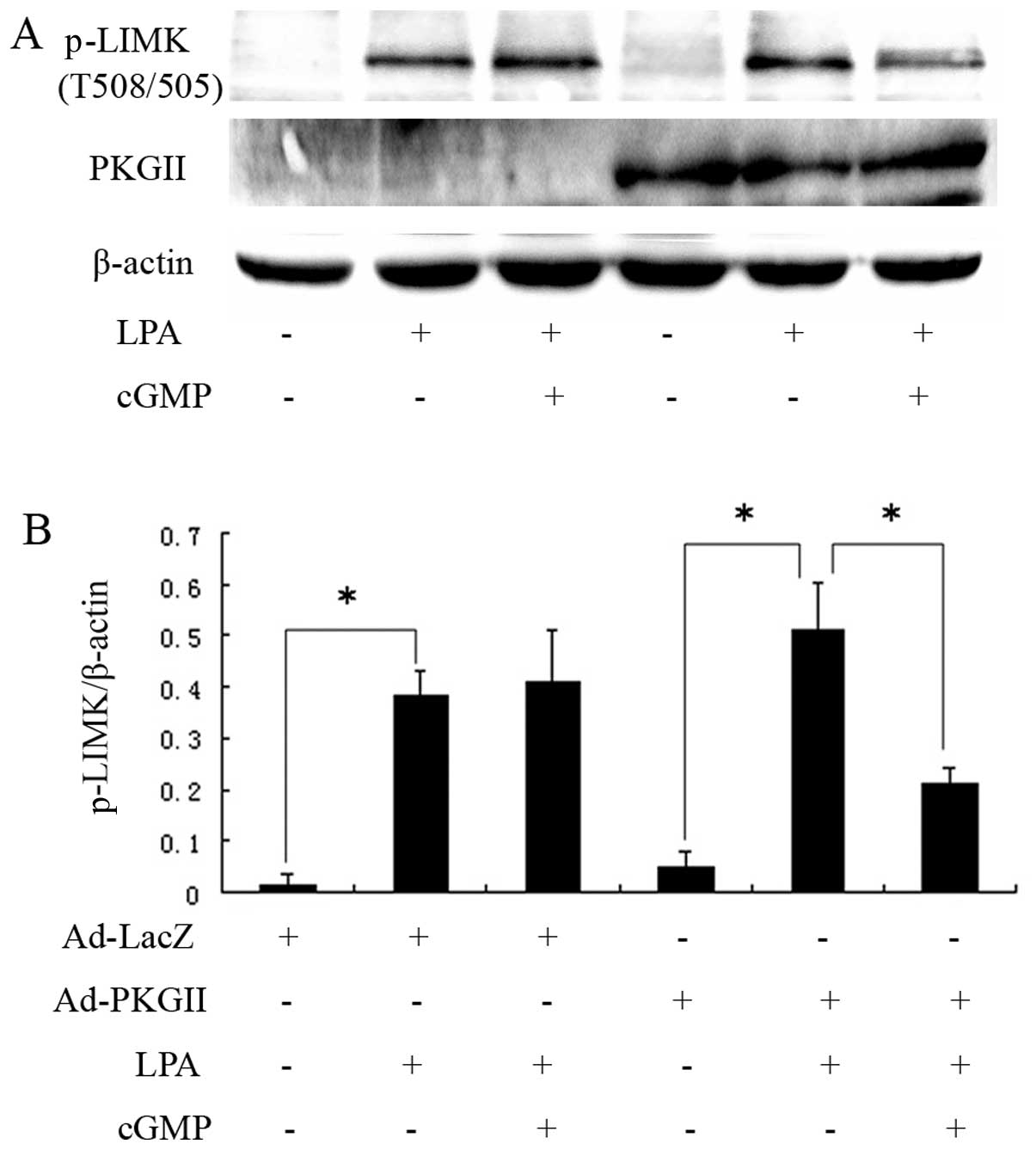
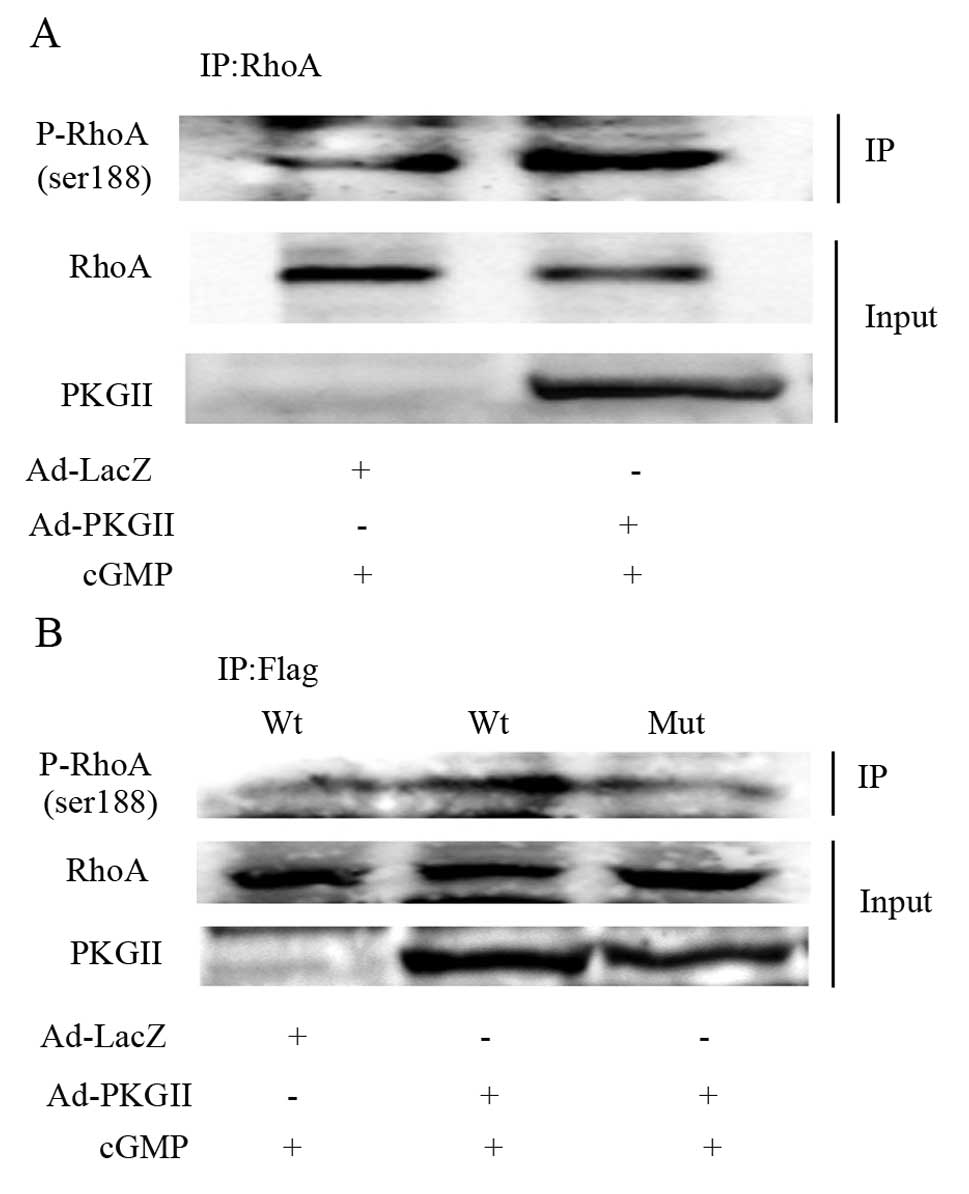
|
1
|
Vega FM and Ridley AJ: Rho GTPases in
cancer cell biology. FEBS Lett. 582:2093–2101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jaffe AB and Hall A: RHO GTPases:
biochemistry and biology. Annu Rev Cell Dev Biol. 21:247–269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar
|
|
4
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bos JL, Rehmann H and Wittinghofer A: GEFs
and GAPs: critical elements in the control of small G proteins.
Cell. 129:865–877. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forget MA, Desrosiers RR, Gingras D and
Beliveau R: Phophorylation states of Cdc42 and RhoA regulate their
interactions with Rho GDP dissociation inhibitor and their
extraction from biological membranes. Biochem J. 361:243–254. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lang P, Gesbert F, Delespine-Carmagnat M,
Stancou R, Pouchelet M and Bertoglio J: Protein kinase A
phosphorylation of RhoA mediates the morphological and functional
effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 15:510–519.
1996.PubMed/NCBI
|
|
8
|
Ellerbroek SM, Wennerberg K and Burridge
K: Serine phosphorylation negatively regulates RhoA in vivo. J Biol
Chem. 278:19023–19031. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hofmann F: The biology of cyclic
GMP-dependent protein kinases. J Biol Chem. 280:1–4. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cook AL and Haynes JM: Protein kinase G
II-mediated proliferative effects in human cultured prostatic
stromal cells. Cell Signal. 16:253–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Swartling FJ, Ferletta M, Kastemar M,
Weiss WA and Westermark B: Cyclic GMP-dependent protein kinase II
inhibits cell proliferation, Sox9 expression and Akt
phosphorylation in human glioma cell lines. Oncogene. 28:3121–3131.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang R, Kwon IK, Thangaraju M, Singh N,
Liu K, Jay P, Hofmann F, Ganapathy V and Browning DD: Type 2
cGMP-dependent protein kinase regulates proliferation and
differentiation in the colonic mucosa. Am J Physiol Gastrointest
Liver Physiol. 303:G209–G219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fallahian F, Karami-Tehrani F, Salami S
and Aghaei M: Cyclic GMP induced apoptosis via protein kinase G in
oestrogen receptor-positive and -negative breast cancer cell lines.
