Introduction
Immunoglobulin A nephropathy (IgAN) is a renal
disorder, which is characterized by symptomatic and pathological
changes caused by diffuse deposition of IgA or IgA-containing
immune complexes in the glomerular mesangial area and capillary
loops. IgAN is increasingly common in males than in females and has
been observed in a high percentage of renal biopsies in Asia. Bi
(1) and Li (2) identified that IgAN accounts for
26–34% of all primary glomerular diseases in China and is one of
the main causes of end stage renal disease (ESRD).
Kidney transplantation is considered the optimal
therapeutic strategy for ESRD. Berger et al (3) were the first to report recurrent IgAN
in kidney allograft recipients and recurrent IgA deposition in the
mesangial area of kidney allograft recipients. Additionally, they
found that recurrent IgAN in kidney allograft recipients had a
relatively benign clinical course. Earlier studies have shown that
recurrent IgAN does not have a negative impact on the clinical
course of kidney transplantation (4). However, studies published in the
1990’s that employed longer follow-up periods reported that
recurrent IgAN was a major cause of graft loss (5,6). In
particular, recurrent IgAN was observed in 20–60% of patients
following transplantation and certain transplant recipients
experienced de novo IgAN (7–11).
At present, the prognostic factors associated with
recurrent IgAN in kidney allograft recipients in China remain to be
fully elucidated. Although there have been numerous Western studies
of the incidence rate and risk factors of recurrent IgAN in kidney
allograft recipients, there are few studies focusing on the
prognostic factors of recurrent IgAN following kidney
transplantation (12,13). In the present study, the prognostic
factors associated with recurrent IgAN in kidney allograft
recipients from a single institution in China were
investigated.
Materials and methods
Clinical data
Patients undergoing renal graft biopsy (n=197)who
were hospitalized at the Organ Transplant Center of the Fuzhou
General Hospital (Fuzhou, China) between June, 2004 and December,
2010 were screened. A total of 202 kidney allograft biopsy
specimens were obtained. Forty-two patients (20.8%, including 31
males and 11 females) met the diagnostic criteria for IgAN
following clinical and pathological examinations. Thirty-eight
cases received one transplantation and four cases received two
transplantations. There were 41 cadaveric donor kidney transplants
and 1 live-donor kidney transplant. Follow-up was every three
months and the final follow-up occured on August 31, 2012. The
study was approved by the Ethics Committee of the Organ Transplant
Institute, Fuzhou General Hospital, Fuzhou, China (Review board
IRB00006161) and written informed consent was obtained from the
patients.
The 42 patients who met the diagnostic criteria for
IgAN were divided into two groups, the graft loss group (n=17) and
the functional graft group (n=25). The graft loss group included
patients with graft dysfunction, those who required resumption of
dialysis or a second transplant. Pathological diagnosis was based
on conventional hematoxylin and eosin, periodic acid-schiff, Masson
and immunohistochemical staining for IgG, IgM, IgA, C3d, C1q and
C4d. The pathological changes of kidney grafts were observed by
light microscopy (Leica Microsystems, Ltd., Hong Kong, China). The
Banff 97 score of allograft nephropathy was calculated as described
previously (14).
Clinical and pathological
characteristics
The following demographic and clinical data were
recorded: Age, gender, date of transplantation, requirement for
repeat transplantation, cadaveric or live donor transplantation,
date of IgAN onset, renal graft biopsy and graft dysfunction,
height, weight, blood pressure, pulse, urine output at the time of
biopsy, edema, gross hematuria and immunosuppressant regimen and
dose. The following clinical indicators were measured 1 week prior
to renal graft biopsy: Routine urine test, 24-h urinary protein
levels for patients with proteinuria, serum creatinine (SCr), blood
urea nitrogen (BUN), uric acid (UA), glomerular filtration rate,
hemoglobin (Hb), triglycerides (TG), total cholesterol (CHO), serum
total protein (TPR) and serum albumin (Alb) levels. The following
pathological characteristics were also recorded: Renal allograft
nephropathy score, glomerular sclerosis, crescent formation,
fibrinoid necrosis, tubular atrophy, interstitial fibrosis and
arteriolopathy.
