Introduction
Immediate-early genes are involved in acute stress
responses in the central nervous system (1). Stress results in the high expression
of immediate-early genes, including c-Fos, c-Jun, JunB and JunD, in
the hypothalamic paraventricular nucleus and the central amygdaloid
nucleus, suggesting that stress may affect neurotransmitter gene
expression through Fos and Jun proteins in these nuclei (2). Stress affects the
hypothalamic-pituitary-adrenal axis, resulting in constant
secretion of corticosterone from the adrenal gland (3). Immediate-early genes have been
considered to be reliable markers for the identification of
activated cells under stressful conditions (4,5).
Running at moderate or high intensity leads to a significant
elevation in the number of c-Fos-positive cells in the
paraventricular nucleus (6). c-Fos
and c-Jun are known to participate in cell growth, mitosis
(7,8), differentiation, development (9,10)
and the regulation of the expression of other genes (11,12).
Immediate-early genes have an important role in the regulation of
the nervous system in response to stress, and the expression of
these genes is used as a marker for neuronal activity in the brain
following exposure to stressful stimuli (13,14).
Exercise can be employed as a model of the temporary
immunosuppression that occurs following severe physical stress,
resulting in deterioration of the immune system and leading to
infection or cancer (15,16). Prolonged exhaustive exercise leads
to central fatigue and this type of fatigue limits endurance
exercise performance through limitation of brain function (17,18).
As a result, exhaustive treadmill running has been used as an
animal model of stressful conditions, and alleviation of brain
stress has been shown to result in an increase in the time to
exhaustion in response to treadmill running (19,20).
β-glucan is the major structural component of the
cell wall in yeast and fungi, including mushrooms. β-glucan
prevents oxidative injury, stimulates innate immune defenses,
exerts anti-tumor responses and increases resistance to a wide
variety of infections (21–23).
β-glucan also exhibits hypocholesterolemic and anti-coagulant
properties, making it a promising candidate for a pharmacological
promoter of health (21). Orally
administered water-soluble glucan is translocated from the
gastrointestinal tract into the systemic circulation and induces
protection against infection (22).
Numerous pharmacological effects of β-glucan have
been reported; however, the effects of β-glucan on the expression
of immediate-early genes under stressful conditions have yet to be
elucidated. In the present study, the effects of β-glucan on the
expression of c-Fos and c-Jun in the hypothalamus, dentate gyrus
and dorsal raphe following exhaustive treadmill running were
assessed.
Materials and methods
Animals
Adult male Sprague Dawley rats, weighing 203.7±12.8
g (seven weeks old) were obtained from a commercial breeder (Dae
Han Bio Link Co., Chungbuk, Korea). The animals were housed at a
controlled temperature (20±2°C) with a 12-h light/dark cycle (light
from 7.00 a.m. to 7.00 p.m.). Food and water were available ad
libitum. All experimental procedures were performed in
accordance with the Animal Care Guidelines of the National
Institutes of Health and the Korean Academy of Medical Sciences
(Seoul, Korea).
Animals were randomly divided into five groups:
Control, exercise, exercise and 50 mg/kg β-glucan treatment,
exercise and 100 mg/kg β-glucan treatment, and exercise and 200
mg/kg β-glucan treatment (n=10 in each group). The water-soluble
glucan (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) used in
this study was β-1,3-glucan in microparticulate form. The β-glucan
solution was diluted with drinking water to concentrations of 50,
100 and 200 mg/kg and was made fresh daily. Rats in the β-glucan
treatment groups were administered β-glucan at three different
doses (50, 100 and 200 mg/kg of body weight, respectively) per day
for seven days. The treatment was administered 60 min prior to the
start of exercise, which was conducted as described below. Rats in
the control and exercise groups received water by oral gavage once
per day for seven days.
Exercise protocols
The physical exercise load applied in the present
study consisted of running on a motor-driven treadmill without
inclination. Rats in the exercise groups were forced to run on a
treadmill for 30 min once per day during a period of six
consecutive days, whereas rats in the control group were left on
the treadmill without running for 30 min. The exercise load
consisted of forced running at a speed of 10 m/min for 10 min,
followed by 16 m/min for 10 min and then 21 m/min for a final 10
min.
