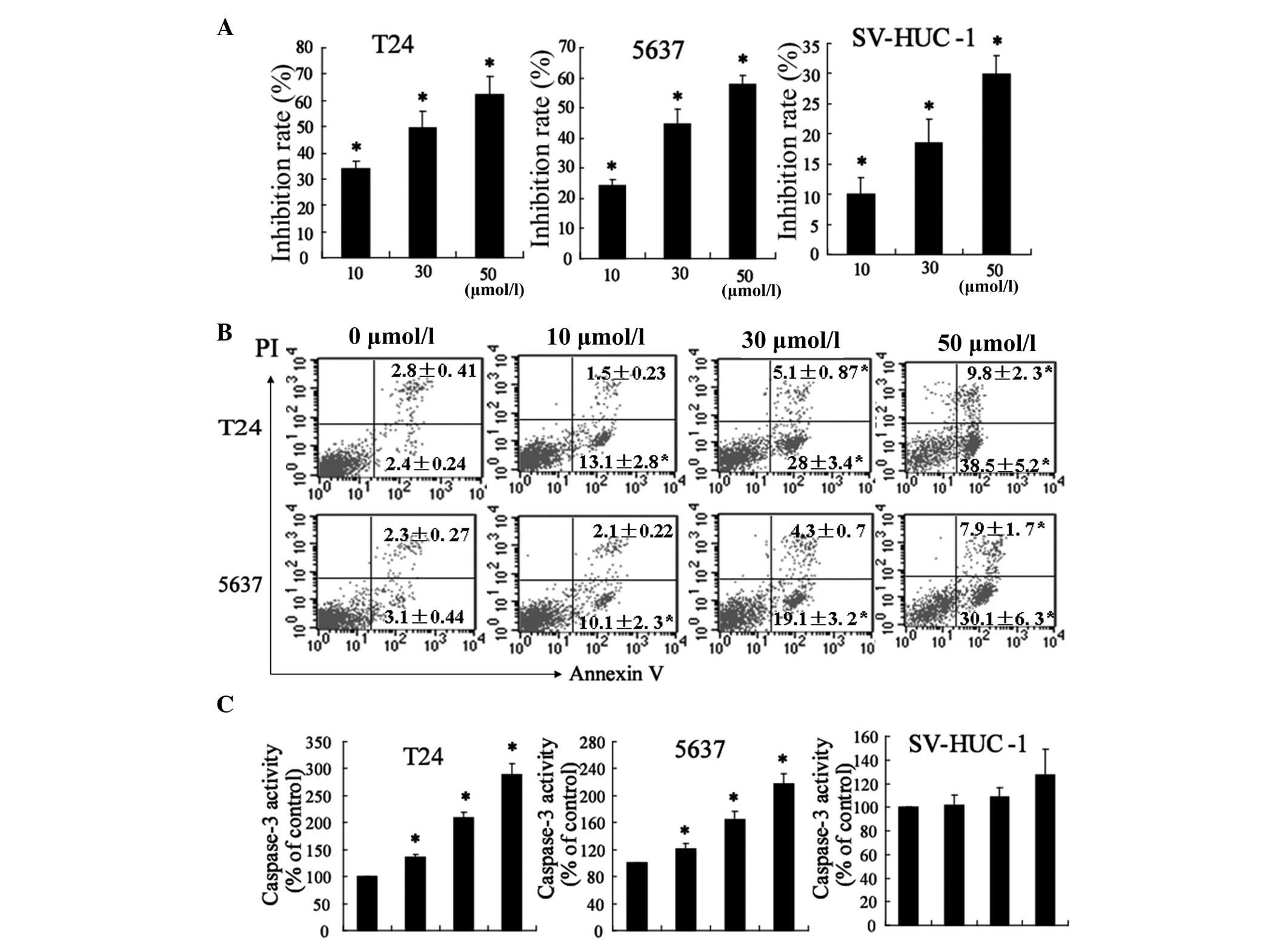
The inhibitory effects of resveratrol on the growth
of bladder cancer cells and normal human urothelial cells were
evaluated using MTT assays. Resveratrol treatment inhibited the
growth of T24 and 5637 cells in a dose-dependent manner (Fig. 1A). For instance, when T24 cells
were treated with 10, 30 and 50 μmol/l resveratrol for 48 h, the
inhibitory rate of cell viability was 34.0, 49.4 and 62.3%,
respectively, and the IC50 value 48 h after treatment
with resveratrol was 26.75 μmol/l in T24 cells. Similar results
were obtained in 5637 cells following treatment with different
concentrations of resveratrol and the IC50 value
following treatment with resveratrol was 35.58 μmol/l for 48 h.