Statistical analysis
All data were recorded in a database. Quantitative
data were compared using Student’s t-test. If the data had
non-normal distribution and/or heterogeneity of variance, a rank
sum test was performed. Qualitative data were compared using the
χ2 test. Renal graft survival rate was calculated using
the Kaplan-Meier method, and differences in survival were examined
with the log-rank test. All data were analyzed using SPSS software,
version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
General characteristics of patients
Between June, 2004 and December, 2010, 197 patients
underwent renal graft biopsy at the Organ Transplant Center, Fuzhou
General Hospital and 202 kidney allograft biopsy specimens were
obtained. Forty-two patients (20.8%; including 31 males and 11
females) met the diagnostic criteria for IgAN. Thirty-eight
patients underwent transplantation once and four patients underwent
transplantation twice. Forty-one cases received cadaveric donor
kidneys and one case received a kidney from a living donor. The
examination results indicated the presence of concomitant
proteinuria and hematuria in 25 patients (59.5%) and proteinuria,
but no hematuria in six patients (14.3%). The concentration of
urinary protein in these 31 patients indicated a mean of 3.6±2.1
g/24 h (range, 0.312–8.13 g/24 h). Analysis of all 42 biopsy
specimens indicated the presence of 17.2±13.0 glomeruli. All
specimens met the Banff 97 criteria for renal biopsy (14).
The graft loss and functional graft groups showed no
statistically significant differences in gender, age, human
leukocyte antigen (HLA) matching, immunosuppressant regimen and
dose, blood pressure and urine output at the time of biopsy
(Table I).
 | Table IDemographic and clinical
characteristics of enrolled patients with IgAN. |
Table I
Demographic and clinical
characteristics of enrolled patients with IgAN.
| Characteristics | Graft loss
(n=17) | Functional graft
(n=25) | P-value |
|---|
| Gender | | | |
| Males | 12 | 19 | |
| Females | 5 | 6 | 0.733 |
| Mean age (years) | 36.1±9.2 | 38.1±4.3 | 0.823 |
| HLA mismatches | 2.29±0.69 | 2.36±0.95 | 0.881 |
| HLA-A | 0.82±0.64 | 0.8±0.82 | 0.804 |
| HLA-B | 0.71±0.69 | 0.76±0.66 | 0.777 |
| HLA-DR | 0.76±0.66 | 0.8±0.82 | 0.989 |
| Immunosuppressive
drugs at time of diagnosis | | | |
| Prednisolone
(mg/day) | 8.6±2.0 | 9.1±2.9 | 0.603 |
| n (%) | 17 (100) | 25 (100) | 1 |
| Ciclosporin
(mg/day) | 225.0±35.4 | 133.3±28.9 | 0.076 |
| n (%) | 4 (23.5) | 9 (36.0) | 0.391 |
| Tacrolimus
(mg/day) | 2.2±1.1 | 2.0±1.0 | 0.606 |
| n (%) | 13 (76.5) | 16 (64.0) | 0.296 |
| MMF (mg/day) | 892.9±349.3 | 863.6±171.9 | 0.961 |
| n (%) | 14 (82.3) | 20 (80.0) | 0.758 |
| Bredinin
(mg/day) | 87.5±17.7 | 75±15.6 | 0.317 |
| n (%) | 2 (11.8) | 2 (8.0) | 0.758 |
| Azathioprine
(mg/day) | 50 | 50 | 1.00 |
| n (%) | 1 (5.9) | 3 (12.0) | 0.758 |
| Blood pressure
(mmHg) | | | |
| Systolic | 136.4±19.4 | 135.8±18.5 | 0.926 |
| Diastolic | 86.4±12.4 | 85.6±12.0 | 0.833 |
| Urine volume (ml/24
h) | 1872.7±392.7 | 1742.5±595.0 | 0.636 |
Analysis of clinical indicators
Analysis of the clinical data of the two groups
indicated that patients in the graft loss group were more likely to
exhibit proteinuria (P=0.014), higher SCr levels at the time of
biopsy (P=0.009), lower epidermal growth factor receptor levels
(P=0.013), lower TPR (P=0.01) and lower serum Alb levels (P=0.043)
(Table II). However, the two
groups showed no significant differences in hematuria, BUN, UA, Hb,
TG and CHO levels (Table II).