On the seventh day of the experiment, the time to
exhaustion during treadmill running was determined for the exercise
groups. The time to exhaustion was defined as the time between the
commencement of exercise and the first occurrence of a failure to
keep up with the treadmill for a period of ≥3 min. The speeds used
for the determination of the time to exhaustion were 10 m/min for 5
min, followed by 16, 18, 21, 24, 26, 29, 32, 34 and 37 m/min for 3
min each and then 40 m/min until exhaustion, as described in a
previous study (19).
Brain preparation
The animals were sacrificed on the seventh day of
the experiments immediately following determination of the
exhaustion time. For preparation of the brains, the animals were
fully anesthetized with Zoletil 50® (10 mg/kg,
intraperitoneal; Vibac, Carros, France). The rats then received a
transcardial perfusion with 50 mM phosphate-buffered saline (PBS)
and were fixed with a freshly prepared solution of 4%
paraformaldehyde in 100 mM phosphate buffer (pH 7.4). The brains
were then removed, post-fixed in the same fixative overnight and
transferred to a 30% sucrose solution for cryoprotection. Coronal
sections with a thickness of 40 μm were prepared using a frozen
microtome (Leica, Nussloch, Germany).
Immunohistochemical detection of c-Jun
and c-Fos
Immunohistochemistry was performed for evaluation of
c-Fos- and c-Jun-positive cells in the dorsal raphe (bregma, from
−7.08 to −7.56 mm), the hypothalamus (bregma, from −1.80 to −1.92
mm) and the hippocampus (bregma, from −2.92 to −3.24 mm), according
to the previously described method (14,24).
The sections were incubated in PBS for 10 min and then washed three
times in the same buffer. The sections were subsequently incubated
in 1% hydrogen peroxide (H2O2) for 30 min,
prior to being incubated overnight with rabbit anti-c-Fos antibody
(1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
rabbit anti-c-Jun antibody (1:1,000; Santa Cruz Biotechnology,
Inc.). The sections were then incubated for 1 h with biotinylated
