Introduction
p53 is a major cell proliferation suppressor with a
central role in a complex regulatory network. p53 inhibits the cell
cycle through several different mechanisms and is capable of
inducing cell death when required (1). These inhibitory functions of p53 on
cell growth are important to prevent tumourigenesis and the loss of
wild-type p53 has been demonstrated to increase the risk of cancer
in humans (2,3). Despite its crucial role in preventing
malignant cell growth, p53 is not fundamental to normal cell
proliferation and differentiation, with wild-type p53 being absent
during embryonic development (4).
Additionally, p53 overexpression in normal cells is capable of
inducing fatal damage due to its excessive activity. Thus, p53
homeostasis is vital for normal cell development and
proliferation.
Mouse double minute 2 homolog (MDM2), which is
encoded by the MDM2 oncogene, modulates the levels of p53 and is
involved in a negative feedback loop, which regulates p53 protein
expression. Upon phosphorylation, p53 has a relatively short
half-life, as MDM2 binds and ubiquitinates the phoshphorylated form
of p53, which is then ultimately degraded (5). Under pathological conditions, the
half-life of p53 is prolonged, due to compromised MDM2-induced
ubiquitination as a result of the activation of multiple inhibitory
pathways, which in certain cases cause p53 to become hyperactive
(6). In addition, numerous p53
target genes regulate the trans-activity of MDM2. p53-MDM2 feedback
is thus important when cells are challenged by factors that may
stimulate development and growth processes. MDM2 has been found to
interact with p53 through three major mechanisms: (i) Through
binding to the N-terminus of p53 to inhibit its function as a
transcription factor (7,8); (ii) through acting as an E3 ubiquitin
ligase and promoting the ubiquitination and degradation of p53
(9) and (iii) through facilitating
p53 translocation from the nucleus to the cytoplasm in order to
separate p53 from regulatory sites within its target genes.
Interruption of the MDM2-p53 feedback loop impacts
cell survival. p14ARF is a tumour suppressor involved in
interrupting this feedback loop (10–12).
It binds to MDM2, retaining MDM2 in the nucleus and separating it
from p53 (13,14), thus impeding the formation of the
MDM2-p53 complex and disrupting the feedback loop. Another protein
that interrupts the MDM2-p53 feedback loop is MDMx; an MDM2
homologue. MDMx competes with p53 for ubiquitination by MDM2,
ultimately resulting in the degradation of MDMx instead of p53
(15).
The present study investigated approaches to screen
for proteins that compete with p53 for binding to MDM2.
Co-immunoprecipitation (Co-IP) was performed using the 1–52 amino
acid region of p53 as bait. The mechanism underlying the effect of
PIAS3 on the MDM2-p53 negative feedback loop, which differs from
the manner in which p53 accumulates under conditions of DNA damage
and proteinase inhibitor treatment, was also investigated.
Understanding the mechanism by which PIAS3 affects the MDM2-p53
feedback loop may provide novel insight for tumour suppression by
increasing the stability of the p53 protein.
Materials and methods
Cell lines and plasmids
Human HEK293, H1299 and A549 cells (American Type
Culture Collection, Manassas, VA, USA) were grown in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% (v/v) foetal
bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin
at 37°, in 5% CO2. To generate truncated p53 N-terminal
proteins and full-length p53 proteins fused to FLAG®
tags (Sigma-Aldrich, St. Louis, MO, USA) at the N-terminus, reverse
transcription-polymerase chain reaction (RT-PCR) was performed
using the following primer sequences: P1,
5′-GGAATTCCATATGTACCCATACGATGTTCCAGATTACGCTGAGGAGCCGCAGTCAGA-3′ and
either P2, 5′-CCG CTCGAGTTACCTGCTCCCCCCTGGCTCCTT-3′, or P3,
5′-CCGCTCGAGGATTACAAGGATGACGACGATAAG-3′ (BGI, Beijing, China). The
respective PCR products were cloned into the pcDNA3.1 vector using
the NheI and XhoI restriction sites. To generate
truncated p53 N-terminal proteins and full-length p53 proteins
fused to haemagglutinin (HA) tags at the N-terminus (Life
Technologies, San Diego, CA, USA), RT-PCR was performed using the
following primer sequences: P4,
5′-GGAATTCCATATGTACCCATACGATGTTCCAGATTACGCTGAGGAGCCGCAGTCAGA-3′ and
either P5, 5′-CCGCTCGAGAGCCCTGCTCCCCCCTGGCTCC-3′, or P6,
5′-CCGCTCGAGTCAGTCTGAGTCAGGCCCTTCTGT-3′ (BGI). The respective PCR
products were then cloned into the pcDNA3.1 vector using the
NheI and XhoI restriction sites.
Antibodies
The mouse monoclonal anti-FLAG and -HA antibodies
(Abcam PLC, Cambridge, MA, USA) were used for western blot analysis
and the detection of the IP products of the Flag- and HA-tagged
proteins, respectively. The mouse monoclonal DO-1 anti-p53 antibody
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used for the
detection of ubiquitinated p53 and p53 immunoprecipitates. The
mouse monoclonal anti-p21 antibody (Abcam PLC) was used for the
detection of endogenous p21 protein. The mouse monoclonal anti-MDM2
and -PIAS3 antibodies (Abcam PLC) were used for the detection and
immunoprecipitation of endogenous MDM2 and PIAS3 proteins,
respectively.
