Introduction
The World Health Organization has reported that
cancer is a primary factor of mortality worldwide with the rate
increasing daily. It is estimated that the overall number of cases
is likely to increase by >11 million until 2030. Cervical cancer
is a type of cancer which is expected to increase in frequency, and
is caused by the human papilloma virus that forms warts in the
throat and genital area (1).
Cervical cancer and other types of cancer-related mortality in
females of the developing countries contribute to >85% of the
global disease burden (2). In
response to this imminent challenge, a number of investigations
have focused on the use of traditional medicine for cancer
treatment. By developing genomic and proteomic technologies in
recent years, cancer research studies have entered to a new era,
and cancer is currently thought to be a genetic disease. This novel
point of view requires novel diagnosis and treatment approaches. It
is commonly accepted that the anti-tumor effect of cancer drugs is
primarily based on the induction of apoptosis. Thus, it is of
importance to determine mechanisms leading to apoptosis to
investigate responses of tumor cells to herbal drugs.
Scientific investigations have indicated that the
bioactive components of medicinal herbs may reduce the risk of
cancer through their anti-microbial, anti-oxidant and
anti-tumorigenic activity, and through their ability to directly
suppress carcinogenic bioactivities (3). Juglans mandshurica Maxim is a
member of the Juglandaceae plant family, has bitter bark, and is
pungent. Its primary chemical constituents include juglones,
flavonoids, tannins and gallic acid (4,5). The
bark of Juglans mandshurica Maxim exhibits detoxifying
effects, improves eyesight, restores consciousness and has
anti-tumor properties (6,7). A previous study showed that under
in vitro conditions, aqueous extracts of bark directly kill
mouse sarcoma 180 (S180) cells and significantly inhibit the growth
of mouse hepatoma 22 cells (8). It
has been reported that juglones, some of the primary components in
Juglans mandshurica Maxim extracts (HT), may extend the life
of HepA mice by 95% and inhibit the growth of S180 cells by 50%
in vivo. Kim et al (9) reported that bark extracts from
Juglans mandshurica Maxim exhibit cytotoxic effects on the
human colon cell line HT-29 and the human lung adenocarcinoma cell
line A599. However, no conclusive evidence of the effect of HT on
cell proliferation, activity of telomerase, apoptosis induction and
cell cycle arrest of HeLa cells in vitro, or its anti-tumor
mechanisms, have been reported to date. The present study aimed to
investigate the anti-tumor effects and mechanisms of HT by
assessing its effect on the cell cycle and apoptosis in HeLa
cells.
Materials and methods
Reagents
HT were provided by the Pharmacy Department of the
School of Life Science, Beijing Institute of Technology (Beijing,
China), Dulbecco’s modified Eagle medium (DMEM) was purchased from
GIBCO-BRL (Invitrogen Life Technologies, Carlsbad, CA, USA), fetal
calf serum was obtained from Sijiqing Biological Engineering
Material Co. Ltd. (Hangzhou, China) and Trypsin (1:250) and MTT
were supplied by Amresco Company (Solon, OH, USA).
Preparation of HT
Crude HT was provided by the Pharmacy Department of
the School of Life Science, Beijing Institute of Technology
(Beijing, China). For further isolation of pure juglone, the dry
powder was extracted in 95% ethanol 3 times for 1 h at a time and
the ethanolic extract was evaporated to dryness using a rotary
evaporator at 60°C. The residue was dissolved in a 10-fold amount
of petroleum ether 3 times. To this extract, 2%
Na2CO3 was added until pH 9.0 was reached,
and the mixture was filtered. Hydrochloric acid was added to the
filtrate to adjust the pH to 4.0, and the mixture was filtered. The
precipitate was collected, neutralized and dried at 60°C. The dried
product was HT. Juglone was identified as the primary active
component. Chromatographic experiments were performed on an Alltima
C18 column (4.6×250 mm, 5 μm) where the mobile phase was
methanol and water (70:30), the detection wavelength was 261 nm,
the column temperature was 27°C and the flow rate was 1.0
ml/min.