anti-rabbit secondary antibody (1:200; Vector Laboratories, Inc.,
Burlingame, CA, USA) for c-Fos and c-Jun immunohistochemistry. The
sections were subsequently incubated with avidin-biotin-peroxidase
complex (1:100; Vector Laboratories, Inc.) for 1 h at room
temperature. For staining, the sections were incubated in a
solution consisting of 0.05% 3,3′-diaminobenzidine
tetrahydrochloride and 0.03% H2O2 in 50 mM
Tris-HCl (pH 7.6) for ~5 min, washed with PBS and mounted onto
gelatin-coated slides. The slides were air-dried overnight at room
temperature and coverslips were mounted using Permount mounting
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
numbers of c-Fos- and c-Jun-positive cells in the dorsal raphe, the
hypothalamus and hippocampus were counted hemilaterally using a
microscope (Olympus, Tokyo, Japan).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Data were analyzed by one-way analysis of variance,
followed by Duncan’s post hoc test using SPSS software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of β-glucan on time to exhaustion
during treadmill running
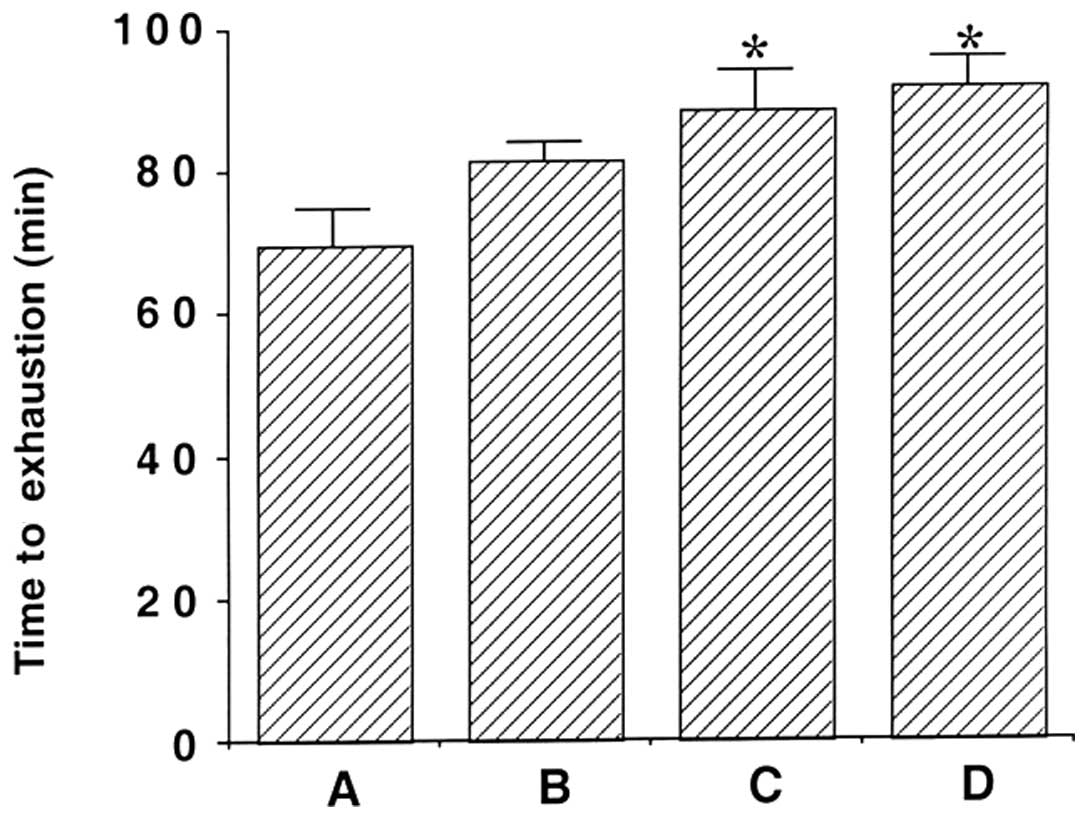
The effects of β-glucan on time to exhaustion are
shown in Fig. 1. The average
running time until fatigue was 69.88±5.16 min in the exercise,
81.66±2.34 min in the 50 mg/kg β-glucan-treated, 88.66±5.42 min in
the 100 mg/kg β-glucan-treated and 91.77±4.04 min in the 200 mg/kg
β-glucan-treated groups. These findings indicate that the time to
exhaustion in response to treadmill running increased following
treatment with β-glucan (P<0.05). In particular, treatment with
100 and 200 mg/kg β-glucan resulted in a significant increase in
the time to exhaustion by treadmill running as compared with the
exercise group (P<0.05).
Effect of β-glucan on the expression of
c-Fos in the hypothalamus, dentate gyrus and dorsal raphe
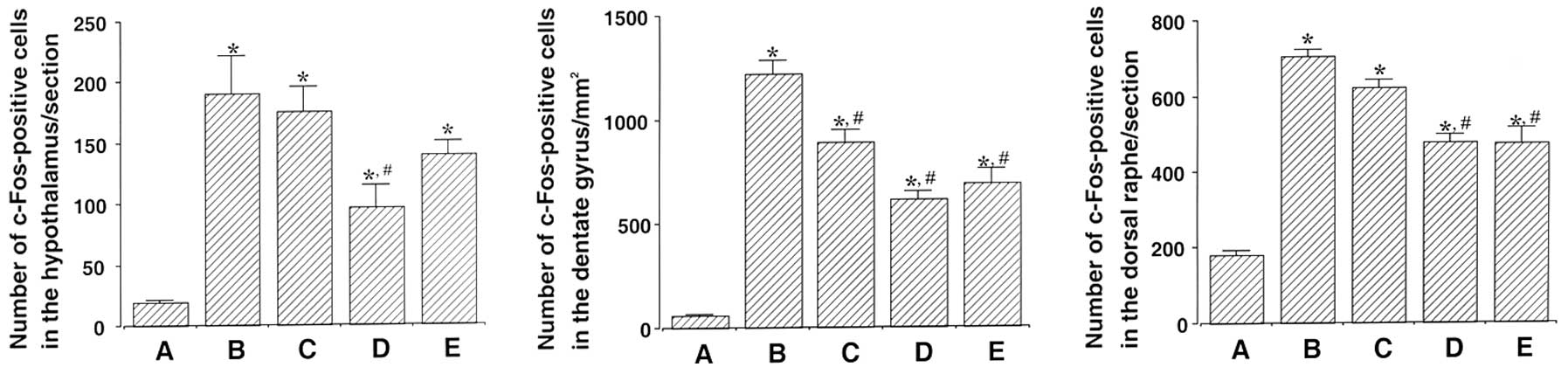
Photomicrographs of c-Fos-positive cells in the
hypothalamus, dentate gyrus and dorsal raphe are shown in Fig. 2. The number of c-Fos-positive cells