Quantitative PCR
Quantitative PCR was performed on the iCycler
instrument (Bio-Rad) as follows: 95°C for 5 min, 40 cycles (95°C
for 30 sec, 60°C for 30 sec and 72°C for 20 sec). Data were
exported from iCycler software (Bio-Rad) and imported into Excel
(Microsoft, Redmond, WA, USA).
Western blot analysis
Cells were harvested and protein concentrations were
quantified using a Bio-Rad protein assay kit (Bio-Rad, Hercules,
CA, USA). Protein samples were then subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to Immunobilon-P polyvinylidene fluoride membranes
(Millipore, Billerica, MA, USA) using a Semi-Dry Transfer kit
(Bio-Rad). Membranes were blocked using 10% (w/v) non-fat dry milk
and subsequently incubated in the aforementioned primary antibodies
and rabbit polyclonal horseradish peroxidase-labeled secondary
antibodies to mouse IgG (Abcam PLC). Immunoreactive signals were
visualised using enhanced chemiluminescence (Amersham Pharmacia
Biotech, Little Chalfont, UK) and exposed to X-ray film.
Co-IP assay
Co-IP of endogenously expressed proteins was
performed using HEK293 cells transfected with pcDNA3.1-Flag-p53NT,
pcDNA3.1-HA-PIAS3 or empty vector. After 24 h, cells were harvested
using 1% NP40 lysis buffer. Lysates were then incubated overnight
with 2.5 μg anti-Flag antibody and Protein G beads using a Protein
G Immunoprecipitation kit (Sigma-Aldrich). The resulting complexes
were washed, denatured and eluted according to the manufacturer’s
instructions.
Silver staining of SDS-polyacrylamide
gels
Following electrophoresis, the SDS-polyacrylamide
gels were soaked in fixative containing 25% ethanol and 10% acetic
acid for 30 min. Gels were then transferred to conditioning medium
containing 0.4 M sodium acetate (pH 6.0), 30% ethanol, 4.4 mM
sodium thiosulfate and 1% glutaraldehyde for 20 min. Following
conditioning, gels underwent at least three 5 min washes in
ddH2O. Each gel was then soaked in silver nitrate
solution (0.1% silver nitrate and 0.01% formaldehyde) for 20 min,
followed by development using developer solution (2.5% sodium
carbonate and 0.015% formaldehyde). Development was terminated with
the addition of acetic acid.
Proliferation assay
Cell proliferation was assayed using the phenazine
methosulfate and
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS)-based Cell Titer 96® AQueous One Solution Cell
Proliferation assay (Promega Corporation, Madison, WI, USA).
Approximately 1000 cells/well were plated onto 96-well plates.
After 24 h, the MTS mix was added at a final volume of 20 μl/100 μl
medium and incubated for an additional 60 min under the same
conditions. Following the addition of 10% SDS, the absorbance of
formazan was measured at 490 nm using an Evolution 60S (Thermo
Scientific, Waltham, MA, USA). All assays were performed in
triplicate every other day for 10 days. The results are expressed
as the mean ± standard error.
Soft agar assay
Efficiency of growth in soft agarose was determined
by plating the cells in DMEM containing 0.33% agarose (Indubiose
A37; IBF, Villeneuve-la-Garenne, France) with 10% FBS above a layer
of 0.5% agarose in the same culture medium. Colonies were counted
two weeks subsequent to plating. Cultures were examined using an
inverted microscope (Leica DM6000 CS; Leica, Hong Kong, China).
Tandem mass spectrometry (MS/MS)
identification of novel p53 binding proteins
MS/MS identification of phosphopeptides was
conducted using a 4800 MALDI TOF/TOF™ analyser (Applied Biosystems,
Carlsbad, CA, USA). Tryptic digests of samples were re-dissolved in
0.1% trifluoroacetic acid and 50% acetonitrile, then mixed 1:1 with
a matrix consisting of 20 mg/ml DHCA, 50% acetonitrile and 5 mM
NH4HPO4, prior to spotting on a sample plate.
The sample plate was externally calibrated using a Mass Standards
kit (Applied Biosystems). The peptide MALDI-TOF/TOF spectra were
acquired manually by inputting the peptide mass into the precursor
mass window. MS/MS spectra were collected using ≥2,000 laser shots
and were searched against a Gossypium peptide sequence database
annotated from a Gossypium expressed sequence tag (EST) database
(downloaded from http://www.ncbi.nlm.nih.gov; release date December 9
2010; including 507,959 EST sequences and 62,267,048 residues)
using MASCOT 2.2 software (Matrix Science, Oxford, UK) with the
following settings: Enzyme, trypsin; MS tolerance, 0.3 Da; MS/MS
tolerance, 0.5 Da; maximum number of missed cleavages, 1; peptide
charge, 1+; fixed modifications, carbamidomethylation of
Cys; variable modifications, oxidation of Met, phosphorylation and
sulphation of Ser, Thr and Tyr. Peptides with an ion score >15
were considered to possess significant homology (P<0.1) rather
than being a random event. Analyses of spectra were then performed
by hand to further validate the identification of these
peptides.
