Introduction
Wound healing and the management of
difficult-to-treat wounds have been the focus of traumatology
departments, particularly the infected wound. It has been well
recognized that negative pressure wound therapy (NPWT) accelerates
wound healing. The NPWT device included a section of foam placed in
the wound and covered with an occlusive dressing (1). The functions of NPWT include
resolving infection, completing wound debridement, reducing the
time required to produce a healthy granulation bed and increasing
wound contraction (2). Although
extensive research has been performed to investigate the mechanisms
of action by which NPWT increases the rate of healing, and its
probable beneficial effects in various basic and clinical
circumstances, the molecular mechanism of NPWT has rarely been
reported to interpret this phenomenon
A crucial link of how the NPWT system promotes wound
healing mainly focuses on the increased blood supply. Angiogenesis
is a complex process, which is controlled and regulated by
angiopoietin and anti-angiogenesis factors. Various growth factors,
including vascular endothelial growth factor (VEGF), fibroblast
growth factors (acidic and basic FGFs) and angiopoietin are
involved in neovascularization (3). The angiogenesis-associated growth
factors that essentially contribute to the maturation,
stabilization and remodeling of the vasculature were hypothesized
to be differentially expressed following treatment with a NPWT
system in an infected wound.
In order to detect the expression of these
angiogenesis-associated growth factors, a protein biochip array
technique was used, which was constructed on the surface of a
biochip. A carrier component transports the biochips to different
treatment stations within the analyzer, and following ligand
binding, a chemiluminescent signal is produced, which is measured
by a charge-coupled camera device and quantified by imaging
software (4). To the best of our
knowledge, this is the first study in which a protein biochip array
technology has been used to detect the molecular mechanism of the
NPWT system.
Materials and methods
Patients
A total of 20 patients (13 males and 7 females) aged
between 28 and 47 years (mean, 36.4) with a full-thickness infected
wound, which could not be closed immediately at the Department of
Orthopedics in Zhongnan Hospital of Wuhan University (Wuhan,
China), were include in this study. Patients consented to partaking
in the study. The etiology of all wounds was trauma. All patients
required initial debridement and necrotic soft tissue excisions.
Spaced holes were drilled in the exposed bone and necrotic exposed
tendons were excised. Following a proper debridement, patients were
administered with a NPWT foam under a continuous pressure of −125
mmHg (all materials from VSD Medical Technology Co., Ltd., Wuhan,
China), no layer of protection was used between the foam and the
wound. Various antibiotics, according to a drug sensitivity test,
were used until the infection had been controlled. All wounds were
finally covered following a number of VSD dressing renewals when a
clean, red granulating wound bed was achieved. A full-thickness
skin graft or a local transposition flap was selected as a final
covering based on the situation of granulation tissue and exposed
deep tissue. The study was approved by the local ethical committee
(approval no. 2011059, Zhongnan Hospital of Wuhan University,
Wuhan, China).
Following primary debridement and on the third day
during NPWT, a Laser Doppler Blood Perfusion imager (Perimed Ltd.,
Beijing, China) and PeriScan PIM 3 system (Perimed Ltd.) was used
to scan the blood flow of each wound, and the PIMsoft software
(version 1.5; (Perimed Ltd.) was used to analyze the blood flow on
the scanned images. At the same time, a 3×5×5 mm granulation
tissues sample in the centre of each wound was collected. Then all
samples were maintained at −80°C until analyzed.
Proteomic analysis
Quantbody human angiogenesis antibody array 1
(RayBiotech Inc., Norcross, GA, USA) was used to assay the samples
mentioned previously, 10 angiogenesis-associated growth factors,
including angiogenin, angiotesin-2 (ANG-2), EGF, heparin-binding
EGF-like growth factor (HB-EGF), leptin, bFGF, placenta growth
factor (PIGF), VEGF, platelet-derived growth factor (PDGF) and
hepatocyte growth factor (HGF) were detected in the current study.
Samples of granulation tissues were treated consulting the
specification of biochip array used. The protein array was
performed following the user manual (version July 2010).