in the hypothalamus was 19.28±1.78 in the control, 190.71±30.56 in
the exercise, 175.42±19.98 in the 50 mg/kg β-glucan-treated,
98.14±18.54 in the 100 mg/kg β-glucan-treated and 133.57±10.35 in
the 200 mg/kg β-glucan-treated groups. These results demonstrate
that treadmill exercise led to increased expression of c-Fos in the
hypothalamus (P<0.05) and that treatment with β-glucan resulted
in the suppression of the exercise-induced expression of c-Fos
(P<0.05). In particular, treatment with β-glucan at 100 mg/kg
resulted in the statistically significant suppression of c-Fos
expression in the hypothalamus as compared with the exercise group
(P<0.05) (Fig. 3).
The number of c-Fos-positive cells in the dentate
gyrus was 57.50±3.43 in the control, 1,218.66±65.97 in the
exercise, 888.66±56.14 in the 50 mg/kg β-glucan-treated,
614.66±37.38 in the 100 mg/kg β-glucan-treated and 688.83±73.88 in
the 200 mg/kg β-glucan-treated groups. These results demonstrate
that treadmill exercise led to increased expression of c-Fos in the
dentate gyrus (P<0.05) and that treatment with β-glucan resulted
in the suppression of the exercise-induced expression of c-Fos
(P<0.05). In particular, treatment with β-glucan at 100 and 200
mg/kg resulted in the statistically significant suppression of
c-Fos expression in the dentate gyrus as compared with the exercise
group (P<0.05) (Fig. 3).
The number of c-Fos-positive cells in the dorsal
raphe was 180.42±13.19 in the control, 705.00±19.11 in the
exercise, 623.00±19.71 in the 50 mg/kg β-glucan-treated,
476.57±20.41 in the 100 mg/kg β-glucan-treated and 475.00±40.13 in
the 200 mg/kg β-glucan-treated groups. These results demonstrate
that treadmill exercise led to increased expression of c-Fos in the
dorsal raphe (P<0.05) and that treatment with β-glucan resulted
in the suppression of the exercise-induced expression of c-Fos
(P<0.05). In particular, treatment with β-glucan at 100 and 200
mg/kg resulted in the statistically significant suppression of
c-Fos expression in the dorsal raphe as compared with the exercise
group (P<0.05) (Fig. 3).