Statistical analysis
All results are presented as the average ± standard
error of the mean. Student’s t-test was used for comparison between
two groups. The difference is considered as statistically
significant when P<0.05.
Results
Selection and identification of a novel
p53 binding protein
pcDNA3.1-Flag-p53NT was constructed and was stably
transfected into H1299 cells along with the pcDNA3.1 empty vector.
H1299 cell lysates were then subjected to immunoprecipitation using
an anti-FLAG antibody. The products of the Co-IP assay were
separated using 10% SDS-PAGE and silver stained. Numerous protein
bands were observed on the pcDNA3.1-Flag-p53NT-transfected H1299
cell gel compared with the empty vector gel (Fig. 1A). The bands were excised from the
gel and the proteins were recovered and sent for MS analysis
(Beckman Coulter, Inc., Brea, CA, USA). MS revealed sequences of
several types of protein. The most significant signal was from the
PIAS3 protein. The corresponding co-IP band of PIAS3 was also the
strongest; therefore, PIAS3 was selected to be the focus of this
study. To assess the interaction between p53 and PIAS3, HEK293
cells were transfected with either pcDNA3.1-FLAG-p53NT and
pcDNA3.1-HA-PIAS3 or pcDNA3.1-FLAG-p53 and pcDNA3.1-HA-PIAS3. Each
group of transfected cells was lysed 24 h after transfection and
the lysates were subjected to Co-IP. Western blot analysis using
antibodies corresponding to the Co-IP products and inputs, revealed
that PIAS3 binds to the truncated p53 NT protein and the wild-type
p53 protein in vitro (Fig.
1B). To further assess the interaction between endogenous p53
and PIAS3, untransfected HEK293 cells were analysed using Co-IP.
Similar results were observed, showing that endogenous PIAS3 also
binds to p53 (Fig. 1C).
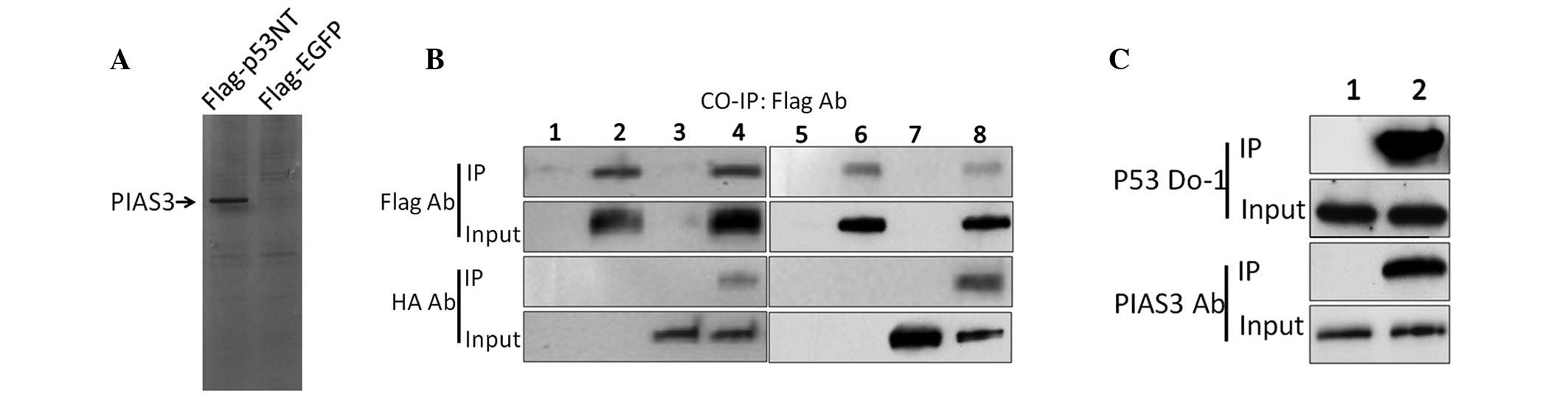 | Figure 1Identification of a novel p53 binding
protein. (A) Identification of a novel p53 binding protein using
silver staining. (B) Co-IP analysis of protein-protein interactions
between p53NT and PIAS3. PcDNA3.1-Flag-p53NT and pcDNA3.1-HA-PIAS3
were transfected into HEK293 together or independently. After 24 h,
Co-IPs were performed using an anti-Flag antibody and the inputs
and IP products were assayed using anti-Flag or -HA antibodies,
respectively. Lanes 1 and 5, untreated HEK293 cells; lane 2, HEK293
cells transfected with pcDNA3.1-Flag-p53NT; lanes 3 and 7, HEK293
cells transfected with pcDNA3.1-HA-PIAS3; lane 4, HEK293 cells
co-transfected with pcDNA3.1-Flag-p53NT and pcDNA3.1-HA-PIAS3; lane
6, HEK293 cells transfected with pcDNA3.1-Flag-p53 and lane 8,
HEK293 cells co-transfected with pcDNA3.1-Flag-p53 and
pcDNA3.1-HA-PIAS3. (C) Co-IP analysis of the endogenous interaction
between p53 and PIAS3. Untransfected HEK293 cells were lysed and
Co-IP analyses were performed using the anti-p53 antibody DO-1
(lane 1) or rabbit immunoglobulin G (lane 2) as negative control.