Statistical analysis
Results are expressed as the mean ± standard
deviation and were compared by analysis of variance. Values of each
angiogenesis factor expression level and blood flows pre-primary
debridement and three days later were compared. Data were analyzed
with the SPSS 17.0 (SPSS. Inc., Chicago, IL, USA) software.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Outcomes of wound repair
No complications were noted regarding NPWT.
Following NPWT, all patients presented with a clean, red,
granulating wound bed and successful reconstruction had been
achieved by skin grafting (15 patients) or using local flaps (5
patients). The average wound healing time was 31 days (range, 21–44
days). The number of dressing changes was between 1 and 4, with an
average of 1.8 times.
Wound blood flow
All wounds were scanned using the PeriScan PIM 3
system, and an image of each wound was captured (Fig. 1). A mean quantitative value of
blood flow in the whole wound was calculated by PIMsoft software
(Table I). A marked boost of blood
flow was observed following NPWT, the mean blood flow prior to NPWT
was 63.38±15.09; and on the third day this increased statistically
to 297.13±54.67 during NPWT (P<0.001).
 | Table IThe records of blood flow of each
wound (n=20) prior to and following NPWT. |
Table I
The records of blood flow of each
wound (n=20) prior to and following NPWT.
| Patient | Wound area, cm | Mean blood flow of
wound prior to NPWTa | Mean blood flow of
wound on the third day during NPWTb |
|---|
| 1 | 3×5×6 | 50.6 | 231.9 |
| 2 | 4×6×9 | 68.7 | 322.7 |
| 3 | 6×7×9 | 88.3 | 309.9 |
| 4 | 7×8×9 | 34.5 | 289.5 |
| 5 | 4×6×7 | 56.7 | 356.7 |
| 6 | 7×7×9 | 55.2 | 332.6 |
| 7 | 2×4×8 | 76.8 | 347.2 |
| 8 | 5×7×8 | 88.5 | 198.6 |
| 9 | 7×8×8 | 56.7 | 334.7 |
| 10 | 6×5×5 | 46.8 | 289.0 |
| 11 | 4×7×10 | 76.8 | 310.4 |
| 12 | 7×8×8 | 43.1 | 209.1 |
| 13 | 8×10×12 | 59.0 | 408.4 |
| 14 | 3×4×5 | 67.4 | 276.9 |
| 15 | 4×7×9 | 77.5 | 344.5 |
| 16 | 5×7×8 | 68.4 | 287.1 |
| 17 | 3×6×9 | 56.7 | 230.9 |
| 18 | 4×5×11 | 77.9 | 339.5 |
| 19 | 5×7×10 | 46.9 | 287.6 |
| 20 | 4×6×6 | 71.1 | 235.3 |
Protein biochip array analysis
The granulation tissue samples collected prior to
and on the third day during NPWT, were observed to detect the