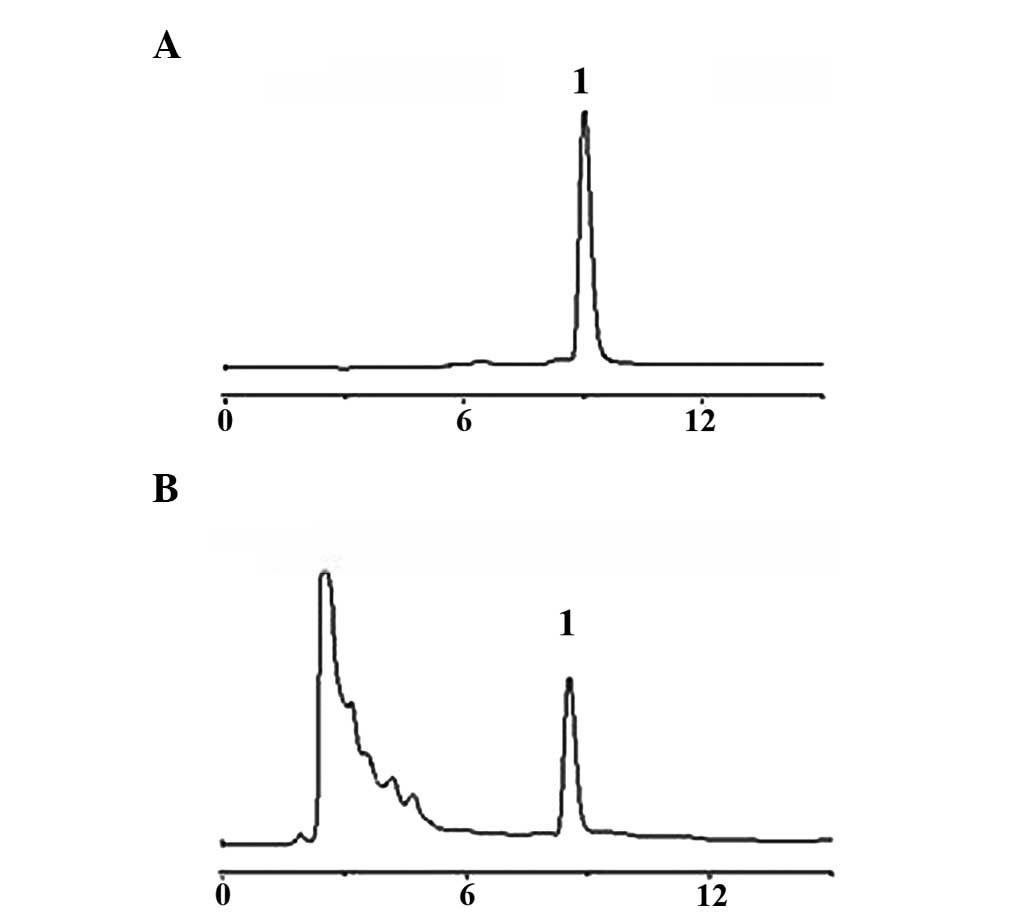
HT extracts were purified by High-performance liquid
chromatography (HPLC). The results showed a single peak at a
retention time of 9.12 min as shown in Fig. 1B, and the purity of the peak was
determined to be 99.5%. It was compared with standard HPLC peak of
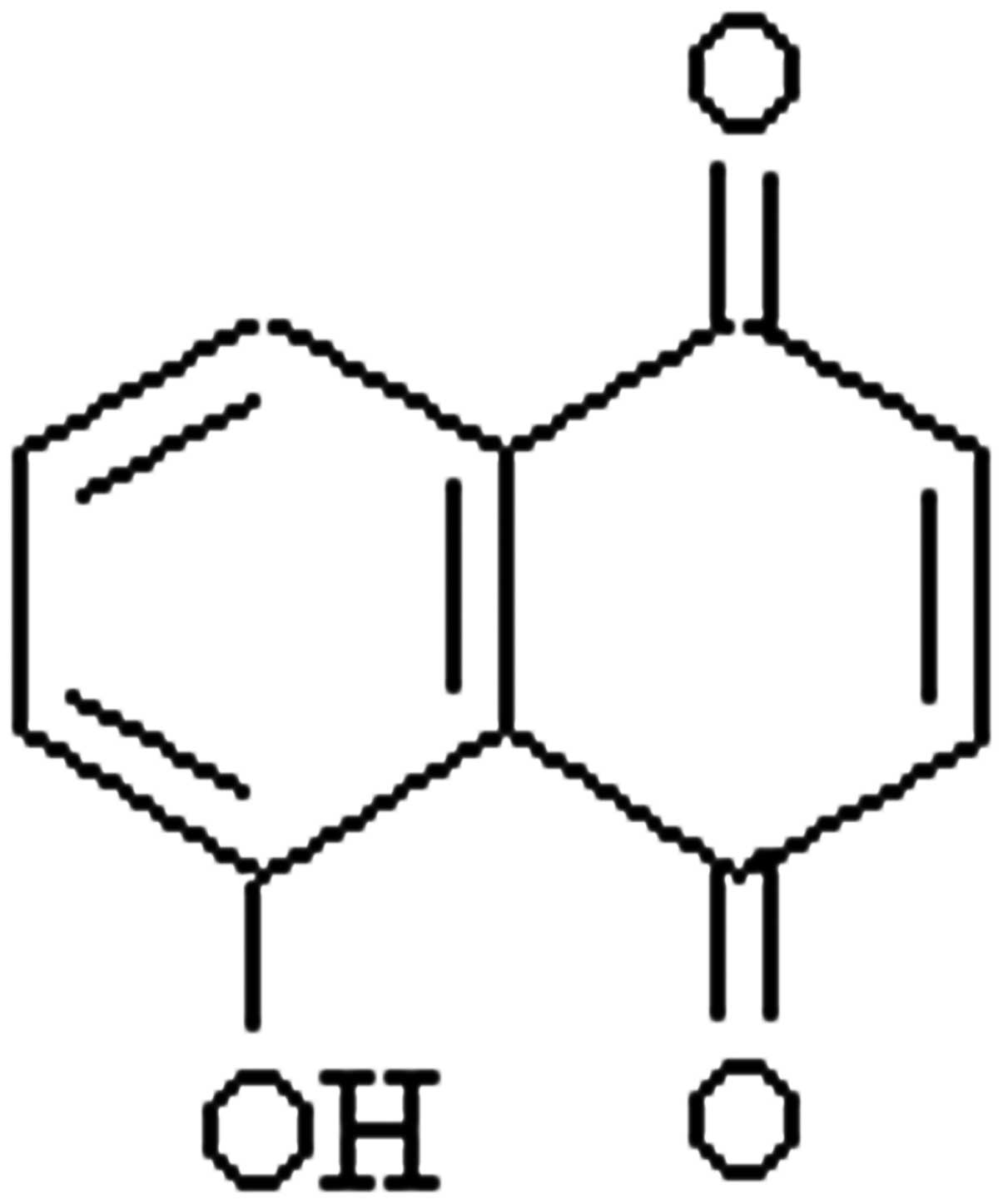
juglone as shown in Fig. 1A. Thus,
the compound was purified by HPLC separation under the
aforementioned, optimized conditions, and its chemical structure is
shown in Fig. 2.
Cell culture
HeLa cells were obtained from the College of Life
Science and Technology, Beijing Normal University (Beijing, China).
Cells were cultured in DMEM medium containing 10% fetal calf serum
in an incubator at 37°C, with 5% CO2 and saturated
humidity. The cells were studied at logarithmic growth phase.
Inhibition of cell proliferation by
HT
Cytotoxicity was assessed using the MTT assay. HeLa
cells, at logarithmic growth phase, were counted using the trypan
blue exclusion method. The cell density was adjusted to
1.0×107 cells/l with DMEM medium. Cells were inoculated
into 96-well plates at 190 μl/well and incubated at 37°C, 5%
CO2 and 100% humidity for 12 h. DMEM (10 μl) containing
specific concentrations of HT was added into each well separately,
and each group was repeated in 5 wells. A negative (cells + medium
without HT) and positive control (cisplatin) were also designed.