PIAS3, protein inhibitor of activated STAT3; p53NT, p53 N-terminus;
HA, haemagglutinin; IP, immunoprecipitation; EGFP, enhanced green
fluorescent protein. |
PIAS3 increases the stability and
transactivity of p53 by inhibiting its ubiquitination
In the present study, PIAS3 was observed to bind to
p53 through the 1–52 amino acid region of p53 (Fig. 1B). This region was previously
reported to be the domain through which p53 binds to MDM2 (16). The p53-MDM2 interaction leads to
the ubiquitination of p53, resulting in p53 degradation (17). Therefore, the present study aimed
to investigate whether PIAS3 affects p53 protein stability. p53
protein expression was assessed in HEK293 cells 12, 24, 36 and 48 h
after transient transfection with pcDNA3.1-PIAS3 or with the empty
vector. Western blot analysis revealed a significant time-dependent
increase in p53 protein expression following transfection, showing
that PIAS3 is capable of increasing p53 protein levels (Fig. 2A). However, no significant
difference was observed in p53 mRNA expression following
transfection in these cells (Fig.
2B). HEK293 cells overexpressing PIAS3 and control HEK293 cells
were then treated with cycloheximide, a translational elongation
inhibitor, and cell lysates were harvested to measure the half-life
of p53 (18). Western blot
analysis revealed that cycloheximide attenuated p53 degradation in
the HEK293 cells overexpressing PIAS3, but not in the HEK293 cells
transfected with the empty pcDNA3.1 vector (Fig. 2C). In vitro Co-IP assay
revealed that overexpression of PIAS3 competitively inhibited
p53-MDM2 binding through binding to the p53 protein (Fig. 2D). These findings suggest that
PIAS3 increases p53 expression in a post-translational manner and
strongly suggests the involvement of PIAS3 in disrupting the
formation of the p53-MDM2 complex and inhibiting MDM2-induced p53
ubiquitination (Fig. 2E). p53 is
an important transcription factor involved in a complex regulatory
network; therefore, its intracellular stability is critical for the
normal functioning of its downstream targets, including p21
(19). Thus, the effect of PIAS3
on p21 expression was investigated. Chromatin IP analysis of HEK293
cells transiently transfected with pcDNA3.1-PIAS3 revealed that the
PIAS3 overexpression-induced p53 accumulation increased the binding
of p53 to its responsive elements in DNA clusters in the p21
promoter region (Fig. 2F). In