Normal human urothelial cells demonstrated greater resistance to
the cytotoxic effect of resveratrol. In addition, the
IC50 value at 48 h was 276 μmol/l for SV-HUC-1
cells.
Flow cytometric analysis demonstrated that the
number of apoptotic T24 and 5637 cells significantly increased
following treatment with resveratrol (Fig. 1B). The effects of resveratrol on
the apoptosis of T24 and 5637 cells were dose-dependent
(P<0.05). Activation of caspases is important in the execution
of apoptosis (20). In order to
determine whether resveratrol was able to alter the activity of
caspases in bladder cancer cells, caspase-3 activity was assessed.
As shown in Fig. 1C, resveratrol
significantly increased caspase-3 activity in T24 and 5637 cells;
however, had no effect on the activity in SV-HUC-1 cells.
Resveratrol inhibits miR-21
expression
To further identify the mechanism for resveratrol on
the cytotoxicity and apoptosis of bladder cancer cells, qPCR was
performed to detect miR-21 expression following treatment with
resveratrol for 12 h. As shown in Fig.
2, resveratrol treatment significantly decreased the expression
of miR-21 in a dose-dependent manner in the two cell lines
(P<0.05).
Resveratrol decreases phosphorylation of
Akt and the level of Bcl-2 protein
It has been reported that resveratrol is able to
inhibit Akt activation in bladder cancer cells (7). Additionally, the effect of