changes in the expression level of the factors mentioned on the
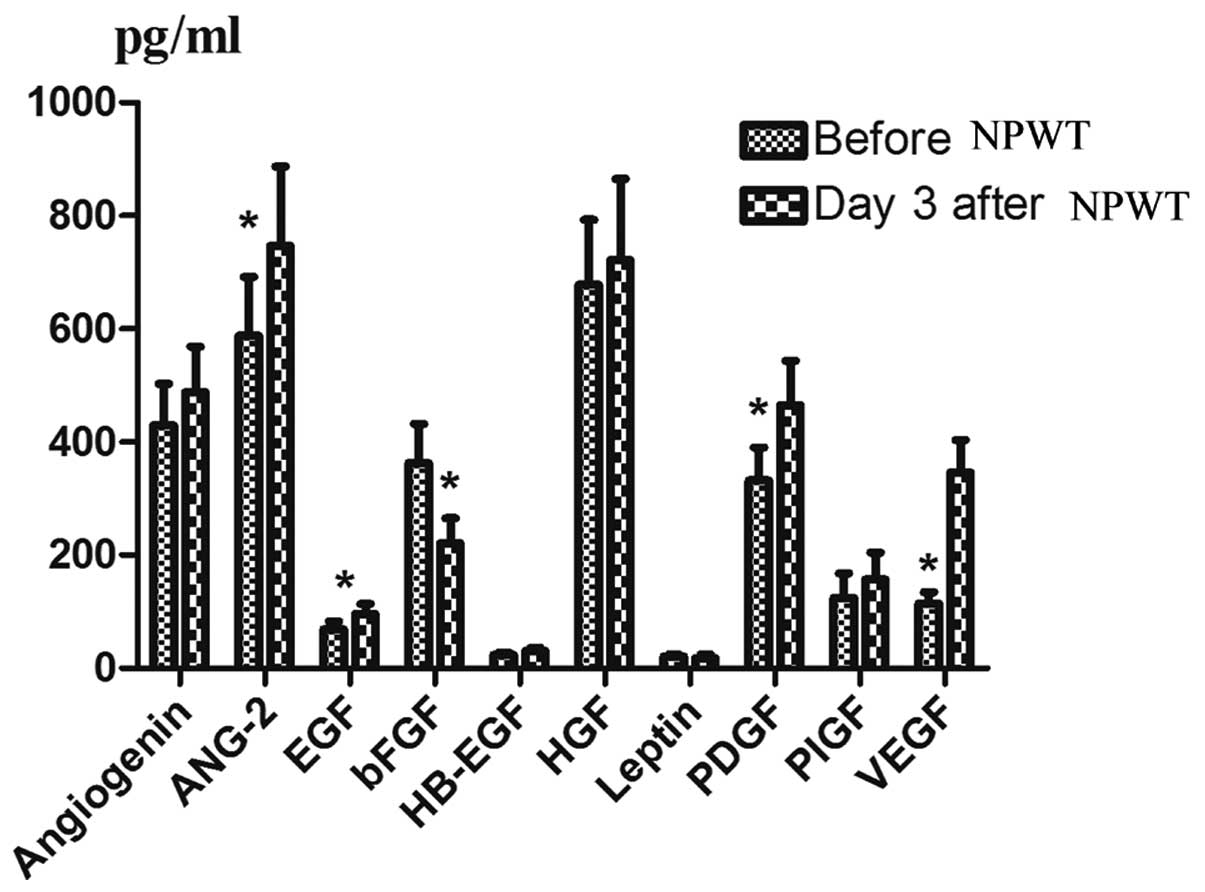
biochip array. Examples of the array are shown in Fig. 2. When angiogenesis factors were
compared pre- and during NPWT therapy, an increase was observed in
the protein levels of angiogenin, EGF, ANG-2, hepatocyte growth
factor (HGF), PDGF, VEGF and PIGF; however, significant variations
only occurred in ANG-2 (P=0.001), EGF (P=0.000), PDGF (P=0.018) and
VEGF (P=0.000). In addition, the levels of bFGF significantly
decreased (P=0.007). The expression levels of HB-EGF and leptin
were too low to measure the variation following NPWT (Fig. 3).
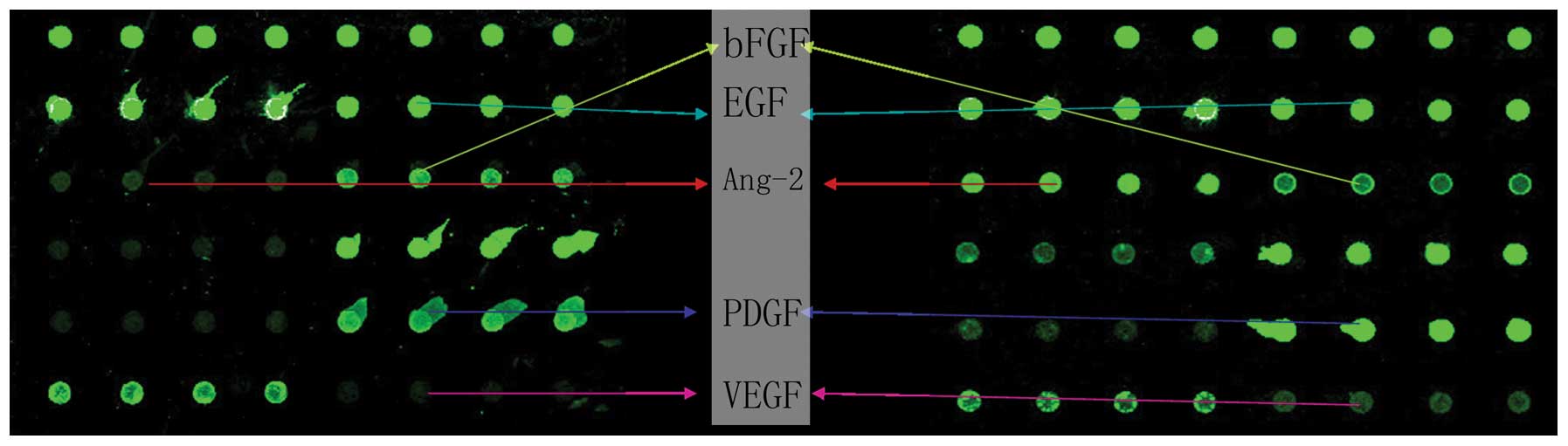 | Figure 2Scanned images of spectrum mean
quantity indicate cytokines using a RayBio Biotin label-based
Quantbody human angiogenesis antibody array 1 from granulation
tissue samples prior to NPWT (left) and on the third day during
NPWT (right). Intensity of every point is associated with the
matching protein expression. Each four sequential points indicate a
factor. All image points reflect the factors of POS1, POS2,
angiogenin, ANG-2, EGF, bFGF, HB-EGF, HGF, leptin, PDGF, PIGF and
VEGF from left to right and top to bottom, while the two factors
topside were reference points. NPWT, negative pressure wound
therapy; ANG-2 angiotesin-2; HB-EGF, heparin-binding EGF-like
growth factor; HGF, hepatocyte growth factor; PDGF,
platelet-derived growth factor; PIGF, placenta growth factor; VEGF,
vascular endothelial growth factor. |
Discussion
A complex soft tissue defect wound is difficult to
manage. Application of NPWT has been shown to exhibit a marked
effect on promoting wound healing (5). Earlier in vivo studies of
wound healing showed that NPWT improved removal of edema fluid and
increased blood flow, granulation tissue formation and bacterial
clearance in wounds (6,7). Lee et al (8) reported that NPWT application produces
successful surgical reconstruction for large, deep skin and soft
tissue defects without extensive radical flap surgery or loss of
skin graft. In the current study, no complications were observed
and high quality granulation tissue was achieved in each patient.
Less local flaps were required during NPWT therapy, the wounds