Effect of β-glucan on expression of c-Jun
in the hypothalamus, dentate gyrus and dorsal raphe
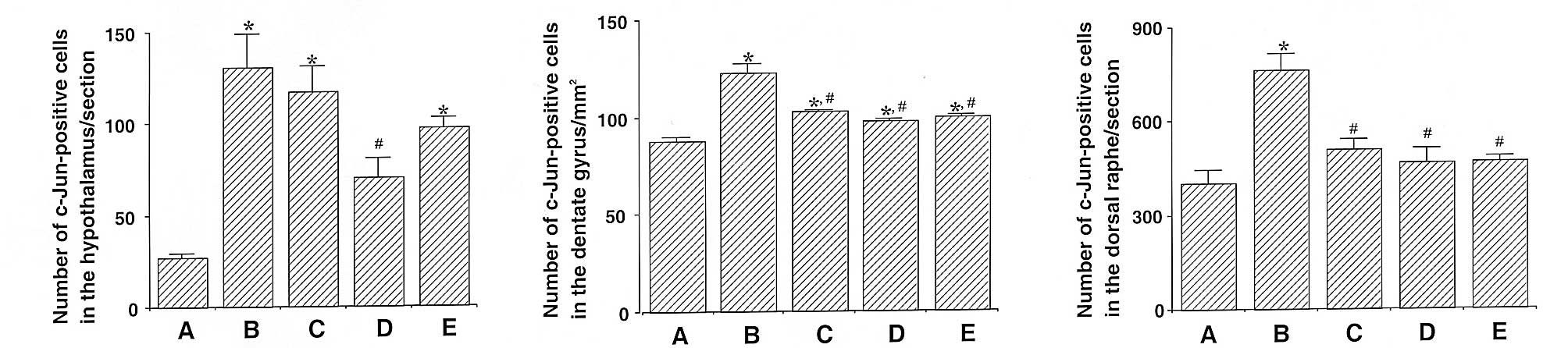
Photomicrographs of c-Jun-positive cells in the
hypothalamus, dentate gyrus and dorsal raphe are shown in Fig. 4. The number of c-Jun-positive cells
in the hypothalamus was 26.88±2.45 in the control, 130.88±17.85 in
the exercise, 117.22±13.73 in the 50 mg/kg β-glucan-treated,
70.55±10.34 in the 100 mg/kg β-glucan-treated and 97.22±5.65 in the
200 mg/kg β-glucan-treated groups. These results demonstrate that
treadmill exercise led to increased expression of c-Jun in the
hypothalamus (P<0.05) and that treatment with β-glucan resulted
in the suppression of the exercise-induced expression of c-Jun
(P<0.05). In particular, treatment with β-glucan at 100 mg/kg
resulted in the statistically significant suppression of c-Jun
expression in the hypothalamus as compared with the exercise group
(P<0.05) (Fig. 5).
The number of c-Jun-positive cells in the dentate
gyrus was 88.00±2.20 in the control, 122.40±4.61 in the exercise,
103.05±0.65 in the 50 mg/kg β-glucan-treated, 97.83±1.02 in the 100
mg/kg β-glucan-treated and 99.59±1.08 in the 200 mg/kg
β-glucan-treated groups. These results demonstrate that treadmill
exercise led to increased expression of c-Jun in the dentate gyrus
(P<0.05) and that treatment with β-glucan resulted in the
suppression of the exercise-induced expression of c-Jun
(P<0.05). In particular, treatment with β-glucan resulted in the
statistically significant suppression of c-Jun expression in the
dentate gyrus as compared with the exercise group (P<0.05)
(Fig. 5).
The number of c-Jun-positive cells in the dorsal
raphe was 401.33±43.19 in the control, 763.50±51.96 in the
exercise, 511.50±31.78 in the 50 mg/kg β-glucan-treated,
468.16±43.82 in the 100 mg/kg β-glucan-treated and 472.16±13.77 in
the 200 mg/kg β-glucan-treated groups. These results demonstrate
that treadmill exercise led to increased expression of c-Jun in the
dorsal raphe (P<0.05) and that treatment with β-glucan resulted
in the suppression of the exercise-induced expression of c-Jun
(P<0.05). In particular, treatment with β-glucan resulted in
statistically significant suppression of c-Jun expression in the
dorsal raphe as compared with the exercise group (P<0.05)
(Fig. 5).
Discussion
Previous studies have used the expression of c-Fos
and c-Jun as a marker for stimuli-induced changes in the metabolic
activity of neurons under various conditions (1,2,25,26).
The application of stressful stimuli resulted in the enhanced
expression of c-Fos and c-Jun (6,14,24,27).