addition, quantitative PCR and western blot analyses showed that
p21 expression was upregulated at the mRNA and protein levels in
the PIAS3 overexpressing cells, while no difference was detected in
the HEK293 cells transfected with the empty pcDNA3.1 vector
(Fig. 2A and B).
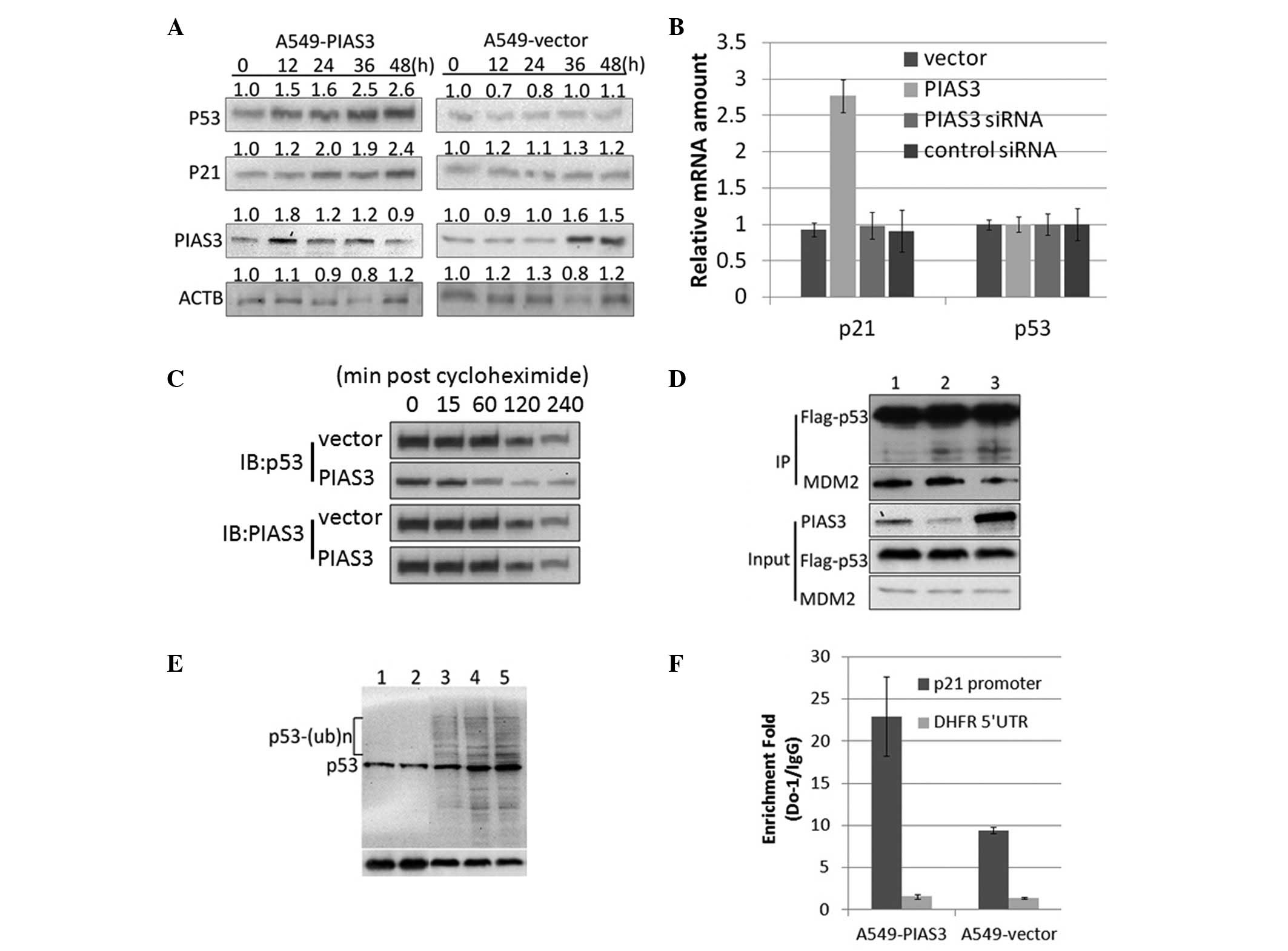 | Figure 2PIAS3 inhibits the ubiquitination and
increases the transactivity of p53. (A) Overexpression of PIAS3
increased p53 protein expression without altering p53 mRNA levels.
(B) Overexpression of PIAS3 was not found to change p21 mRNA
expression. A549 cells were stably transfected with PcDNA3.1-PIAS3
and p53 and p21 mRNA levels were detected using quantitative
polymerase chain reaction analysis. (C) Overexpression of PIAS3
increases the half-life of p53. (D) Competitive binding of PIAS3 to
p53 inhibits p53-MDM2 binding. HEK293 cells were co-transfected
with: lane 1, Flag-p53 and MDM2; lane 2, Flag-p53, MDM2 and the
empty vector and lane 3, Flag-p53, MDM2 and PIAS3. (E)
Overexpression of PIAS3 inhibits MDM2-mediated p53 ubiquitination.
Lane 1, A549-PIAS3; lane 2, A549-PIAS3 incubated with ALLN; lane 3,
A549 incubated with ALLN; lane 4, A549-empty vector incubated with
ALLN and lane 5, A549-pcDNA31-Flag incubated with ALLN. (F)
Overexpression of PIAS3 was observed to promote the binding
activity of p53 to the p21 promoter. Chromatin IP using an anti-p53
antibody followed by qPCR analysis was performed on the p21
promoter. PIAS3, protein inhibitor of activated STAT3; IP,
immunoprecipitation; siRNA, small interfering RNA; DFHR,
dihydrofolate reductase; UTR, untranslated region; ACTB, actin
beta; MDM2, mouse double minute 2 homolog. |
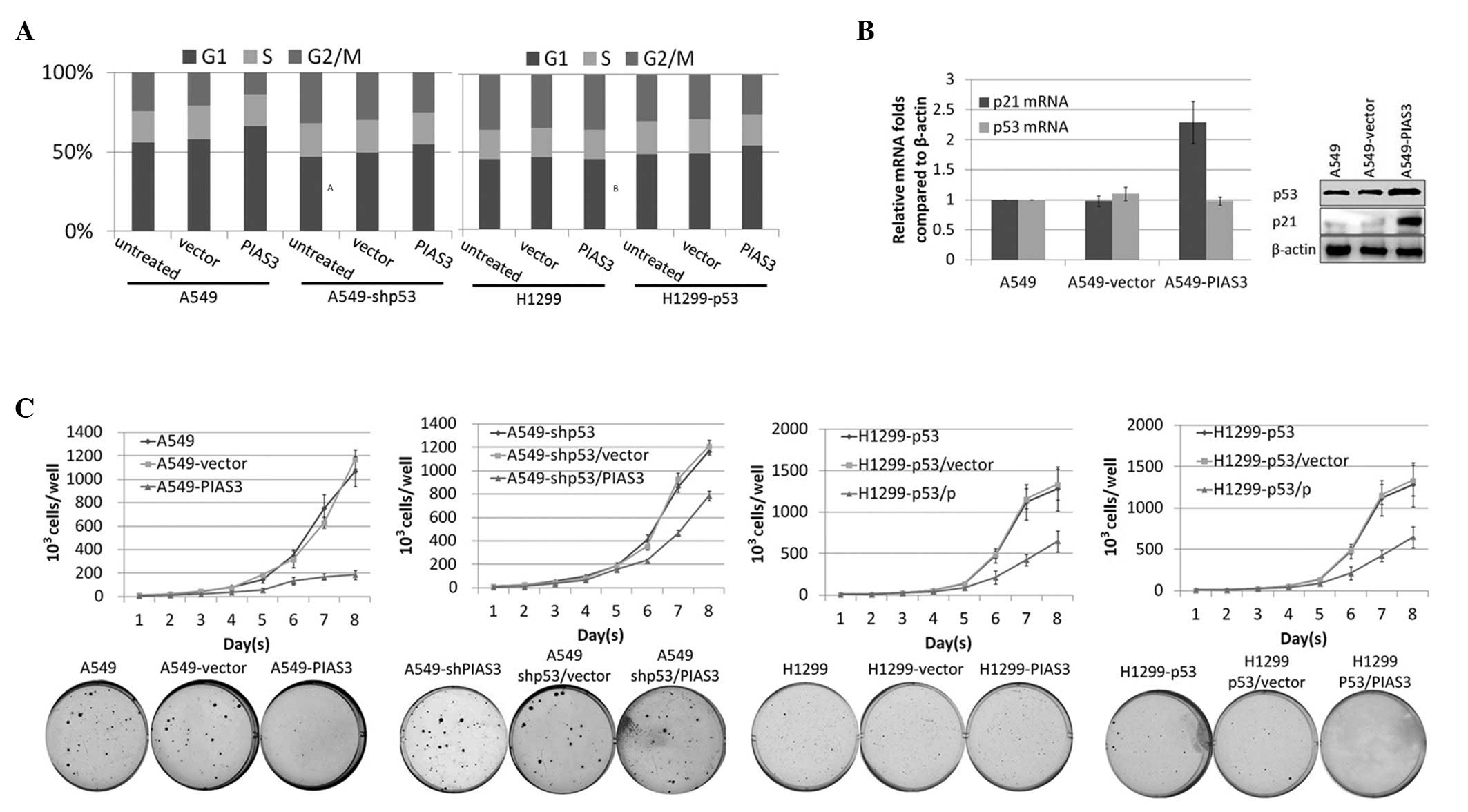
PIAS3 overexpression inhibits cell growth
rate and tumour formation in a p53-dependent manner
It is well established that p53 overexpression
enhances the induction of apoptosis and cell cycle arrest. In the
present study, PIAS3 was observed to stabilise the p53 protein
through disrupting the formation of the p53-MDM2 complex;
therefore, the effect of PIAS3 on cell proliferation was
investigated. To assess the effect of PIAS3 on cell proliferation,
A549 cells were stably transfected with pcDNA3.1-Flag-PIAS3 or the
empty pcDNA3.1 vector and cell growth curves were constructed. In
order to determine whether PIAS3 overexpression induces cell cycle
arrest in a p53-dependent manner, A549 cells stably transfected