The nine final concentrations of HT were 10, 20, 50, 100, 200, 400,
600, 800 and 1,000 μg/ml. Cells were cultured for 24 h with HT.
Subsequently, an MTT assay (5 mg/ml, 20 μl/well for 4 h) was
used to detect the inhibitory effect of HT. Optical density values
were detected at 570 nm to obtain the IC50 value.
Effect of HT on the necrosis of HeLa
cells assessed using fluorescence staining
HeLa cells in were seeded into 6-well plates,
allowed to attach, and treated with HT for 48 h. Cells were fixed
in 4% paraformaldehyde for 20 min at a ratio of 3:1, followed by
staining with Hoechst 33342 (10 μg/ml) for 30 min. Following
mounting with glycerol, images were captured by fluorescent
microscopy (BX60; Olympus Corporation, Tokyo, Japan).
Assessment of apoptosis in HeLa cells
using DNA agarose gel electrophoresis
HeLa cells treated with HT for 48 h were trypsinised
and collected by centrifugation at 4,000 × g for 5 min and DNA was
extracted with phenol. Following the addition of 10 μl of the
sample into 1% agarose gel with ethidium bromide (0.5 mg/ml) and
electrophoresis, images were captured by ultraviolet
transillumination.
Effect of HT on the cell cycle of HeLa
cells assessed using flow cytometry
HeLa cells (2×105 cells/well) were
cultured in 6-well plates for 12 h. The cells were incubated for 24
and 48 h with 300 and 600 μg/ml HT, and were then centrifuged at
250 × g for 5 min and washed with PBS. Cells were fixed with 70%
ethanol for 12 h at 4°C, and centrifugation and washing were
repeated. The DNA distribution was detected by flow cytometry (BD
FACSCalibur; BD Bioscience, Franklin Lakes, NJ, USA) following the
addition of 1 ml PI staining solution to the cells at 4°C in the
dark for 30 min.
Telomerase activity in HeLa cells treated
with HT
Total RNA was extracted from HeLa cells following
treatment with different concentrations of HT for 24 and 48 h, and
quantitative polymerase chain reaction was performed. The primer
sequences were as follows: Ts primer: 5′-AATCCGTCG AGCAGAGTT-3′ and
Cx primer: 5′-CCCTTACCCTTACCC TTACCCTAA-3′. cDNA was amplified by
PCR and its product was purified by ethanol precipitation.
Following the addition of 2 μl sample into 12.5% non-denaturing
polyacrylamide gel for electrophoresis and silver nitrate staining,
the image was immediately captured using a gel imaging system
(BIO-BEST200M, SIM International Group Co, Ltd., Newark, DE,
USA).
Statistical analysis
SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was
used for statistical analysis. Values are expressed as the mean ±
standard deviation. Data were analyzed using a one-way analysis of
variance, followed by Student’s two-tailed t-test for comparison
between two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibition of the cell proliferation of
HeLa cells by HT
Initially, the growth inhibitory effect of HT on
HeLa cells was assessed. HeLa cells were treated with various
concentrations of HT (20–1,000 μg/ml) for 24 and 48 h. A MTT assay
was employed to assess cell proliferation. As shown in Table I, HT inhibited the cellular
proliferation in a dose- and time-dependent manner. Compared with
the control group, HT significantly inhibited cell proliferation.
With increasing HT concentration and time incubation time, the
inhibitory effect was gradually and statistically enhanced
(P<0.01–0.001). It was indicated that HT markedly inhibited the
proliferation of HeLa cells in a time- and dose-dependent manner.