 | Table IIClinical indicators of patients in the
graft loss and functional graft groups. |
Table II
Clinical indicators of patients in the
graft loss and functional graft groups.
| Indicators | Graft loss
(n=17) | Functional graft
(n=25) | P-value |
|---|
| Proteinuria, n
(%) | 16 (94.1) | 15 (60) | 0.014a |
| Hematuria, n (%) | 13 (76.5) | 16 (64) | 0.391 |
| Proteinuria (g/24
h) | 4.1±2.4 | 2.6±1.7 | 0.047a |
| SCr (μmol/l) | 373.6±267.5 | 220.9±164.9 | 0.009a |
| BUN (mmol/l) | 16.9±9.5 | 13.5±10.7 | 0.081 |
| UA (mmol/l) | 489.9±157.8 | 480.7±133.8 | 0.769 |
| eGFR (ml/min) | 29.3±18.2 | 51.9±32.1 | 0.013a |
| Hb (g/l) | 97.8±24.8 | 107.9±21.8 | 0.173 |
| TG (mmol/l) | 1.5±1.0 | 1.5±0.7 | 0.397 |
| CHO (mmol/l) | 5.8±3.5 | 6.1±2.1 | 0.182 |
| TPR (g/l) | 57.9±7.5 | 66.5±10.9 | 0.01a |
| Alb (g/l) | 34.3±5.1 | 38.7±8.2 | 0.043a |
Analysis of the pathological
characteristics
Analysis of the pathological characteristics of the
two groups indicated that patients in the graft loss group were
more likely to have a high Banff score (P=0.001), severe glomerular
sclerosis (P=0.002), increased crescent formation (P=0.01), greater
tubular atrophy (P=0.013) and interstitial fibrosis (P=0.033)
compared with those of the functional graft group (Table III).
 | Table IIIPathological characteristics of
patients in the graft loss and functional graft groups. |
Table III
Pathological characteristics of
patients in the graft loss and functional graft groups.
|
Characteristics | Graft loss
(n=17) | Functional graft
(n=25) | P-value |
|---|
| Banff score
(0–30) | 15.6±6.0 | 8.8±4.6 | 0.001a |
| Concomitant CAN, n
(%) | 4 (23.5) | 8 (32.0) | 0.551 |
| Concomitant AR, n
(%) | 1 (5.9) | 2 (8.0) | 0.794 |
| Calcineurin
inhibitor toxicity (%) | 0 (0.0) | 1 (4.0) | 0.404 |
| Glomerular
sclerosis n (%) | | | 0.002a |
| 0% | 2 (11.8) | 7 (28.0) | |
| 1–25% | 3 (17.7) | 14 (56.0) | |
| >25% | 12 (70.6) | 4 (16.0) | |
| Crescent | 7 (41.8) | 2 (8.0) | 0.01a |
| Tubular
atrophy | | | 0.013a |
| 0% | 0 (0) | 3 (12.0) | |
| 1–25% | 2 (11.8) | 11 (44.0) | |
| >25% | 12 (70.6) | 11 (44.0) | |
| Interstitial
fibrosis | | | 0.033a |
| 0–5% | 0 (0) | 4 (16.0) | |
| 6–25% | 4 (23.5) | 12 (48.0) | |
| >25% | 13 (76.5) | 10 (40.0) | |
| Vascular |
| Normal | 1 | 7 | |
| Hyalinosis | 1 | 18 | 0.073 |
Analysis of renal graft survival
During the same period (between June, 2004 and
December, 2010), in 88 kidney allograft recipients who underwent
renal graft biopsy at Fuzhou General Hospital, pathological
examination confirmed the presence of other glomerular diseases,
including acute diffuse proliferative glomerulonephritis, rapidly
progressive glomerulonephritis, membranous glomerulonephritis,
minimal change glomerulonephritis, focal segmental
glomerulosclerosis, membranoproliferative glomerulonephritis,
mesangial proliferative glomerulonephritis and chronic
glomerulonephritis, based on the diagnostic criteria previously
described (15). In addition, 36
of these patients demonstrated glomerular lesions, including renal
interstitial lesions free of glomerular lesions, tubular lesions,
arteriolopathy, acute and chronic rejection, and immunosuppressant
toxicity.