repaired using local flaps were one third of those that recieved
skin grafting, and the success rate of the skin grafts were 100%,
due to the several advantages, including stabilizing the skin graft
and preventing the collection of fluid under the skin graft
(9).
The current study suggests that the increase of
vasculogenesis and angiogenesis may be a significant effect of
NPWT. Increased blood supply enhances the migration of inflammatory
cells in and around the wound. Previous studies focus on the effect
of angiogenesis-related factors during vasculogenesis and
angiogenesis. These factors act on endothelial cells within blood
vessels around the injured tissue leading to the sprouting of new
capillaries to perfuse the wound tissue. These capillaries function
to mobilize a diverse collection of cell types into the peripheral
blood and promote angiogenesis, either by directly incorporating
into neovasculature or indirectly by serving as an additional
source of angiogenic growth factors/cytokines. In addition, they
have been show to exhibit direct effects on keratinocytes (10,11).
Jacobs et al (12) used an
in vivo application of the vacuum-assisted closure foam with
negative pressure, which resulted in increased growth factor
production, improved angiogenesis and collagen deposition. However,
no previous study has used system to explore these cytokines
following NPWT. The ability to detect a number of factors at once
may have been problematic previously; however, protein chip
detection has been broadly used in various fields (13) due to its advantages of high
sensitivity, simultaneous detection of multiple protein expression
levels, and ease of use. The use of a protein biochip array in the
current study may provide a novel insight into molecular events,
which may improve wound healing.
VEGF, a mitogenic factor, which acts on endothelial
cells, is crucial in vasculogenesis and angiogenesis (14), and is regarded as one of the most
important molecules involved in the process of angiogenesis
(15). Labler et al
(16) reported that the levels of
VEGF in wound fluid were significantly higher in patients treated
with NPWT compared with those treated with gauze. Our previous
study showed an increase in VEGF levels using NPWT compared with a
traditional gauze treatment (17).