The IC50 was 413.50 (24 h) and 391.30 μg/ml (48 h)
(Table I).
 | Table IInhibitory effect of HT on the
proliferation of HeLa cells. |
Table I
Inhibitory effect of HT on the
proliferation of HeLa cells.
| | Optical density
value | Inhibition rate
(%) |
|---|
| |
|
|
|---|
| Group | Dose (μg/ml) | /24 h | /48 h | /24 h | /48 h |
|---|
| Control | 0 | 0.378±0.039 | 0.642±0.021 | - | - |
| Cisplatin | 25 |
0.078±0.008c |
0.065±0.008c | 79.6 | 89.9 |
| HT | 10 |
0.334±0.015a | 0.605±0.018 | 11.7 | 3.9 |
| 20 |
0.302±0.026b |
0.531±0.017b | 20.1 | 15.6 |
| 50 |
0.276±0.016b |
0.436±0.029c | 27.1 | 30.7 |
| 100 |
0.249±0.014b |
0.378±0.037b | 34.3 | 39.9 |
| 200 |
0.198±0.013c |
0.342±0.071c | 47.5 | 45.6 |
| 400 |
0.172±0.009c |
0.300±0.062c | 54.5 | 52.3 |
| 600 |
0.132±0.011c |
0.225±0.024c | 65.1 | 64.2 |
| 800 |
0.091±0.008c |
0.105±0.008c | 76.2 | 83.3 |
| 1,000 |
0.083±0.008c |
0.078±0.008c | 77.9 | 87.7 |
Effect of HT on the necrosis of HeLa
cells assessed usingfluorescence staining
It was observed that the percentage of necrotic
bodies of HeLa cells increased rapidly in a time-dependent manner
following treatment with 600 μg/ml HT for 24 and 48 h. It was
observed that HT was capable of inducing apoptosis and necrosis in
HeLa cells, as shown in Fig. 3A.
Treatment of HeLa cells for 24 and 48 h with 600 μg/ml HT resulted
in apoptotic cell death. HeLa cells treated with HT and stained
with Hoechst 33258 (Fig. 3B) were
used to determine the cell death rate. Apoptotic bodies were
studied morphologically and included features such as nuclear
fragmentation and condensation of chromatin following treatment
with 600 μg/ml for 24 and 48 h as compared with the control group.
Nuclear staining with Hoechst 33258 was assessed by fluorescence
microscopy. The percentages of necrotic cells induced by 600 μg/ml
HT were 36.80 and 45.71% following 24 and 48 h treatment,
respectively (Fig. 3).
Effect of HT on the apoptosis of HeLa
cells assessed using DNA agarose gel electrophoresis
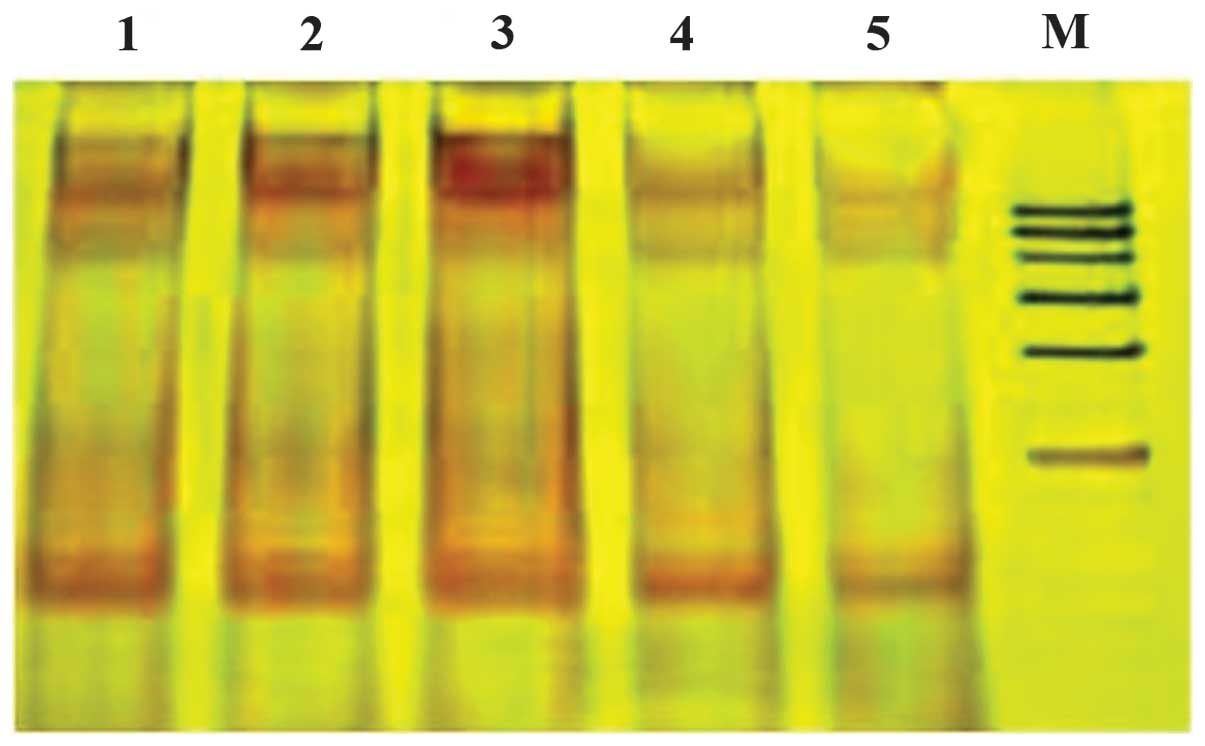
The induction of apoptosis by DNA fragmentation was
assessed in HeLa cells incubated with HT. A DNA ladder was observed
in HeLa cells treated with 100, 200, 400 and 600 μg/ml HT for 24 h.