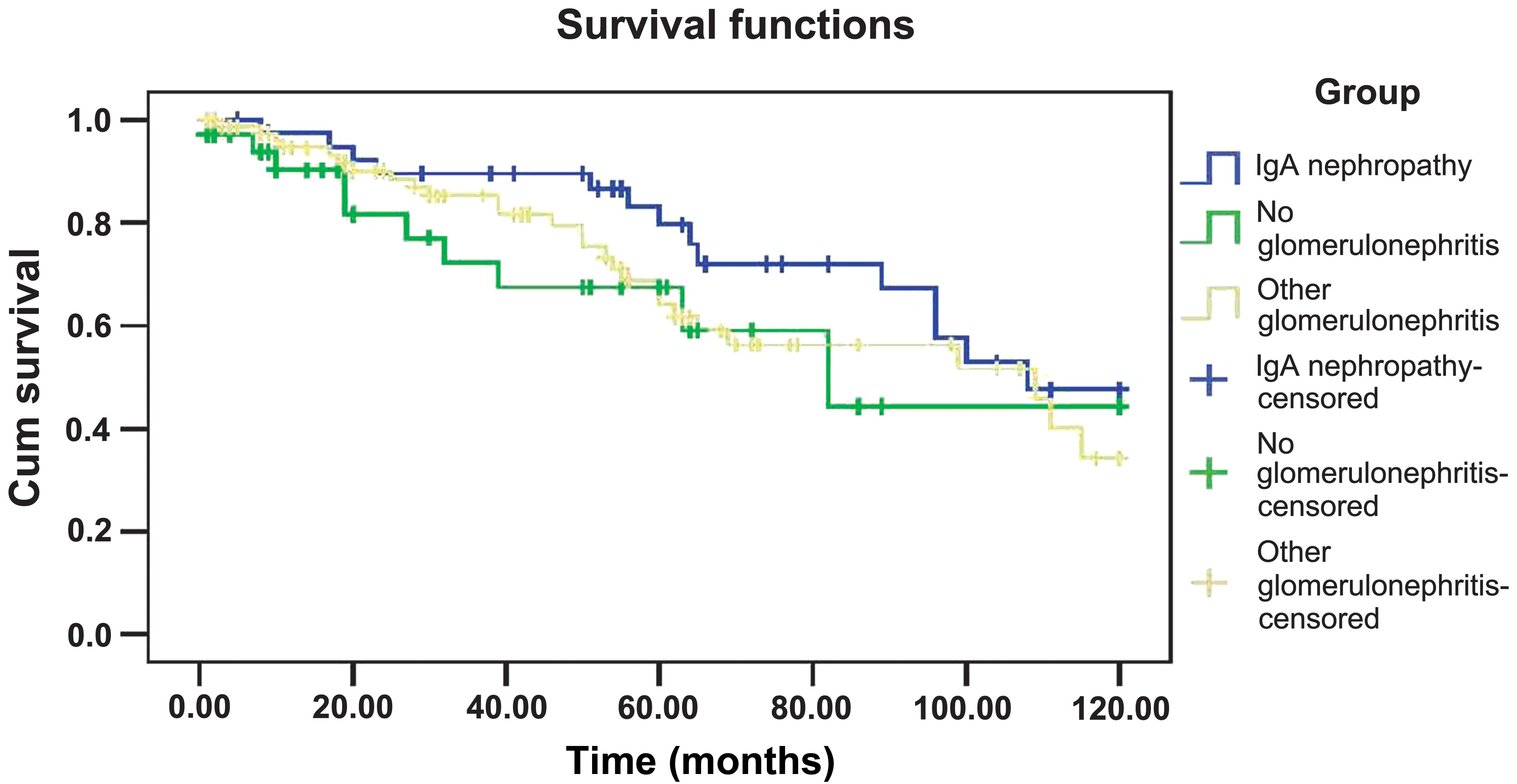
The cumulative graft survival rates of the three
groups (patients with IgAN and patients with or without other
glomerular diseases) over 10 years are shown in Fig. 1. The renal graft survival rate was
calculated using the Kaplan-Meier method and identified no
significant differences in the graft survival rates of the three
groups over 10 years (P=0.122).