resveratrol on the PI3K/Akt pathway was examined by measuring the
levels of phospho-Akt and total Akt. According to western blot
analysis, the treatment of T24 and 5637 cells with resveratrol led
to a dose-dependent decrease in the expression of phospho-Akt,
while total Akt protein levels remained constant (Fig. 3A). Furthermore, resveratrol
decreased Bcl-2 protein expression as shown in Fig. 3B.
Downregulation of miR-21 expression
decreases phosphorylation of Akt and Bcl-2 expression
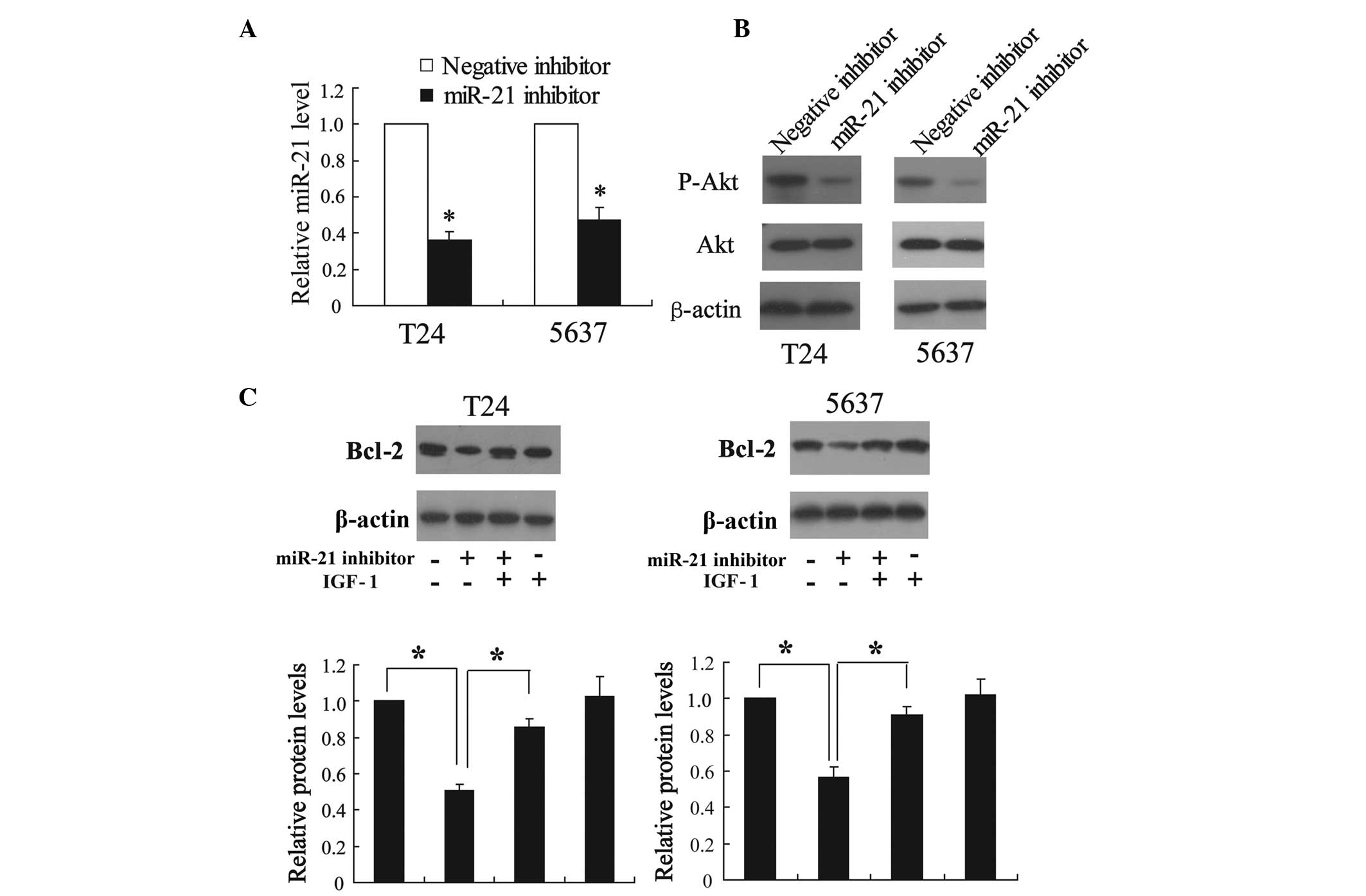
To determine whether miR-21 is able to regulate Akt
activity and Bcl-2 expression in T24 and 5637 cells, the modulation
of the levels of phosphorylation of Akt and Bcl-2 protein
expression in cells transfected with the miR-21 inhibitor was
investigated. The results from qPCR revealed that the miR-21
inhibitor is able to significantly decrease the expression of
miR-21 in T24 and 5637 cells (P<0.01; Fig. 4A), suggesting that the miR-21
inhibitor is efficiently introduced into the cells and acts to
knock down miR-21 expression. Furthermore, the inhibition of miR-21
expression decreased the expression of phospho-Akt as shown in
Fig. 4B. The Bcl-2 protein
expression was also reduced by the miR-21 inhibitor, which was able
to be reversed by IGF-1, a strong stimulator of Akt in bladder
cancer cells (Fig. 4C) (21).