In addition, in this study significantly higher levels of VEGF were
observed in the wound following NPWT.
ANG-2 is an angiogenic factor associated with
maturation, stabilization and remodeling of vasculature (18), and promotion of endothelial cell
survival (19). Shyu et al
(20), observed a synergistic
effect of angiopoietin and VEGF in promoting angiogenesis. This
effect was implicated in the FOXC2-Ang2 signaling system, which is
crucial for controlling vascular function (21). It has not been previously reported
whether angiopoietin-2 levels alter following NPWT; however, in the
current study, a significant increase was observed following
NPWT.
PDGF is a co-factor that acts with VEGF to generate
stable angiogenesis (22), and it
has been reported to stimulate and enhance novel tissue formation
in bone and soft tissue models (23). Combination of Ang-2 and PDGF may
increase urokinase plasminogen-activator receptor expression
(24), which is significant in the
signaling pathways involved in wound healing. Shah et al
(25) observed that the
application of growth factors, such as PDGF, may aid in the healing
of chronic, non-healing wounds. EGF is secreted by platelets,
macrophages and fibroblasts, which act in a paracrine manner on
keratinocytes (26). An in
vitro study has shown that EGF was upregulated following acute
injury, thus significantly accelerating reepithelialization
(27). EGF and transforming growth
factor β (TGF-β) increase the tensile strength of the wound
(28). PlGF, an angiogenic
mediator promoting pathophysiological neovascularization, is
expressed during cutaneous wound healing (29). The diabetic mouse model of Erba
et al (30) indicated that
the NPWT device was associated with a significant activation of the
wound, expression of PDGF and EGF, superior granulation tissue
formation rich in collagen I, as well as superior wound
epithelialization. Shah et al (25) found that the application of growth
factors, including PDGF may aid in the healing of chronic,
non-healing wounds. In the current study, all three cytokines were
expressed at elevated levels following NPWT, however the increase
of PIGF was not identified to be statistically significant. A study
period longer than three days may demonstrate a significant
increase in PIGF due to its involvement in improving in wound
closure by enhancing angiogesis (29). A similar trend was observed with
angiogenin, a plasma protein with angiogenic and ribonucleolytic
activity implicated in wound healing. Further studies are required
to investigate the lack of significant difference in the present
study.
HGF is a multifunctional cytokine that is capable of
stimulating multiple intracellular signaling pathways to induce a
marked variety of biological activities in a wide spectrum of cell
types. HGF stimulates migration and proliferation of keratinocytes
and has been suggested to be involved in wound healing (31). Chen et al (32) also observed that HGF exhibits a
potential role in reepithelialization and cilium hyperplasia.
However, in the current study HGF levels were not identified to be
significantly different following NPWT, however it remained at a
high level and exhibited a rising tendency.
Leptin is a recently identified cell factor, which
promotes novel angiogenesis, and modulates inflammatory and immune
function (33). Murad et al
(34) reported that leptin is
acutely upregulated in the injured skin, and proposed that this
local production of leptin serves a critical functional role as an
autocrine/paracrine regulator of normal wound healing. A burn wound
rat model suggested that topical application of leptin promotes
reepithelization to shorten the wound healing time (35). An animal study by Kim et al
(36) indicated that HB-EGF is
involved in the wound-healing process. HB-EGF may reduce the
required frequency of EGF application since it may cause prolonged
EGFR signaling when immobilized in the wound (37). To the best of our knowledge, the
current study was the first to demonstrate the influence of NPWT on
leptin and HB-EGF; however, the results showed a low expression
level and the changes were not significant. This may indicate that
NPWT has less influence on leptin and HB-EGF.