Discussion
The presence of IgAN in kidney allograft recipients
may be due to recurrence of IgAN, de novo IgAN or
transmission of IgA deposits from the donor kidney. The majority of
patients with kidney disease also presented with ESRD at medical
examination and such patients are not suitable for biopsy. Thus,
histopathological diagnosis of the primary disease is not available
for the majority of kidney allograft recipients. In addition,
time-zero biopsy data of donor grafts are generally not available.
This renders it difficult or even impossible to differentiate
recurrent IgAN, de novo IgAN and donor-transmitted IgAN.
However, long term clinical observations at Fuzhou General Hospital
indicated that the majority of donor kidneys with IgA deposition at
the baseline biopsy did not result in IgA deposition during
postoperative allograft biopsy. In addition, Sanfilippo et
al (16) showed that mesangial
IgA deposition can quickly dissipate following transplantation.
Thus, it was hypothesized that the majority of patients in the
present study had recurrent IgAN or de novo IgAN.
This study screened 197 hospitalized patients who
underwent renal graft biopsy at the Organ Transplant Center of the
Fuzhou General Hospital (Fuzhou, China) between June, 2004 and
December, 2010. Of these, 42 patients (20.8%) met the diagnostic
criteria for IgAN, indicating that IgAN was common among the kidney
allograft recipients. Previous studies did not identify any
clinical abnormalities in approximately one-third of patients with
confirmed IgAN (5,17,18);
therefore, it was suggested that there may have been increased
cases of IgAN if renal allograft biopsies were performed for all
kidney allograft recipients.
Abnormal routine urine test results were common in
the renal allograft recipients with IgAN (83.3%), and microscopic
examination indicated concomitant proteinuria and hematuria in 25
patients (59.5%). Statistical analysis indicated that proteinuria,
but not hematuria, was significantly associated with graft loss.
The 24-h urinary protein level was also significantly greater in
the graft loss group than that in the functional graft group.
Moreover, the results indicated that serum TRP and Alb levels were
significantly lower in the graft loss group compared with those in
the functional graft group. Collectively, these results suggested
that the substantial loss of protein in the urine is associated
with poor graft outcome.
The results also indicated that higher SCr levels
were significantly associated with graft loss. In particular, the
mean SCr level was 373.6±267.5 μmol/l in the graft loss group and
220.9±164.9 μmol/l in the functional graft group at the time of
biopsy. BUN and UA levels were not correlated with renal graft
prognosis. Concurrent with the results of the present study, Wang
et al (19) demonstrated
that patients with graft loss exhibited higher SCr levels at the
time of renal graft biopsy and Kiattisunthorn et al
(13) suggested that higher SCr
and urinary protein levels were associated with poor graft
function.
The present study did not identify a significant
correlation between HLA matching and the prognosis of kidney
allografts in patients with IgAN. However, previous studies have
shown that females and patients with positive HLA matching were
more prone to recurrent IgAN and had a lower 5-year graft survival
rate (20,21). In addition, Andresdottir et
al (22,23) investigated the graft survival of
1,207 patients with IgAN and 7,935 patients with no glomerular
disease, and reported that the presence of the HLA-B8 and DR3
haplotype increased the risk of graft loss.
Namba et al (9) found that progressive graft loss in
patients with recurrent IgAN was associated with the deterioration
of renal graft function, hypertension and angiotensin converting
enzyme inhibitor deficiency at the time of biopsy. However, the
present study demonstrated no significant correlation between graft
loss and hypertension, hypotension or pulse rate at the time of
biopsy. The results also indicated that the levels of serum TG and
CHO were not significantly associated with graft loss.