However, apoptosis was not observed in the control group. The
result showed that HT induces apoptosis in HeLa cells and this
effect was enhanced with increasing HT concentrations, as shown in
Fig. 4.
Effect of HT on the cell cycle of HeLa
cells
Compared with the control group (21.2%), the S phase
fraction of HeLa cells treated with 300 and 600 μg/ml HT for 24 h
was markedly increased to 23 and 28.4%, respectively. S phase
arrest was further increased following 48 h of incubation (37 and
39.4%) and exhibited significant differences (P<0.05–0.001). The
G1-phase fraction decreased and the G2-phase fraction increased
significantly (P<0.05–0.001). The results showed that S phase
arrest appeared in HeLa cells treated with HT, in a time- and
dose-dependent manner and was greatest at 48 h (Table II).
 | Table IIEffect of HT on the cell cycle and
apoptosis of HeLa cells. |
Table II
Effect of HT on the cell cycle and
apoptosis of HeLa cells.
| Group | Dose (μg/ml) | G1 (%) | S (%) | G2 (%) |
|---|
| Control | - | 67.7±5.7 | 21.2±9.7 | 11.1±8.7 |
| HT treated for 24
h | 300 |
61.7±8.7a |
23.0±4.1a |
15.3±6.3a |
| HT treated for 48
h | 300 |
47.3±10.9b |
37.0±9.9b |
15.7±5.9a |
| HT treated for 24
h | 600 |
55.4±13.8b |
28.4±9.2b |
16.2±8.2b |
| HT treated for 48
h | 600 |
41.9±11.3b |
39.4±8.8b |
18.7±3.9b |
Telomerase activity in HeLa cells treated
with HT
Compared with the control group, 300 and 600 μg/ml
HT inhibited telomerase activity in HeLa cells, and the higher
concentration exhibited a marked effect. The inhibitory effect
increased in a time- and dose-dependent manner. The results
indicated that HT decreased HeLa cell proliferation by inhibiting
telomerase activity (Fig. 5).
Discussion
A large number of bioactive compounds synthesized by
marine organisms, plants and microorganisms have been studied for
their potential biological activities at the cellular level, which
may be employed for novel therapies. The normal growth of the cell
depends on strictly controlled stages of the cell cycle (10). According to the present study, HT
has the potential to influence the cell cycle stage, and a number
of anti-cancer agents have previously been reported to arrest the
cell cycle at specific points, and thereby induce apoptotic cell
death (11–13). The cell cycle is arrested at the G2
phase when DNA is damaged (14,15).
In the present study, HT was shown to have an anti-proliferative
effect. The genesis and development of tumors is associated with
uncontrolled cell proliferation and evasion of apoptosis,
particularly the uncontrolled cell growth caused by multiple gene
mutation (16). In addition,
numerous statistical outcomes have shown that telomerase activity
in the malignant tumors was higher compared with the majority of
normal tissue types, and telomerase was involved in the genesis and
development of malignant tumors (17). Two mechanisms are important in this
context: Telomerase is a reverse transcriptase and may directly
interact with telomeres to trigger cell survival. Furthermore,
telomerase has a role in the mechanism of DNA damage repair and
ensures cell proliferation and survival. These two mechanisms
indicate that inhibition of telomerase activity in tumor cells may
achieve an anti-tumor effect.