As a single polypeptide, bFGF is one of the 22
members of the FGF family and is produced by a variety of cell
populations, mainly by activated macrophages and thrombocytes
(38). bFGF is involved in a
number of physiological and pathophysiological processes, including
growth, wound and bone healing, cell differentiation and
proliferation. Local administration of bFGF in skin flaps markedly
increased tissue viability and accelerated the wound healing
process (39). Jacobs et al
(12) designed an animal model in
which NPWT led to increased expression of VEGF and bFGF in the
first 5 days. However, in the current study, bFGF was observed at
low levels on the third day during NPWT. A clinical phenomenon may
indicate that incipient granulation tissue under NPWT easily
collapses if the negative pressure is removed. The granulation
tissue may lack collagen, which is not generated without bFGF. It
perhaps performs at a higher level in the later phase during NPWT,
thus, the tendency of bFGF under NPWT is hypothesized to increased
following initial decrease. To the best of our knowledge, this is
the first study to report this, thus, further studies are required
to confirm this mechanism.
In addition, the angiogenesis-associated growth
factors were not individually involved in angiogenesis, but
interacted with and activated each other to promote angiogenesis.
For example, in a study by Ko et al (40), VEGF enhanced neoangiogenesis,
however, this was not effective on the maturation of organized
reepithelization. The neoangiogenesis induced by EGF was not
dominant, but did enhance the maturation of organized
reepithelization. The key ability of bFGF is to induce angiogenesis
via stimulation of VEGF expression (41). There are a number of studies of
this, however these require further investigation. Investigation of
the association and effects in disparate wounds and different
phases may indicate whether angiogenesis modulation is involved in
combined wound therapy.
The cause of these changes in the
angiogenesis-associated growth factor levels and the underlying
mechanisms were not mentioned in the current study. Quinn et
al (42) showed that
mechanical stretching regulates VEGF and bFGF gene expression in
cultured pulmonary artery smooth muscle cells; however, the
specific mechanisms remain unclear. The authors hypothesize that
further research may provide an explanation. The factors detected
in the current study are limited, as a number of other cytokines,
including interleukin, TGF-β, insulin-like growth factor (IGF),
GM-CSF, matrix metal proteinase 9 (MMP-9) are also involved in
wound angiogenesis. Whether these cytokines are expressed
differently under subatmospheric pressure also requires further
investigation.
In conclusion, NPWT therapy can promote wound
healing, and the mechanism may include: significantly increasing
blood flows, and significant increases in VEGF, EGF, PDGF, ANG-2
and decreased bFGF after NPWT therapy. However, how these
angiogenesis-associated growth factors act under subatmospheric
pressure in wounds also requires further investigation.
Acknowledgements
This study was funded by grants from the National
Science Funds of China (project code, 81171713), and by the
Doctoral candidate Independent research of Wuhan University (grant
no. 2012303020203).
Reference
|
1
|
Armstrong DG, Lavery LA, Abu-Rumman P,
Espensen EH, Vazquez JR, Nixon BP and Boulton AJ: Outcomes of
subatmospheric pressure dressing therapy on wounds of the diabetic
foot. Ostomy Wound Manage. 48:64–68. 2002.PubMed/NCBI
|
|
2
|
Argenta LC and Morykwas MJ:
Vacuum-assisted closure: a new method for wound control and
treatment: clinical experience. Ann Plas Surg. 38:563–577. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murugesan S, Mousa SA, O’connor LJ,
Lincoln DW II and Linhardt RJ: Carbon inhibits vascular endothelial
growth factor- and fibroblast growth factor-promoted angiogenesis.
FEBS Lett. 581:1157–1160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fitzgerald SP, Lamont JV, McConnell RI and
Benchikh el O: Development of a high-throughput automated analyzer
using biochip array technology. Clin Chem. 51:1165–1176. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Laat EH, van den Boogaard MH, Spauwen
PH, van Kuppevelt DH, van Goor H and Schoonhoven L: Faster wound
healing with topical negative pressure therapy in difficult-to-heal
wounds: a prospective randomized controlled trial. Ann Plast Surg.
