Introduction
Tea is known for its laxative effects and may be
found in a variety of dietary supplements, weight loss teas and
colon cleaning preparations (1).
In general, tea is used as a drink, food or medicine. Tea has been
recognized in different systems of traditional medicine for the
treatment of different diseases and ailments, however there are a
few studies available on the clinical uses of herbs as constipation
treatments that have demonstrated promising results, particularly
Pu-erh tea. Pu-erh tea is commonly known as the large leaves of
C. sinensis O. kuntze var. assamica Kitamura, a plant
native to China (2). Drinking
Pu-erh tea for a long period of time is able to aid the maintenance
of mental and physical health. This tea is believed to reduce high
blood pressure and cholesterol levels, and to be important in
preventing heart disease and cancer (3). However, the preventive effect of
Pu-erh tea on activated carbon-induced constipation in mice has not
been clearly studied.
Constipation is medically defined as fewer than
three stools per week and severe constipation as less than one
stool per week. Constipation occurs when the colon absorbs too much
water (4). In the present study,
mice were orally administered activated carbon, which attached to
the gastrointestinal mucosal surfaces and reduced the drainage of
the gastrointestinal tract, reduced gastrointestinal fluid and
slowed down gastrointestinal movement, and above all resulted in
weakness of the spleen and the stomach, thereby establishing a
model of constipation.
In previous studies, constipation induced by
activated carbon was used to demonstrate the effects of drugs for
the treatment of constipation (5,6). A
large number of studies also demonstrated that a megadose of
activated carbon is able to induce digestive tract obstruction
(7). Therefore, in the present
study, we examined the functional effects of Pu-erh tea in the
enteron alimentary tract by adopting the activated carbon-induced
constipation mice model to provide insights into to the inhibitory
effect of Pu-erh tea using various concentrations. We examined body
weight, dietary intake, drinking water amount, gastrointestinal
transit, first black stool defecation time and used a serum assay
to measure motilin (MTL), gastrin (Gas), endothelin (ET),
somatostatin (SS), acetylcholine enzyme (AchE), substance P (SP)
and vasoactive intestinal peptide (VIP) levels. In the present
study, we used bisacodyl, a laxative drug that is able to stimulate
intestinal peristalsis by working directly on the colon to produce
a bowel movement, as a positive control. It is typically prescribed
for the relief of constipation and for the management of neurogenic
bowel dysfunction as well as part of bowel preparation prior to
medical examinations (8,9).
Materials and methods
Preparations of Pu-erh tea
Seven-son tea cake Pu-erh tea was purchased from
Menghai County Yunhai Tea Factory (Yunan, China). The Pu-erh tea
was stored at −80°C and freeze-dried to produce a powder. A 20-fold
volume of boiling water was added to the powdered sample and
extracted twice. The water extract was evaporated using a rotary
evaporator (N-1100; Eyela-Tokyo Rikakikai Co., Ltd., Tokyo, Japan),
concentrated and then dissolved in dimethylsulfoxide (DMSO;
Amresco, Solon, OH, USA) to adjust to the stock concentration (20%,
w/v).
Liquid chromatography-mass spectrometry
(LC-MS) analysis
The tea extracts were dissolved in DMSO to produce a
final concentration of 10 mg/ml and then diluted with 50% methanol
to make a final concentration of 2 mg/ml. The diluted sample (5 μl)
was analyzed by liquid chromatography followed by tandem mass
spectrometry (LC-MS/MS). LC-MS/MS was performed using a Finnigan
LCQ Advantage MAX ion trap mass spectrometer (Thermo Electron Co.,
Waltham, MA, USA) equipped with an electrospray ionization source.
Separation by HPLC was performed with a Finnigan Surveyor Modular
HPLC System (Thermo Electron Co.) using an Xterra MS C18 column (5
μm, 2.1×150 mm; Waters Corp., Dublin, Ireland). Mobile phase A was
water and mobile phase B was acetonitrile; the two contained 0.1%
formic acid. Gradient elution at a flow rate of 0.2 ml/min was
performed as follows: 0–5 min with 0–40% B (linear gradient), 5–20
min with 40–80% B (linear gradient), 20–25 min with 80–100% B
(linear gradient) and 25–30 min with 100% B (isocratic). Full-scan
mass spectra were obtained in the positive and negative ion modes
at a range of m/z 100–1,000. For identifying compound structures,
data obtained from the MS/MS analysis were compared with that from
an MS/MS spectral library search (10).
Animals
This study followed a protocol approved by the
Animal Ethics Committee of Chongqing Medical University (Chongqing,
China). Seven-week-old female ICR mice (n=120) were purchased from
the Experimental Animal Center of Chongqing Medical University.
They were maintained in a temperature controlled (temperature,
25±2°C; relative humidity, 50±5%) facility with a 12 h light/dark
cycle and free access to a standard rat chow diet and water.
Induction of constipation in mice
To investigate the preventive effects of Pu-erh tea
against activated carbon-induced constipation, the animals were
divided into 6 groups of 10 mice each. The experimental design was
as follows: the normal group was administered arabic gum water for
9 days and a single dose of vehicle treatment (0.2 ml of 10% arabic
gum, w/w). The activated carbon control group received oral
administration of activated carbon (0.2 ml of 10% activated carbon,
w/w; activated carbon dissolved in 10% arabic gum) at 6:00 p.m.
from the sixth to ninth day to induce constipation. The sample and
activated carbon groups were orally administered a dose of 200, 400
and 800 mg/kg body weight of Pu-erh tea extract and 100 mg/kg body
weight of bisacodyl dissolved in water at 10:00 a.m. everyday for 9
days and constipation was induced as mentioned. The body weight,
diet intake, water intake, stool weight and stool moisture were
determined at 9:00 a.m. everyday.