Downregulation of miR-21 expression
increases apoptosis of bladder cancer cells
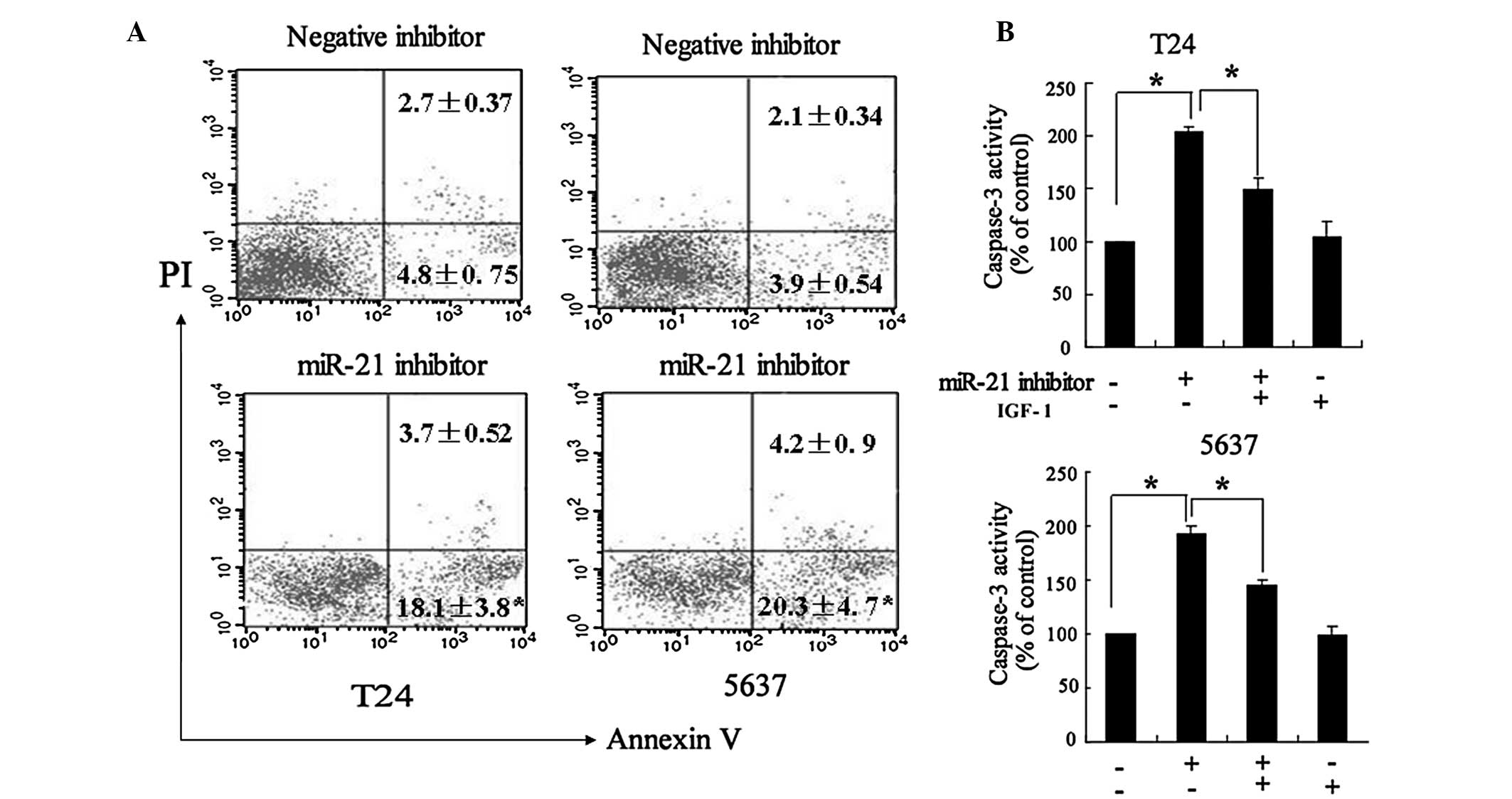
Flow cytometric analysis demonstrated that the
number of apoptotic cells significantly increased following
transfection of the miR-21 inhibitor (Fig. 5A). In addition, the miR-21
inhibitor significantly increased caspase-3 activity in T24 and
5637 cells, which was able to be reversed by IGF-1 (Fig. 5B).