The expression of four immediate-early genes (c-Fos, c-Jun, JunB
and NGFI-B) was increased following 30 min of global ischemia
(24). Rats receiving predator
stress during pregnancy exhibited anxiety-like behavior with higher
expression of c-Fos in the hypothalamus and locus coeruleus
following delivery (14). Running
at a moderate speed (25 m/min) led to increased induction of c-Fos
in various hypothalamic regions; however, running at a low speed
(15 m/min) had no effect on the expression of c-Fos (6). Expression of c-Fos in the brain areas
associated with micturition was enhanced by the induction of stress
urinary incontinence and the suppression of c-Fos expression led to
amelioration of the symptoms of stress urinary incontinence
(27). These results suggest that
neuronal activation in the micturition centers was induced by
stress urinary incontinence. The performance of acute exercise led
to upregulated expression of c-Fos and c-Jun through activation of
the mitogen-activated protein kinase signaling pathway (28). According to the present study,
expression of c-Fos and c-Jun in the hypothalamus, dentate gyrus
and dorsal raphe was enhanced by exhaustive treadmill running
(Figs. 3 and 5), indicating that strenuous exercise
acted as a form of physical stress and resulted in the enhancement
of c-Fos and c-Jun expression in the brain.
Pharmacological agents acting as ergogenic aids
through a reduction in fatigue have been widely investigated
(17–19). Caffeine and Paeonia radix exhibited
ergogenic effects during exhaustive treadmill running (17,18).
Seo et al (19) reported
that Phellinus linteus enhanced the time to exhaustion in
response to treadmill running. Phellinus linteus has been
used for the treatment of gastroenteric disorders, inflammation and
lymphatic diseases (29–31). β-glucan also originates from
Phellinus linteus, and the immunological response to
β-glucans isolated from medicinal mushrooms has been well
documented (32). Prolonged and
high-intensity exercise in endurance athletes leads to a
deterioration in immune function and increased risk of upper
respiratory tract infections. The positive immunomodulatory effect
of β-glucan on immunocompetent cells has been reported (33). Treatment with β-glucan increased
production of interleukin-2, -4 and -5 and interferon-γ in blood
leucocytes, suggesting that β-glucan may alter immune function
following strenuous exercise (34). β-glucan from dietary oats reduced
the body weight and increased the maximum running time compared
with that in control rats (35).
In addition, β-glucan from dietary oats decreased the levels of
blood urea nitrogen, lactic acid and creatine kinase activity in
the serum and increased the levels of non-esterified fatty acids
and lactic dehydrogenase activity in the serum as well as the
content of liver glycogen. These results suggest that β-glucan from
dietary oats can enhance the endurance capacity through the
facilitation of recovery from fatigue (35). In the present study, treatment with
β-glucan resulted in enhanced time to exhaustion in treadmill
running (Fig. 1). Treatment with
β-glucan also led to the alleviation of the exercise-induced
increase in c-Fos and c-Jun expression in the hypothalamus, dentate
gyrus and dorsal raphe (Figs. 3
and 5). These results suggest that
β-glucan acted as an ergogenic aid during strenuous exercise
through the suppression of stress-related neuronal parameters,
including c-Fos and c-Jun, in the brain.
In conclusion, exposure to stressful stimuli, such
as strenuous exercise, results in the enhanced expression of c-Fos
and c-Jun in the brain. β-glucan reduces exercise-induced stress
through downregulation of c-Fos and c-Jun following exhaustive
exercise.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea Grant funded by the Korean Government
(no. NRF-2009-351-G00080).
References
|
1
|
Schreiber SS, Tocco G, Shors TJ and
Thompson RF: Activation of immediate early genes after acute
stress. Neuroreport. 2:17–20. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Honkaniemi J, Kainu T, Ceccatelli S,
Rechardt L, Hökfelt T and Pelto-Huikko M: Fos and jun in rat
central amygdaloid nucleus and paraventricular nucleus after
stress. Neuroreport. 3:849–852. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zamora-González EO, Santerre A,
Palomera-Avalos V and Morales-Villagrán A: A chronic combinatory
stress model that activates the HPA axis and avoids habituation in
BALB/C mice. J Neurosci Methods. 213:70–75. 2013.PubMed/NCBI
|
|
4
|
Imaki T, Shibasaki T, Chikada N, Harada S,
Naruse M and Demura H: Different expression of immediate-early
genes in the rat paraventricular nucleus induced by stress:
relation to corticotropin-releasing factor gene transcription.
Endocr J. 43:629–638. 1996. View Article : Google Scholar
|
|
5
|
Singewald N, Salchner P and Sharp T:
Induction of c-Fos expression in specific areas of the fear
circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry.