with pcDNA3.1-Flag-PIAS3 or the empty vector were subjected to flow
cytometric analysis subsequent to DNA staining. Overexpression of
PIAS3 was found to increase the proportion of cells in
G0/G1-phase (Fig. 3A). By contrast, PIAS3
overexpression had no effect on the cell cycle in the p53-null
H1299 cell line or in p53-knockdown A549 cells. The accumulation of
p53 was observed to cause cell cycle changes through the
upregulation of p21 (Fig. 3B).
Furthermore, PIAS3 overexpression was found to decelerate cell
growth rate (Fig. 3C). Soft agar
assay on A549 cells overexpressing PIAS3 and A549 cells transfected
with the empty vector revealed that PIAS3 inhibits the rate of
tumour formation. By contrast, PIAS3 overexpression in NCl-H1299
cells, a p53-null cell line, had no effect on cell growth or tumour
formation. To further investigate this finding, H1299 cells were
transfected with pcDNA3.1-Flag-p53 to generate a stably transfected
wild-type p53-expressing cell line, H1299-wtp53. PcDNA5-PIAS3 or
the pcDNA5 empty vector were then transfected into H1299-wtp53
cells and stably overexpressing cell lines were obtained. These
cell lines were utilised to construct cell growth curves and
perform the soft agar assay. Exogenous wt-p53 was found to inhibit
cell growth rate and tumour formation through PIAS in the
H1299-wtp53 cell line.
Discussion
Investigation into the p53 network has revealed
increasing numbers of proteins that are involved in the MDM2-p53
negative feedback circuit. Such proteins include, p300, E2F1 and
the p38, casein kinase 1 and ataxia telangiectasia mutated kinases
(20–23). Notably, these proteins are capable
of mediating the function of MDM2 by regulating p53. Phosphorylated
MDM2 loses its capacity to regulate p53, as phosphorylation induces
MDM2 self-ubiquitination and subcellular translocation.
Accumulation of the p53 protein is vital for p53
activation; triggering its DNA binding, transcriptional regulation
and other regulatory functions. Under normal conditions, MDM2
controls the accumulation of p53 by regulating its half-life and
maintains a relatively low level of p53 protein expression
(9,24,25).
The p53 binding domain in MDM2 binds to a transcriptional
regulatory domain at the N-terminus of p53. Upon p53-MDM2 binding,
p53 is phosphorylated by the RING domain of MDM2 (26). Ubiquitinated p53 is then
translocated to the cytoplasm or the nucleus and is ultimately
degraded by 26s proteasomes (27,28).