Overexpression of miR-21 is able to
reverse the effects of resveratrol on bladder cancer cells
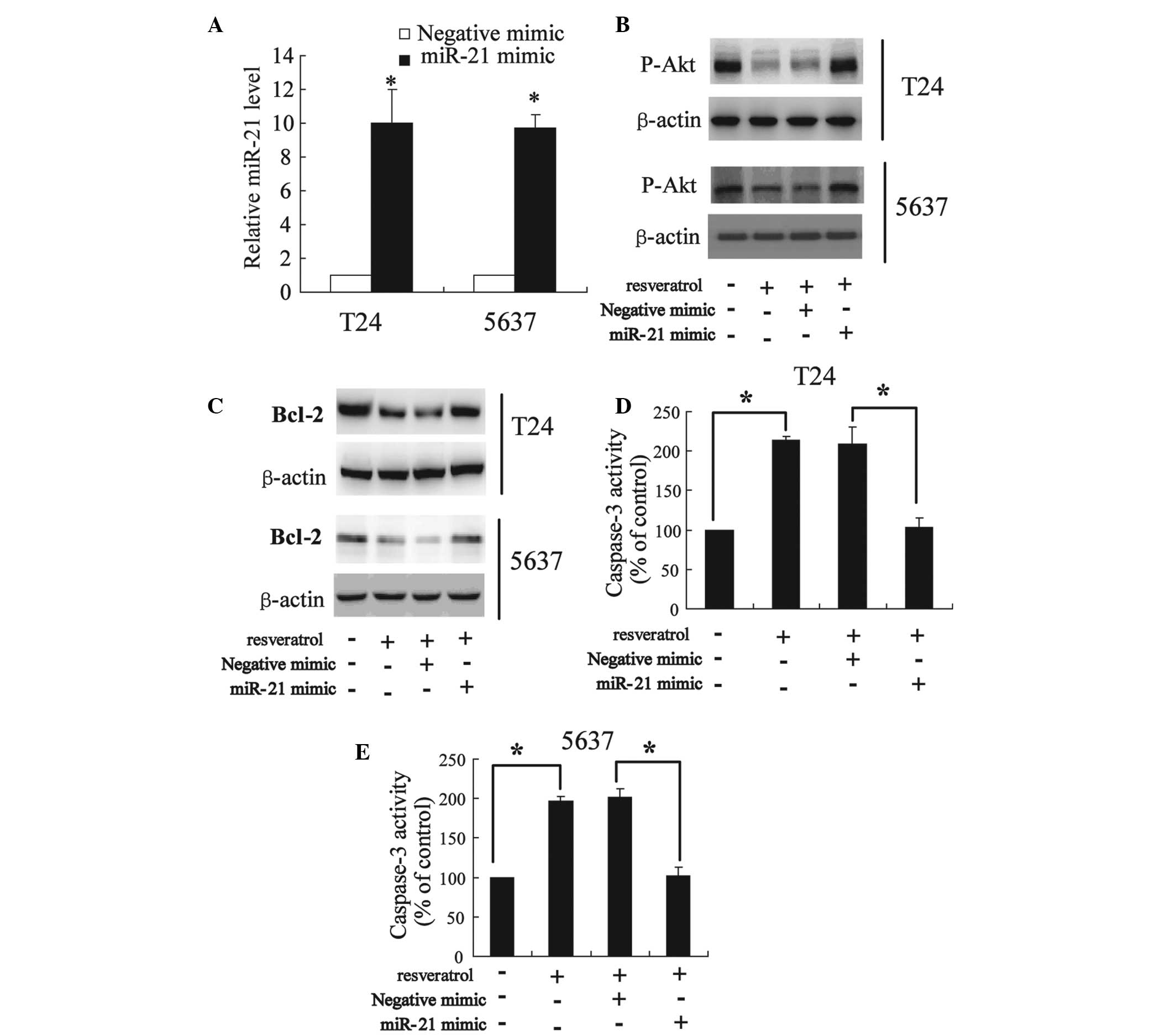
In order to evaluate the role of miR-21 on the
effect of resveratrol in bladder cancer cells, the cells were
treated with resveratrol following transfection with the miR-21
mimic. As shown in Fig. 6A, the
miR-21 mimic is able to significantly increase the expression of
miR-21 in T24 and 5637 cells. Notably, the present study revealed
that the overexpression of miR-21 was able to restore the decrease
of phospho-Akt and Bcl-2 expression induced by resveratrol
(Fig. 6B and C). In addition,
caspase-3 activity significantly increased following treatment with
50 μmol/l resveratrol, which was able to be reversed by the miR-21
mimic (Fig. 6D and E).