FEBS J. 278:3360–3369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YC, Ren F, Sang JR, Tao Y and Xu WR:
Type II cGMP-dependent protein kinase inhibits proliferation of the
gastric cancer cell line BGC-823. Mol Med Rep. 3:361–366.
2010.PubMed/NCBI
|
|
15
|
Wu Y, Chen Y, Qu R, Lan T and Sang J: Type
II cGMP-dependent protein kinase inhibits EGF-triggered signal
transduction of the MAPK/ERK-mediated pathway in gastric cancer
cells. Oncol Rep. 27:553–558. 2012.PubMed/NCBI
|
|
16
|
Jiang L, Lan T, Chen Y, Sang J, Li Y, Wu
M, Tao Y, Wang Y, Qian H and Gu L: PKG II inhibits EGF/EGFR-induced
migration of gastric cancer cells. PLoS One. 8:e616742013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren F, Gao Q, Tao Y, Sang J, Yang X, Fan S
and Chen Y: Impacts of cGMP dependent protein kinase II on gastric
cancer cell BGC-823 migration. J Jiangsu Univ (Med Ed). 20:113–116.
2010.
|
|
18
|
Bian D, Mahanivong C, Yu J, Frisch SM, Pan
ZK, Ye RD and Huang S: The G12/13-RhoA signaling pathway
contributes to efficient lysophosphatidic acid-stimulated cell
migration. Oncogene. 25:2234–2244. 2006.
|
|
19
|
Kodama Y and Hu CD: An improved
bimolecular fluorescence complementation assay with a high
signal-to-noise ratio. Biotechniques. 49:793–805. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kato M, Blanton R, Wang GR, Judson TJ, Abe
Y, Myoishi M, Karas RH and Mendelsohn ME: Direct binding and
regulation of RhoA protein by cyclic GMP-dependent protein kinase
Iα. J Biol Chem. 287:41342–41351. 2012.
|
|
21
|
Keilbach A, Ruth P and Hofmann F:
Detection of cGMP dependent protein kinase isozymes by specific
antibodies. Eur J Biochem. 208:467–473. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deguchi A, Thompson WJ and Weinstein IB:
Activation of protein kinase G is sufficient to induce apoptosis
and inhibit cell migration in colon cancer cells. Cancer Res.
64:3966–3973. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hou Y, Gupta N, Schoenlein P, Wong E,
Martindale R, Ganapathy V and Browning D: An anti-tumor role for
cGMP-dependent protein kinase. Cancer Lett. 240:60–68. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lohmann SM, Vaandrager AB, Smolenski A,
Walter U and De Jonge HR: Distinct and specific functions of
cGMP-dependent protein kinases. Trends Biochem Sci. 22:307–312.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hofmann F, Feil R, Kleppisch T and
Schlossmann J: Function of cGMP-dependent protein kinases as
revealed by gene deletion. Physiol Rev. 86:1–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maekawa M, Ishizaki T, Boku S, Watanabe N,
Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K and Narumiya S:
Signaling from Rho to the actin cytoskeleton through protein
kinases ROCK and LIM-kinase. Science. 285:895–898. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yiu G and He Z: Glial inhibition of CNS
axon regeneration. Nat Rev Neurosci. 7:617–627. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Caramel J, Quignon F and Delattre O:
RhoA-dependent regulation of cell migration by the tumor suppressor
hSNF5/INI1. Cancer Res. 68:6154–6161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sawada N, Itoh H, Yamashita J, Doi K,
Inoue M, Masatsugu K, Fukunaga Y, Sakaguchi S, Sone M, Yamahara K,
et al: cGMP-dependent protein kinase phosphorylates and inactivates
RhoA. Biochem Biophys Res Commun. 280:798–805. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sauzeau V, Le Jeune H, Cario-Toumaniantz
C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P and
Loirand G: Cyclic GMP-dependent protein kinase signaling pathway
inhibits RhoA-induced Ca2+ sensitization of contraction
in vascular smooth muscle. J Biol Chem. 275:21722–21729. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pellegrin S and Mellor H: Actin stress
fibres. J Cell Sci. 120:3491–3499. 2007. View Article : Google Scholar
|
|
32
|
Vaandrager AB, Hogema BM and de Jonge HR:
Molecular properties and biological functions of cGMP-dependent
protein kinase II. Front Biosci. 10:2150–2164. 2005. View Article : Google Scholar : PubMed/NCBI
|