Defects in apoptosis signaling cause tumor cells to
exceed the normal life expectancy, which provides an increased
opportunity for gene mutations, interfering with differentiation
and increasing invasiveness. Apoptotic defects promote tumor cell
metastasis and escape recognition by immune cells, including
cytotoxic lymphocytes and natural killer cells. A number of studies
have focused on tumor cell apoptosis mechanisms and excessive
proliferation inhibition (18–21).
The present study indicated that HT significantly inhibited HeLa
cell proliferation, and the highest inhibition rate observed was
83.7%. Results of the trap-silver staining assay showed that HT
markedly decreased telomerase activity of HeLa cells and the
inhibition occurred in a time- and dose-dependent manner. Thus, the
mechanism of the anti-tumor activity of HT may involve the
inhibition of telomerase activity in tumor cells. Apoptosis
generally features marked morphological changes, including
chromatin condensation, nuclear fragmentation and formation of
apoptotic bodies. Therefore, it is possible to visually detect
apoptosis. Following treatment with HT, the cell walls of HeLa
cells disappeared and chromatin condensed. The Hoechst 33258
staining assay showed that HT readily induced apoptosis in HeLa
cells as indicated by a bright blue fluorescence staining of the
nuclei, while faint blue fluorescence was observed in cells without
HT treatment. Furthermore, a DNA ladder, which is characteristic
for apoptosis-specific DNA fragmentation, was observed by agarose
gel electrophoresis of DNA of HeLa cells incubated with HT. These
results indicate that HT markedly induced HeLa cell apoptosis.
Notably, S phase arrest was observed in HeLa cells
treated with HT. This increase not only demonstrated the effect of
HT on cellular activity, but also reflected a time- and
dose-dependent pattern for HeLa cell cycle arrest in S phase. The
release of DNA from cells during late apoptosis and necrosis
indicated that the nuclear DNA contents decreased in the cells
(22). The distribution of the DNA
content detected by flow cytometry may thus be able to indicate the
number of apoptotic cells. The preliminary results showed that HT
blocked HeLa cells in S phase.
In conclusion, the current study, evidenced that HT
has a marked effect on HeLa cells in vitro. It markedly
inhibited telomerase activity in HeLa cells, blocked cells in S
phase and induced apoptosis in tumor cells in a time- and
dose-dependent manner. However, genesis and development of tumors
are complicated processes, which are proceed via a variety of
pathways. The complex mechanism of the anti-tumor and
apoptosis-inducing effect of HT requires further study.
Acknowledgements
The present study was supported by the Beijing
Municipal Science and Technology Commission of P.R. China (no.
60217289).
References
|
1
|
Chen HC, Schiffman M, Lin CY, Pan MH, You
SL, Chuang LC, Hsieh CY, Liaw KL, Hsing AW and Chen CJ; CBCSP-HPV
Study group. Persistence of type-specific human papillomavirus
infection and increased long-term risk of cervical cancer. J Natl
Cancer Inst. 103:1387–1396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM: The global health burden of
infection-associated cancers in the year 2002. Int J Cancer.
118:3030–3044. 2006.PubMed/NCBI
|
|
3
|
Kaefer CM and Milner JA: The role of herbs
and spices in cancer prevention. J Nutr Biochem. 19:347–361. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun ML, Wang YM, Song ZQ and Fang GZ:
Insecticidal activities and active components of the alcohol
extract from green peel of Juglans mandshurica. J Forestry
Res. 18:62–64. 2007. View Article : Google Scholar
|
|
5
|
Zhou YY and Wang D: Advances in study on
the compounds of Juglans mandshurica Maxim. J Chin Mod
Tradit Chin Med. 3:8–10. 2007.(In Chinese).
|
|
6
|
Li Y, Xin N, Li YJ and Zhang JY:
Investigation into anti-tumor and immunomodulatory effects of
Juglans mandshurica maxim extracts (HT). Transactions
Beijing Inst Technol. 31:618–621. 2011.(In Chinese).
|
|
7
|
Li SZ, Wang YC, Jiang DF and Wang Y:
Screening effective part of qinglongyi and Walnut sticks on
anti-tumor by Brine Shrimp Lethelity Bioassay. Northwest Pharm J.