Discussion
It has been reported that resveratrol has a strong
inductive effect on the apoptosis of bladder cancer cells through
the intrinsic mitochondrial-dependent pathway (6). The present study, aimed to further
examine the molecular mechanisms of apoptosis induced by
resveratrol in T24 and 5637 cells. Firstly, the effect of
resveratrol supplementation on cytotoxicity and apoptosis in
bladder cancer cells was evaluated and it was revealed that
resveratrol significantly inhibited proliferation and induced
apoptosis of these cells in a dose-dependent manner. Furthermore,
the present study revealed that resveratrol was able to reduce
miR-21 expression in T24 and 5637 cells and decrease Akt
phosphorylation and Bcl-2 protein expression. Notably,
overexpression of miR-21 was able to reverse the effect of
resveratrol on bladder cancer cells.
Resveratrol has been demonstrated to be effective as
a potential cancer chemoprevention agent against numerous types of
tumor (22). As a natural product,
resveratrol possesses anti-tumor activity with a low toxicity in
normal cells (23). The results of
the present study demonstrated that the IC50 value 48 h
after treatment was 26.75 μmol/l for T24 cells, 35.58 μmol/l for
5637 cells but 276 μmol/l for SV-HUC-1 cells. These results
indicated that resveratrol was able to specifically kill bladder
cancer cells without exhibiting a cytotoxic effect on the normal
cells.
To further clarify the mechanisms involved in the
cytotoxicity and apoptosis of bladder cancer cells, the effect of
resveratrol on the expression of miR-21 was investigated. Previous
studies indicated that miR-21 was significantly upregulated in
advanced bladder cancer tissues and the overexpression of miR-21
promoted the proliferation of bladder cancer cell lines (15). The present study revealed that
resveratrol is able to inhibit the expression of miR-21 in T24 and
5637 cells, which indicated that miR-21 may be involved in
resveratrol-induced apoptosis. The hypothesis that overexpression
of miR-21 is able to reverse the effects of resveratrol on
cytotoxicity and apoptosis was confirmed.
Akt is activated and regulates the process of
proliferation and survival in bladder cancer cells (24). Inhibition of Akt activity is
considered to be an effective strategy for bladder cancer treatment
(25). Bcl-2 is also a direct
participant in the apoptotic pathway and has tumorigenic potential
(18). The present study
demonstrated that resveratrol treatment resulted in dose-dependent
inhibition of the increase in the levels of Akt phosphorylation and
Bcl-2 protein expression, which was restored by downregulation of
miR-21 expression. Our data also supported that miR-21 was
important in the regulation of Akt activity and Bcl-2 expression in
T24 and 5637 cells. The downregulation of miR-21 expression
markedly decreased the phosphorylation of Akt and Bcl-2. The
inhibition of Bcl-2 expression was significantly counteracted by
treatment with an Akt stimulator of IGF-1. These results suggested
that resveratrol may induce apoptosis by the regulation of the
Akt/Bcl-2 signaling pathway by miR-21.
In conclusion, the present study established miR-21
as a novel target of resveratrol for mediating the cytotoxicity and
apoptosis of bladder cancer cells. The data support the hypothesis
that resveratrol inhibits miR-21 expression, leading to the
reduction of Akt activity, which results in a decrease in Bcl-2
expression. These data indicate that resveratrol may be a potent
agent for the treatment of human bladder cancer.
References
|
1
|
Kirkali Z, Chan T, Manoharan M, Algaba F,
et al: Bladder cancer: epidemiology, staging and grading, and
diagnosis. Urology. 66:4–34. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lei AQ, Cheng L and Pan CX: Current
treatment of metastatic bladder cancer and future directions.
Expert Rev Anticancer Ther. 11:1851–1862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borriello A, Cucciolla V, Della Raqione F
and Galletti P: Dietary polyphenols: Focus on resveratrol, a
promising agent in the prevention of cardiovascular diseases and
control of glucose homeostasis. Nutr Metab Cardiovasc Dis.