53:275–283. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soya H, Mukai A, Deocaris CC, Ohiwa N,
Chang H, Nishijima T, Fujikawa T, Togashi K and Saito T:
Threshold-like pattern of neuronal activation in the hypothalamus
during treadmill running: establishment of a minimum running stress
(MRS) rat model. Neurosci Res. 58:341–348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kovary K and Bravo R: Expression of
different Jun and Fos proteins during the G0-to-G1 transition in
mouse fibroblasts: in vitro and in vivo associations. Mol Cell
Biol. 11:2451–2459. 1991.PubMed/NCBI
|
|
8
|
Kovary K and Bravo R: The jun and fos
protein families are both required for cell cycle progression in
fibroblasts. Mol Cell Biol. 11:4466–4472. 1991.PubMed/NCBI
|
|
9
|
Caubet JF: c-fos proto-oncogene expression
in the nervous system during mouse development. Mol Cell Biol.
9:2269–2272. 1989.PubMed/NCBI
|
|
10
|
Wilkinson DG, Bhatt S, Ryseck RP and Bravo
R: Tissue-specific expression of c-jun and junB during
organogenesis in the mouse. Development. 106:465–471.
1989.PubMed/NCBI
|
|
11
|
Akins PT, Liu PK and Hsu CY: Immediate
early gene expression in response to cerebral ischemia. Friend or
foe? Stroke. 27:1682–1687. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheng M and Greenberg ME: The regulation
and function of c-fos and other immediate early genes in the
nervous system. Neuron. 4:477–485. 1990. View Article : Google Scholar
|
|
13
|
Jung JA, Yoo KS and Hwang KK: Changes of
c-Fos immunoreactivity in midbrain by deep pain and effects of
aspirin. Korean J Pediatr. 46:695–701. 2003.
|
|
14
|
Seo JH, Kim TW, Kim CJ, Sung YH and Lee
SJ: Treadmill exercise during pregnancy ameliorates post-traumatic
stress disorder-induced anxiety-like responses in maternal rats.
Mol Med Rep. 7:389–395. 2013.PubMed/NCBI
|
|
15
|
Kohut ML, Boehm GW and Moynihan JA:
Prolonged exercise suppresses antigen-specific cytokine response to
upper respiratory infection. J Appl Physiol (1985). 90:678–684.
2001.PubMed/NCBI
|
|
16
|
Pedersen BK and Hoffman-Goetz L: Exercise
and the immune system: regulation, integration, and adaptation.
Physiol Rev. 80:1055–1081. 2000.PubMed/NCBI
|
|
17
|
Hong JA, Chung SH, Lee JS, Kim SS, Shin
HD, Kim H, Jang MH, Lee TH, Lim BV, Kim YP and Kim CJ: Effects of
Paeonia radix on 5-hydroxytryptamine synthesis and tryptophan
hydroxylase expression in the dorsal raphe of exercised rats. Biol
Pharm Bull. 26:166–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim BV, Jang MH, Shin MC, Kim HB, Kim YJ,
Kim YP, Chung JH, Kim H, Shin MS, Kim SS, et al: Caffeine inhibits
exercise-induced increase in tryptophan hydroxylase expression in
dorsal and median raphe of Sprague-Dawley rats. Neurosci Lett.
308:25–28. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seo JH, Sung YH, Kim KJ, Shin MS, Lee EK
and Kim CJ: Effects of Phellinus linteus administration on
serotonin synthesis in the brain and expression of monocarboxylate
transporters in the muscle during exhaustive exercise in rats. J
Nutr Sci Vitaminol (Tokyo). 57:95–103. 2011.
|
|
20
|
Lee MK, Lim HH, Song YK, Ko IG, Kim H,
Shin MS, Lee TH, Kim CJ, Joo KJ, Park JK and Choi HH: Effects of
the mixture of red ginseng and Paeonia radix on the treadmill
running and swimming exercise in rats. J Exerc Nutr Biochem.