Furthermore, p53 regulates MDM2 transcription, forming an automatic
negative feedback loop together with MDM2-mediated p53 degradation
(29). Thus, the MDM2-p53 feedback
loop is an important mechanism, which must be overcome in order for
p53 to become activated. Upon DNA damage, the p53 protein is highly
phosphorylated by a series of kinases, including ATM/ataxia
telangiectasia and Rad3 related and checkpoint kinase 2 (30). Previous reports have demonstrated
that p53 phosphorylation at Ser20 promotes p53 stability and
activates the DNA repair process (31–33).
The p53-MDM2 interaction is essential to the ubiquitination and
degradation of p53; therefore, mechanisms blocking the formation of
the p53-MDM2 feedback loop enhance p53 stability.
PIAS3 is an E3 small ubiquitin-like modifier
(SUMO)-protein ligase, which belongs to the PIAS family and
covalently attaches SUMO proteins to specific target substrates.
SUMO modification of p53 modulates p53 transcriptional activity
(34) and PIAS3-mediated
heterogeneous nuclear ribonucleoprotein K (hnRNP-K) SUMOylation has
been reported to increase hnRNP-K stability, as well as its
interaction with p53 and p21, leading to cell cycle arrest
(35). Notably, in the present
study, PIAS3 was observed to bind directly to the N terminus of
p53. In addition, the interaction between PIAS3 and P53 was found
to increase the stability of p53 and stimulate p53-induced p21
transcription, which may lead to cell cycle arrest (data not
shown).
The interaction between MDM2 and p53 is a highly
regulated process. MDM2 binds directly to the first transactivation
domain (amino acids, 20–40) of p53, inhibiting its capacity to
interact with transcriptional co-activators (36). However, it has also been shown that
the p53 C-terminal domain interacts directly with the MDM2
N-terminus (37). C-terminal
modification of p53 has been reported to induce full dissociation
of MDM2-p53 complexes in cells. Thus, the N-terminus, as well as
the C-terminus bind to the N-terminus of MDM2. Further research on
the interaction between the C-terminus of p53 and PIAS3 is
required.
In the present study, a novel p53 binding protein,
PIAS3, was identified and binding of PIAS3 to p53 was found to
activate the transactivity of p53. Two different approaches were
used to analyse the effect of PIAS3 on p53 transactivation.
Chromatin IP assays were performed to demonstrate the increased DNA
binding activity of p53 in PIAS3 overexpressing cells. In addition,
the expression of the p53 target gene p21 was detected using
western blot analysis and indicated that PIAS3 induced functional
activation of the p53 protein. Furthermore, the overexpression of
PIAS3 was found to inhibit MDM2-induced p53 ubiquitination and
increase the half-life of p53. In addition, DNA binding and the
transactivation of the p21/waf1 promoter were induced maximally
when the stabilised p53 levels had returned to control levels.
References
|
1
|
Shaper NJ, Harrison M and Bates T: Impact
of laparoscopic cholecystectomy on surgical training. Ann R Coll
Surg Engl. 78:39–42. 1996.PubMed/NCBI
|
|
2
|
Evans SC and Lozano G: The Li-Fraumeni
syndrome: an inherited susceptibility to cancer. Mol Med Today.
3:390–395. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Attardi LD and Jacks T: The role of p53 in
tumour suppression: lessons from mouse models. Cell Mol Life Sci.
55:48–63. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi J and Donehower LA: p53 in embryonic
development: maintaining a fine balance. Cell Mol Life Sci.
55:38–47. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piette J, Neel H and Maréchal V: Mdm2:
keeping p53 under control. Oncogene. 15:1001–1010. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karanicolas J and Brooks CL III: The
structural basis for biphasic kinetics in the folding of the WW
domain from a forming-binding protein: Lessons for protein design?
Proc Natl Acad Sci USA. 100:3954–3959. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kussie PH, Gorina S, Marechal V, Elenbaas
B, Moreau J, Levine AJ and Pavletich NP: Structure of the MDM2
oncoprotein bound to the p53 tumor suppressor transactivation
domain. Science. 274:948–953. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Momand J, Zambetti GP, Olson DC, George D
and Levine AJ: The mdm-2 oncogene product forms a complex with the
p53 protein and inhibits p53-mediated transactivation. Cell.
69:1237–1245. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haupt Y, Maya R, Kazaz A and Oren M: Mdm2
promotes the rapid degradation of p53. Nature. 387:296–299. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamijo T, Weber JD, Zambetti G, Zindy F,
Roussel MF and Sherr CJ: Functional and physical interactions of
the ARF tumor suppressor with p53 and Mdm2. Proc Natl Acad Sci USA.
95:8292–8297. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pomerantz J, Schreiber-Agus N, Liégeois
NJ, Silverman A, Alland L, Chin L, Potes J, Chen K, Orlow I, Lee
HW, Cordon-Cardo C and DePinho RA: The Ink4a tumor suppressor gene
product, p19Arf, interacts with MDM2 and neutralizes MDM2’s
inhibition of p53. Cell. 92:713–723. 1998.PubMed/NCBI
|
|
12
|
Honda R and Yasuda H: Association of
p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for
tumor suppressor p53. EMBO J. 18:22–27. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tao W and Levine AJ: P19(ARF) stabilizes
p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc Natl
Acad Sci USA. 96:6937–6941. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weber JD, Taylor LJ, Roussel MF, Sherr CJ
and Bar-Sagi D: Nucleolar Arf sequesters Mdm2 and activates p53.