15:1142006.(In Chinese).
|
|
8
|
Guo JH, Cui LM, Li SH, Liu L, He RH and
Liu B: Inhibitory effects and immunoregulation of Juglans
mandshurica maxim extract on S180mouse sarcoma. J
Jilin Univ (Med Ed). 33:286–289. 2007.(In Chinese).
|
|
9
|
Kim SH, Lee KS, Son JK, Je GH, Lee JS, Lee
CH and Cheong CJ: Cytotoxic compounds from the roots of Juglans
mandshurica. J Nat Prod. 61:643–645. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murray AW: Recycling the cell cycle:
cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu YJ, Yang SH, Chien CM, Lin YH, Hu XW,
Wu ZZ, Wu MJ and Lin SR: Induction of G2/M phase arrest and
apoptosis by a novel enediyne derivative, THDB, in chronic myeloid
leukemia (HL-60) cells. Toxicol in Vitro. 21:90–98. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gamet-Payrastre L, Li P, Lumeau S, Cassar
G, Dupont MA, Chevolleau S, Gasc N, Tulliez J and Tercé F:
Sulforaphane, a naturally occurring isothiocyanate, induces cell
cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer
Res. 60:1426–1433. 2000.PubMed/NCBI
|
|
13
|
Fujimoto K, Hosotani R, Doi R, Wada M, Lee
JU, Koshiba T, Miyamoto Y, Tsuji S, Nakajima Y and Imamura M:
Induction of cell-cycle arrest and apoptosis by a novel
retinobenzoic-acid derivative, TAC-101, in human pancreatic-cancer
cells. Int J Cancer. 81:637–644. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taylor WR and Stark GR: Regulation of the
G2/M transition by p53. Oncogene. 20:1803–1815. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luk SC, Siu SW, Lai CK, Wu YJ and Pang SF:
Cell cycle arrest by a natural product via G2/M checkpoint. Int J
Med Sci. 2:64–69. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu HL, Yu XF, Qu SC, Zhang R, Qu XR, Chen
YP, Ma XY and Sui DY: Anti-proliferative effect of Juglone from
Juglans mandshurica Maxim on human leukemia cell HL-60 by
inducing apoptosis through the mitochondria-dependent pathway. Eur
J Pharmacol. 645:14–22. 2010.PubMed/NCBI
|
|
17
|
Blasco MA: Telomerase beyond telomeres.
Nat Rev Cancer. 2:627–633. 2002. View
Article : Google Scholar
|
|
18
|
Shi H and Ding ZS: Study progress of the
chemical composition and pharmacological effects of the pecan
species. Chin Tradit Patent Med. 31:924–928. 2009.
|
|
19
|
Chen FH and Tang WM: Studies on the
Constituents and Biological Activity of the Bark of Juglans regia.
Natural Product Research and Development. 20:16–18. 2008.
|
|
20
|
Zhang YL, Zhan M, Pan YF, Fan W, Li HZ and
Huang QS: Study on the contribution to the cytokines of the carya
bark decoction to the BALb/c tumor-bearing mice. Strait Pharm J.
21:40–43. 2009.
|
|
21
|
Han LK, Li W, Narimatsu S, Liu LJ, Fu HW,
Okuda H and Koike K: Inhibitory effects of compounds isolated from
fruit of Juglans mandshurica on pancreatic lipase. J Nat
Med. 61:184–186. 2007. View Article : Google Scholar
|
|
22
|
Shi YY, Li H and Yang SJ: Induction of
apoptosis by panax quinquefolium effective parts (PQEP) on K562
cells. Chin Pharmacol Bull. 21:1494–1497. 2005.
|