10:255–263. 2006.(In Korean).
|
|
21
|
Mantovani MS, Bellini MF, Angeli JP,
Oliveira RJ, Silva AF and Ribeiro LR: beta-Glucans in promoting
health: prevention against mutation and cancer. Mutat Res.
658:154–161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rice PJ, Adams EL, Ozment-Skelton T,
Gonzalez AJ, Goldman MP, Lockhart BE, Barker LA, Breuel KF, Deponti
WK, Kalbfleisch JH, et al: Oral delivery and gastrointestinal
absorption of soluble glucans stimulate increased resistance to
infectious challenge. J Pharmacol Exp Ther. 314:1079–1086. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hong F, Yan J, Baran JT, Allendorf DJ,
Hansen RD, Ostroff GR, Xing PX, Cheung NK and Ross GD: Mechanism by
which orally administered beta-1,3-glucans enhance the tumoricidal
activity of antitumor monoclonal antibodies in murine tumor models.
J Immunol. 173:797–806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neumann-Haefelin T, Wiessner C, Vogel P,
Back T and Hossmann KA: Differential expression of the immediate
early genes c-fos, c-jun, junB, and NGFI-B in the rat brain
following transient forebrain ischemia. J Cereb Blood Flow Metab.
14:206–216. 1994. View Article : Google Scholar
|
|
25
|
Hiroi N, Brown JR, Haile CN, Ye H,
Greenberg ME and Nestler EJ: FosB mutant mice: loss of chronic
cocaine induction of Fos-related proteins and heightened
sensitivity to cocaine’s psychomotor and rewarding effects. Proc
Natl Acad Sci USA. 94:10397–10402. 1997.PubMed/NCBI
|
|
26
|
Dragunow M and Faull R: The use of c-Fos
as a metabolic marker in neuronal pathway tracing. J Neurosci
Methods. 29:261–265. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ko IG, Kim SE, Kim CJ, Jung JH, Lee SJ,
Kim DH, Lee KY and Kim KH: Effect of treadmill exercise on
leak-point pressure and neuronal activation in brain of rats with
stress urinary incontinence. Int Neurourol J. 14:141–148. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoene M, Franken H, Fritsche L, Lehmann R,
Pohl AK, Häring HU, Zell A, Schleicher ED and Weigert C: Activation
of the mitogen-activated protein kinase (MAPK) signalling pathway
in the liver of mice is related to plasma glucose levels after
acute exercise. Diabetologia. 53:1131–1141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han SB, Lee CW, Jeon YJ, Hong ND, Yoo ID,
Yang KH and Kim HM: The inhibitory effect of polysaccharides
isolated from Phellinus linteus on tumor growth and
metastasis. Immunopharmacology. 41:157–164. 1999. View Article : Google Scholar
|
|
30
|
Song YS, Kim SH, Sa JH, Jin C, Lim CJ and
Park EH: Anti-angiogenic, antioxidant and xanthine oxidase
inhibition activities of the mushroom Phellinus linteus. J
Ethnopharmacol. 88:113–116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim SH, Song YS, Kim SK, Kim BC, Lim CJ
and Park EH: Anti-inflammatory and related pharmacological
activities of the n-BuOH subfraction of mushroom Phellinus
linteus. J Ethnopharmacol. 93:141–146. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zaidman BZ, Yassin M, Mahajna J and Wasser
SP: Medicinal mushroom modulators of molecular targets as cancer
therapeutics. Appl Microbiol Biotechnol. 67:453–468. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Majtan J: Pleuran (β-glucan from
Pleurotus ostreatus): an effective nutritional supplement
against upper respiratory tract infections? Med Sport Sci.
59:57–61. 2012.
|
|
34
|
Carpenter KC, Breslin WL, Davidson T,
Adams A and McFarlin BK: Baker’s yeast β-glucan supplementation
increases monocytes and cytokines post-exercise: implications for
infection risk? Br J Nutr. 109:478–486. 2013.
|
|
35
|
Xu C, Lv J, Lo YM, Cui SW, Hu X and Fan M:
Effects of oat β-glucan on endurance exercise and its anti-fatigue
properties in trained rats. Carbohydr Polym. 92:1159–1165.
2013.
|