67:626–631. 2011.PubMed/NCBI
|
|
6
|
Morykwas MJ, Argenta LC, Shelton-Brown EI
and McGuirt W: Vacuum-assisted closure: a new method for wound
control and treatment: animal studies and basic foundation. Ann
Plast Surg. 38:553–562. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mouës CM, Vos MC, van den Bemd GJ, Stijnen
T and Hovius SE: Bacterial load in relation to vacuum-assisted
closure wound therapy: a prospective randomized trial. Wound Repair
Regen. 12:11–17. 2004.
|
|
8
|
Lee DL, Ryu AY and Rhee SC: Negative
pressure wound therapy: an adjuvant to surgical reconstruction of
large or difficult skin and soft tissue defects. Int Wound J.
8:406–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanasono MM and Skoracki RJ: Securing skin
grafts to microvascular free flaps using the vacuum-assisted
closure (VAC) device. Ann Plast Surg. 58:573–576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu L, Marti GP, Wei X, Zhang X, Zhang H,
Liu YV, Nastai M, Semenza GL and Harmon JW: Age-dependent
impairment of HIF-1alpha expression in diabetic mice: Correction
with electroporation-facilitated gene therapy increases wound
healing, angiogenesis, and circulating angiogenic cells. J Cell
Physiol. 217:319–327. 2008. View Article : Google Scholar
|
|
11
|
Wilgus TA, Matthies AM, Radek KA, Dovi JV,
Burns AL, Shankar R and DiPietro LA: Novel function for vascular
endothelial growth factor receptor-1 on epidermal keratinocytes. Am
J Pathol. 167:1257–1266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jacobs S, Simhaee DA, Marsano A, Fomovsky
GM, Niedt G and Wu JK: Efficacy and mechanisms of vacuum-assisted
closure (VAC) therapy in promoting wound healing: a rodent model. J
Plast Reconstr Aesthet Surg. 62:1331–1338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roesli C and Neri D: Methods for the
identification of vascular markers in health and disease: from the
bench to the clinic. J Proteomics. 11:2219–2229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferrara N: Molecular and biological
properties of vascular endothelial growth factor. J Mol Med (Berl).
77:527–543. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kadowaki I, Ichinohasama R, Harigae H,
Ishizawa K, Okitsu Y, Kameoka J and Sasaki T: Accelerated
lymphangiogenesis in malignant lymphoma: possible role of VEGF-A
and VEGF-C. Br J Haematol. 130:869–877. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Labler L, Rancan M, Mica L, Härter L,
Mihic-Probst D and Keel M: Vacuum-assisted closure therapy
increases local interleukin-8 and vascular endothelial growth
factor levels in traumatic wounds. J Trauma. 3:749–757. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou M, Yu A, Wu G, Xia C, Hu X and Qi B:
Role of different negative pressure values in the process of
infected wounds healing treated by vacuum-assisted closure: an
experimental study. Int Wound J. 10:508–515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maisonpierre PC, Suri C, Jones PF, et al:
Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo
angiogenesis. Science. 277:55–60. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harfouche R and Hussain SN: Signaling and
regulation of endothelial cell survival by angiopoietin-2. Am J
Physiol Heart Circ Physiol. 291:H1635–H1645. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shyu KG, Chang H and Isner JM: Synergistic
effect of angiopietin-1 and vascular endothelial growth factor on
neoangiogenesis in hypercholesterolemic rabbit model with acute
hindlimb ischemia. Life Sci. 73:563–579. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xue Y, Cao R, Nilsson D, et al: FOXC2
controls Ang-2 expression and modulates angiogenesis, vascular
patterning, remodeling, and functions in adipose tissue. Proc Natl
Acad Sci USA. 105:10167–10172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heldin CH and Westermark B: Mechanism of
action and in vivo role of platelet-derived growth factor. Physiol
Rev. 79:1283–1316. 1999.PubMed/NCBI
|
|
23
|
Jin Q, Wei G, Lin Z, Sugai JV, Lynch SE,
Ma PX and Giannobile WV: Nanofibrous scaffolds incorporating
PDGF-BB microspheres induce chemokine expression and tissue
neogenesis in vivo. PLoS One. 3:e17292008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bezuidenhout L, Bracher M, Davison G,
Zilla P and Davies N: Ang-2 and PDGF-BB cooperatively stimulate
human peripheral blood monocyte fibrinolysis. J Leukoc Biol.