Increased crescent formation suggested a poor
prognosis of the renal allograft. In the present study, a
significant increase in the number of patients with crescent
formation was observed in the graft loss group (n=7; 41.8%)
compared with the functional graft group (n=2; 8.0%). Tang et
al (24) proposed that IgAN
with crescent formation is increasingly common in patients who
received renal grafts or were diagnosed with primary
glomerulonephritis in China. Such patients developed rapid
progression of renal insufficiency, progressed to kidney
dysfunction and ultimately required hemodialysis. Kowalewska et
al (25) found that early
renal dysfunction or resumption of dialysis occurred in
approximately half of renal allograft recipients with IgAN who
presented with crescent formation. Soler et al (21) showed that the 5-year graft survival
rate was 71% in patients with crescentic glomerulonephritis who
were diagnosed with IgA or Henoch-Schonlein purpura, but was 100%
in patients with glomerular mesangial hyperplasia or focal
segmental glomerulosclerosis.
Oka et al (26) showed that the pathological
characteristics of IgAN in renal graft recipients were different
from those in patients with IgAN who had not undergone
transplantation. In particular, graft recipients with IgAN seldom
displayed active damage, such as extracapillary proliferation, but
often exhibited chronic damage, such as glomerulosclerosis and
interstitial fibrosis. It was speculated that this difference was
associated with the use of immunosuppressive agents. In the present
study, the incidence of glomerulosclerosis, tubular atrophy,
interstitial fibrosis and arteriosclerosis were compared in the
graft loss and functional graft groups. The results showed that
glomerulosclerosis, tubular atrophy and interstitial fibrosis were
significantly more common in the graft loss group than in the
functional graft group. However, the rate of arteriosclerosis was
similar in the two groups.
Furthermore, the results of the current study showed
no significant difference between the renal graft survival rate in
patients with IgAN and patients with or without other glomerular
diseases. It was suggested that these results may be explained by
the similar disease severity of the three groups. Concurrent with
these results, Kiattisunthorn et al (13) found no significant difference in
renal graft survival among these three groups. However, the large
population study of Andresdottir et al (21) demonstrated a greater 1-year graft
survival rate in renal graft recipients with IgAN than in those
without glomerular diseases. They speculated that the results may
be due to the better immune status of patients with IgAN. However,
the results also indicated that the graft survival rate tended to
decrease more rapidly in renal graft recipients with IgAN than in
those without IgAN.
In conclusion, renal transplantation is considered
the optimum treatment for patients with IgAN-induced ESRD as such
patients have a high 5- and 10-year graft survival rate even in the
presence of recurrent IgAN. Following renal transplantation, the
presence of numerous prognostic factors indicated poor graft
survival, which should result in caution and the implementation of
appropriate interventions.
Acknowledgements
The authors would like to thank Dr Feng Zheng for
their input regarding the experimental procedures. This study was
supported in part by grants from the Key Laboratory of Fujian
Province (grant no. 2010Y2006), the Fujian Natural Science
Foundation (grant no. 2012J01404) and the Fujian Science and
Technology Project Foundation (grant no. 2012Y5006).
References
|
1
|
Bi ZQ: Clinical and pathological analysis
of 40 cases of IgA nephropathy. Zhonghua Nei Ke Za Zhi. 23:136–140.
1984.(In Chinese).
|
|
2
|
Li LS: Clinicopathological correlation of
34 cases of IgA nephropathy. Zhonghua Nei Ke Za Zhi. 23:336–339.
3971984.(In Chinese).
|
|
3
|
Berger J, Yaneva H, Nabarra B and Barbanel
C: Recurrence of mesangial deposition of IgA after renal
transplantation. Kidney Int. 7:232–241. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Odum J, Peh CA, Clarkson AR, et al:
Recurrent mesangial IgA nephritis following renal transplantation.
Nephrol Dial Transplant. 9:309–312. 1994.PubMed/NCBI
|
|
5
|
Kessler M, Hiesse C, Hestin D, et al:
Recurrence of immunoglobulin A nephropathy after renal
transplantation in the cyclosporin era. Am J Kidney Dis. 28:99–104.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohmacht C, Kliem V, Burg M, et al:
Recurrent immunoglobulin A nephropathy after renal transplantation:
a significant contributor to graft loss. Transplantation.