Nat Cell Biol. 1:20–26. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shadfan M, Lopez-Pajares V and Yuan ZM:
MDM2 and MDMX: Alone and together in regulation of p53. Transl
Cancer Res. 1:88–89. 2012.PubMed/NCBI
|
|
16
|
Chen J, Marechal V and Levine AJ: Mapping
of the p53 and mdm-2 interaction domains. Mol Cell Biol.
13:4107–4114. 1993.PubMed/NCBI
|
|
17
|
Momand J, Zambetti GP, Olson DC, George D
and Levine AJ: The mdm-2 oncogene product forms a complex with the
p53 protein and inhibits p53-mediated transactivation. Cell.
69:1237–1245. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takimoto R, Wang W, Dicker DT, Rastinejad
F, Lyssikatos J and el-Deiry WS: The mutant p53-conformation
modifying drug, CP-31398, can induce apoptosis of human cancer
cells and can stabilize wild-type p53 protein. Cancer Biol Ther.
1:47–55. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
el-Deiry WS, Tokino T, Velculescu VE, Levy
DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW and
Vogelstein B: WAF1, a potential mediator of p53 tumor suppression.
Cell. 75:817–825. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin K, Trouche K, Hagemeier C, Sørensen
TS, La Thangue NB and Kouzarides T: Stimulation of E2F1/DP1
transcriptional activity by MDM2 oncoprotein. Nature. 375:691–694.
1995. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lughran O and La Thangue NB: Apoptotic and
growth-promoting activity of E2F modulated by MDM2. Mol Cell Biol.
20:2186–2197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grossman SR, Perez M, Kung AL, et al:
P300/MDM2 complexes participate in Mdm2-mediated p53 degradation.
Mol Cell. 2:405–415. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Avantaggiati ML, Ogryzko V, Gardner K,
Giordano A, Levine AS and Kelly K: Recruitment of p300/CBP in
p53-dependent signal pathways. Cell. 89:1175–1184. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kubbutat MH, Jones SN and Vousden KH:
Regulation of p53 stability by Mdm2. Nature. 387:299–303. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu ZK, Geyer RK and Maki CG:
MDM2-dependent ubiquitination of nuclear and cytoplasmic P53.
Oncogen. 19:5892–5897. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang S, Jensen JP, Ludwig RL, Vousden KH
and Weissman AM: Mdm2 is a RING finger-dependent ubiquitin protein
ligase for itself and p53. J Biol Chem. 275:8945–8951. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Geyer RK, Yu ZK and Maki CG: The MDM2
RING-finger domain is required to promote p53 nuclear export. Nat
Cell Biol. 2:569–573. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xirodimas DP, Stephen CW and Lane DP:
Compartmentalization of p53 and Mdm2 is a major determinant for
Mdm2-mediated degradation of p53. Exp Cell Res. 270:66–77. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barak Y, Juven T, Haffner R and Oren M:
Mdm2 expression is induced by wild type p53 activity. EMBO J.
12:461–468. 1993.PubMed/NCBI
|
|
30
|
Durocher D and Jackson SP: DNA-PK, ATM and
ATR as sensors of DNA damage: variations on a theme? Curr Opin Cell
Biol. 13:225–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chehab NH, Malikzay A, Stavridi ES and
Halazonetis TD: Phosphorylation of Ser-20 mediates stabilization of
human p53 in response to DNA damage. Proc Natl Acad Sci USA.
96:13777–13782. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dumaz N, Milne DM, Jardine LJ and Meek DW:
Critical roles for the serine 20, but not the serine 15,
phosphorylation site and for the polyproline domain in regulating
p53 turnover. Biochem J. 359:459–464. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Unger T, Juven-Gershon T, Moallem E,
Berger M, Vogt Sionov R, Lozano G, Oren M and Haupt Y: Critical
role for Ser20 of human p53 in the negative regulation of p53 by
Mdm2. EMBO J. 18:1805–1814. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stindt MH, Carter S, Vigneron AM, Ryan KM
and Vousden KH: MDM2 promotes SUMO-2/3 modification of p53 to
modulate transcriptional activity. Cell Cycle. 10:3176–3188. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee SW, Lee MH, Park JH, Kang SH, Yoo HM,
Ka SH, Oh YM, Jeon YJ and Chung CH: SUMOylation of hnRNP-K is
required for p53-mediated cell-cycle arrest in response to DNA
damage. EMBO J. 31:4441–4452. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin J, Chen J, Elenbaas B and Levine AJ:
Several hydrophobic amino acids in the p53 amino-terminal domain
are required for transcriptional activation, binding to mdm-2 and
the adenovirus 5 E1B 55-kD protein. Genes Dev. 8:1235–1246. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Poyurovsky MV, Katz C, Laptenko O,
Beckerman R, Lokshin M, Ahn J, Byeon IJ, Gabizon R, Mattia M,
Zupnick A, Brown LM, Friedler A and Prives C: The C-terminus of p53
binds the N-terminal domain of MDM2. Nat Struct Mol Biol.
17:982–989. 2010. View Article : Google Scholar : PubMed/NCBI
|