20:618–625. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Albani D, Polito L and Forloni G: Sirtuins
as novel targets for Alzheimer’s disease and other
neurodegenerative disorders: experimental and genetic evidence. J
Alzheimers Dis. 19:11–26. 2010.
|
|
5
|
Harikumar KB and Aggarwal BB: Resveratrol:
a multitargeted agent for age-associated chronic diseases. Cell
Cycle. 7:1020–1035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin X, Wu G, Huo WQ, Zhang Y and Jin FS:
Resveratrol induces apoptosis associated with mitochondrial
dysfunction in bladder carcinoma cells. Int J Urol. 19:757–764.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bai Y, Mao QQ, Qin J, Zheng XY, Wang YB,
Yang K, Shen HF and Xie LP: Resveratrol induces apoptosis and cell
cycle arrest of human T24 bladder cancer cells in vitro and
inhibits tumor growth in vivo. Cancer Sci. 101:488–493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Winter J, Jung S, Keller S, Gregory RI and
Diederich S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Si ML, Zhu S, Wu H, Lu Z, Wu F and Mo YY:
miR-21-mediated tumor growth. Oncogene. 26:2799–2803. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Link A, Becker V, Goel A, Wex T and
Malfertheiner P: Feasibility of fecal microRNAs as novel biomarkers
for pancreatic cancer. PLoS One. 7:e429332012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Buscaglia LE and Li Y: Apoptosis and the
target genes of microRNA-21. Chin J Cancer. 30:371–380. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tili E, Michaille JJ, Alder H, Volinia S,
Delmas D, Latruffe N and Croce CM: Resveratrol modulates the levels
of microRNAs targeting genes encoding tumor-suppressors and
effectors of TGFβ signaling pathway in SW480 cells. Biochem
Pharmacol. 80:2057–2065. 2010.PubMed/NCBI
|
|
14
|
Liu P, Liang H, Xia Q, Li P, Kong H, Lei
P, Wang S and Tu Z: Resveratrol induces apoptosis of pancreatic
cancers cells by inhibiting miR-21 regulation of BCL-2 expression.
Clin Transl Oncol. 15:741–746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tao J, Lu Q, Wu D, Li P, Xu B, Qing W,
Wang M, Zhang Z and Zhang W: microRNA-21 modulates cell
proliferation and sensitivity to doxorubicin in bladder cancer
cells. Oncol Rep. 25:1721–1729. 2011.PubMed/NCBI
|
|
16
|
Ching CB and Hansel DE: Expanding
therapeutic targets in bladder cancer: the PI3K/Akt/mTOR pathway.
Lab Invest. 90:1406–1414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oka N, Tanimoto S, Taue R, Nakatsuji H,
Kishimoto T, Izaki H, Fukumori T, Takahashi M, Nishitani M and
Kanayama HO: Role of phosphatidylinositol-3 kinase/Akt pathway in
bladder cancer cell apoptosis induced by tumor necrosis
factor-related apoptosis-inducing ligand. Cancer Sci. 97:1093–1098.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pietenpol JA, Paradopoulos N, Markowitz S,
Willson JK, Kinzler KW and Vogelstein B: Paradoxical inhibition of
solid tumor cell growth by bcl2. Cancer Res. 54:3714–3717.
1994.PubMed/NCBI
|
|
19
|
Kunze D, Wuttig D, Fuessel S, Kraemer K,
Kotzsch M, Meye A, Grimm MO, Hakenberg OW and Wirth MP: Multitarget
siRNA inhibition of antiapoptotic genes (XIAP, BCL2, BCL-XL) in
bladder cancer cells. Anticancer Res. 28:2259–2263. 2008.PubMed/NCBI
|
|
20
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jung JI, Cho HJ, Kim J, Kwon DY and Park
JH: trans-10, cis-12 conjugated linoleic acid inhibits insulin-like
growth factor-I receptor signaling in TSU-Pr1 human bladder cancer
cells. J Med Food. 13:13–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, Moon RC, et al: Cancer chemopreventive activity of resveratrol,
a natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: the in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang YB, Qin J, Zheng XY, Bai Y, Yang K
and Xie LP: Diallyl trisulfide induces Bcl-2 and
caspase-3-dependent apoptosis via downregulation of Akt
phosphorylation in human T24 bladder cancer cells. Phytomedicine.
17:363–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shahjee HM, Koch KR, Guo L, Zhang CO and
Keay SK: Antiproliferative factor decreases Akt phosphorylation and
alters gene expression via CKAP4 in T24 bladder carcinoma cells. J
Exp Clin Cancer Res. 29:1602010. View Article : Google Scholar : PubMed/NCBI
|