64:1493–1496. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mafsugami K, Naito T, Nitta K, et al: A
clinicopathological study of recurrent IgA nephropathy following
renal transplantation. Nippon Jinzo Gakkai Shi. 40:322–328.
1998.(In Japanese).
|
|
8
|
Berthoux B, Diconne E, Mariat C, et al:
Risk factors for primary IgA nephropathy recurrence after renal
transplantation (RTx). Nephrol Dial Transplant. 18:801–802.
2003.
|
|
9
|
Namba Y, Oka K, Moriyama T, et al: Risk
factors for graft loss in patients with recurrent IgA nephropathy
after renal transplantation. Transplant Proc. 36:1314–1316. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Couser W: Recurrent glomerulonephritis in
the renal allograft: an update of selected areas. Exp Clin
Transplant. 3:283–288. 2005.PubMed/NCBI
|
|
11
|
Ng YS, Vathsala A, Chew ST, et al: Long
term outcome of renal allografts in patients with immunoglobulin A
nephropathy. Med J Malaysia. 62:109–113. 2007.PubMed/NCBI
|
|
12
|
Moriyama T, Nitta K, Suzuki K, et al:
Latent IgA deposition from donor kidney is the major risk factor
for recurrent IgA nephropathy in renal transplantation. Clin
Transplant. 19:41–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kiattisunthorn K, Premasathian N,
Wongwiwatana A, et al: Evaluating the clinical course and
prognostic factors of posttransplantation immunoglobulin A
nephropathy. Transplant Proc. 40:2349–2354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Racusen LC, Solez K, Colvin RB, et al: The
Banff 97 working classification of renal allograft pathology.
Kidney Int. 55:713–723. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Briganti EM, Russ GR, McNeil JJ, et al:
Risk of renal allograft loss from recurrent glomerulonephritis. N
Engl J Med. 347:103–109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sanfilippo F, Croker BP and Bollinger RR:
Fate of four cadaveric donor renal allografts with mesangial IgA
deposits. Transplantation. 33:370–376. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji S, Liu M, Chen J, et al: The fate of
glomerular mesangial IgA deposition in the donated kidney after
allograft transplantation. Clin Transplant. 18:536–540. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berger J: Recurrence of IgA nephropathy in
renal allografts. Am J Kidney Dis. 12:371–372. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang AY, Lai FM, Yu AW, et al: Recurrent
IgA nephropathy in renal transplant allografts. Am J Kidney Dis.
38:588–596. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McDonald SP and Russ GR: Recurrence of IgA
nephropathy among renal allograft recipients from living donors is
greater among those with zero HLA mismatches. Transplantation.
82:759–762. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soler MJ, Mir M, Rodriguez E, et al:
Recurrence of IgA nephropathy and Henoch-Schönlein purpura after
kidney transplantation: risk factors and graft survival. Transplant
Proc. 37:3705–3709. 2005.
|
|
22
|
Andresdottir MB, Haasnoot GW, Doxiadis II,
et al: Exclusive characteristics of graft survival and risk factors
in recipients with immunoglobulin A nephropathy: a retrospective
analysis of registry data. Transplantation. 80:1012–1018. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andresdottir MB, Haasnoot GW, Persijn GG
and Claas FH: HLA-B8, DR3: a new risk factor for graft failure
after renal transplantation in patients with underlying
immunoglobulin A nephropathy. Clin Transplant. 23:660–665. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang Z, Ji SM, Chen DR, et al: Recurrent
or de novo IgA nephropathy with crescent formation after renal
transplantation. Ren Fail. 30:611–616. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kowalewska J, Yuan S, Sustento-Reodica N,
et al: IgA nephropathy with crescents in kidney transplant
recipients. Am J Kidney Dis. 45:167–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oka K, Imai E, Moriyama T, et al: A
clinicopathological study of IgA nephropathy in renal transplant
recipients: beneficial effect of angiotensin-converting enzyme
inhibitor. Nephrol Dial Transplant. 15:689–695. 2000. View Article : Google Scholar
|