81:1496–1503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shah JM, Omar E, Pai DR and Sood S:
Cellular events and biomarkers of wound healing. Indian J Plast
Surg. 45:220–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shiraha H, Glading A, Gupta K and Wells A:
IP-10 inhibits epidermal growth factor-induced motility by
decreasing epidermal growth factor receptor-mediated calpain
activity. J Cell Biol. 146:243–254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brown GL, Curtsinger L III, Brightwell JR,
et al: Enhancement of epidermal regeneration by biosynthetic
epidermal growth factor. J Exp Med. 163:1319–1324. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brown GL, Curtsinger LJ, White M, et al:
Acceleration of tensile strength of incisions treated with EGF and
TGF-beta. Ann Surg. 208:788–794. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cianfarani F, Zambruno G, Brogelli L, et
al: Placenta growth factor in diabetic wound healing: altered
expression and therapeutic potential. Am J Pathol. 169:1167–1182.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Erba P, Adini A, Demcheva M, Valeri CR and
Orgill DP: Poly-N-acetyl glucosamine fibers are synergistic with
vacuum-assisted closure in augmenting the healing response of
diabetic mice. J Trauma. 71:S187–S193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakamura T, Sakai K, Nakamura T and
Matsumoto K: Hepatocyte growth factor twenty years on: Much more
than a growth factor. J Gastroenterol Hepatol. 26:188–202.
2011.PubMed/NCBI
|
|
32
|
Chen M, Guan M, Li J, Wang H and Yang B:
Effects of hepatocyte growth factor on wound healing of rabbit
maxillary sinus mucosa. J Otolaryngol Head Neck Surg. 41:253–258.
2012.PubMed/NCBI
|
|
33
|
Goldberg AC, Goldberg-Eliaschewitz F,
Sogayar MC, Genre J and Rizzo LV: Leptin and the immune response:
an active player or an innocent bystander? Ann NY Acad Sci.
1153:184–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murad A, Nath AK, Cha ST, Demir E,
Flores-Riveros J and Sierra-Honigmann MR: Leptin is an
autocrine/paracrine regulator of wound healing. FASEB J.
17:1895–1897. 2003.PubMed/NCBI
|
|
35
|
Wen H, Wu G, Chen W, Yang H and Fu J:
Topical application of leptin promotes burn wound healing in rats.
Nan Fang Yi Ke Da Xue Xue Bao. 32:703–706. 2012.(In Chinese).
|
|
36
|
Kim JM, Bak EJ, Chang JY, Kim ST, Park WS,
Yoo YJ and Cha JH: Effects of HB-EGF and epiregulin on wound
healing of gingival cells in vitro. Oral Dis. 17:785–793. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tolino MA, Block ER and Klarlund JK: Brief
treatment with heparin-binding EGF-like growth factor, but not with
EGF, is sufficient to accelerate epithelial wound healing. Biochim
Biophys Acta. 1810:875–878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Robson MC, Phillips LG, Lawrence WT, et
al: The safety and effect of topically applied recombinant basic
fibroblast growth factor on the healing of chronic pressure sores.
Ann Surg. 216:401–406. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fayazzadeh E, Ahmadi SH, Rabbani S,
Boroumand MA, Salavati A and Anvari MS: A comparative study of
recombinant human basic fibroblast growth factor (bFGF) and
erythropoietin (EPO) in prevention of skin flap ischemic necrosis
in rats. Arch Iran Med. 15:553–556. 2012.PubMed/NCBI
|
|
40
|
Ko J, Jun H, Chung H, et al: Comparison of
EGF with VEGF non-viral gene therapy for cutaneous wound healing of
streptozotocin diabetic mice. Diabetes Metab J. 35:226–235. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hayward P, Hokanson J, Heggers J, Fiddes
J, Klingbeil C, Goeger M and Robson M: Fibroblast growth factor
reserves the bacterial retardation of wound contraction. Am J Surg.
163:288–293. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Quinn TP, Schlueter M, Soifer SJ and
Gutierrez JA: Cyclic mechanical stretch induces VEGF and FGF-2
expression in pulmonary vascular smooth muscle cells. Am J Physiol
Lung Cell Mol Physiol. 282:L897–L903. 2002.PubMed/NCBI
|