Gastrointestinal transit and defecation
time
Mice fasted for 16 h from the ninth day at 6:00
a.m., but were not deprived of water. Following 16 h, the mice from
the control and sample groups received oral administration of 10%
activated carbon and the mice in the normal group were administered
10% arabic gum. After 30 min, mice were sacrificed by cervical
dislocation under anesthesia with diethyl ether. The 10 mice from
each group were dissected and the small intestine from the pylorus
to the blind intestine was carefully removed. The gastrointestinal
transit of each mouse was calculated as the percentage of the
distance traveled by the activated carbon meal relative to the
total length of the small intestine. The equation was used to
calculate GI transit (%): GI transit (%) = (distance traveled by
the activated carbon)/(total length of the small intestine) × 100.
The last 10 mice of each group were used to determine the first
black stool time following 10% oral administration with activated
carbon.
MTL, Gas, ET, SS, AchE, SP and VIP levels
in serum
MTL, Gas, ET, SS, AchE, SP and VIP levels in the
serum were determined using radioimmunoassay kits (Beijing Puer
Weiye Biotechnology Co., Ltd., Beijing, China).
Statistical analysis
Data are presented as the mean ± SD. Differences
between the mean values for individual groups were assessed using
one-way ANOVA with Duncan’s multiple range test. P<0.05 was
considered to indicate a statistically significant difference. SAS
version 9.1 (SAS Institute Inc., Cary, NC, USA) was used for
statistical analyses.
Results
Component identification of Pu-erh
tea
In an initial LC-MS/MS analysis of the methanol
extract of Pu-erh tea, there were 14 important peaks of Pu-erh tea.
These peaks demonstrated that the important components of Pu-erh
tea were gallic acid (1.70 min, m/z 169), resorcylic acid
(3.43 min, m/z 153), epigallocatechin (9.10 min, m/z
305), catechin (9.30 min, m/z 289), caffeine (9.62 min,
m/z 195), epicatechin (10.73 min, m/z 289),
epigallocatechin gallate (10.87 min, m/z 457), epicatechin
gallate (12.51 min, m/z 441), quercetin-3-galactoside (12.74
min, m/z 463), kaempferol-3-rutinoside (13.24 min,
m/z 593), kaempferol-3-glucoside (13.51 min, m/z
447), quercetin (15.39 min, m/z 301), kaempferol (17.37 min,
m/z 285) and lutein (25.09 min, m/z 568) (Fig. 1).
 | Figure 1Mass spectra (upper ones of each peak)
and MS/MS fragmentation patterns (lower ones of each peak) of 14
important peaks of Pu-erh tea. (A) Gallic acid [m/z 169,
(M−H)−]; (B) resorcylic acid (m/z 153); (C)
epigallocatechin [m/z 305, (M−H)−]; (D) catechin
(m/z 289, (M−H)−]; (E) caffeine [m/z 195,
(M+H)+]; (F) epicatechin [m/z 269,
(M−H)−]; (G) epigallocatechin gallate [m/z 457,
(M−H)−]; (H) epicatechin gallate [m/z 441,
(M−H)−]; (I) quercetin-3-galactoside [m/z 463,
(M−H)−]; (J) kaempferol-3-rutinoside [m/z 593,
(M−H)−]; (K) kaempferol-3-glucoside [m/z 447,
(M−H)−]; (L) quercetin [m/z 301,
(M−H)−]; (M) kaempferol (m/z 285); (N) lutein
[m/z 568, (M+H)+]. |
Body weight, diet intake, water intake,
stool weight and stool moisture of mice
The weight of mice among the normal, control,
bisacodyl and Pu-erh tea 200, 400 and 800 mg/kg dose groups
increased from the first to the sixth day (Fig. 2). Taking activated charcoal induced
constipation from the sixth day and the weight of normal group mice
increased continually. The weight of the bisacodyl group mice
increased less than that in the normal group and the weight of the
mice in the control group decreased substantially. The body weight
of mice in the Pu-erh tea dose groups also decreased and the weight
of the higher concentration dose group mice reduced slowly.
Constipation refers to bowel movements that are
infrequent or hard to pass. Therefore, defecation is the most
important criterion of constipation. From the first to the sixth
day, defecation weight, particle counts of defecation and water
content of defecation in each group was not significantly
different. Defecation weight and particle counts of defecation in
the bisacodyl group mice were slightly higher than the normal group
and the water content of defecations was slightly higher (Table I). Induced constipation, starting
from the seventh to the ninth day, decreased defecation weight,
particle counts and the water content of defecation. The defecation
weight, particle counts and water content of defecation of higher
concentration Pu-erh tea and bisacodyl dose mice demonstrated
little influence, close to those of the normal mice. Through the
observation of the samples treated mice which reduce anorexia, it
was determined that Pu-erh tea has a powerful inhibitory effect on
constipation, as does bisacodyl.
 | Table IDefecation status of mice during the
experiment when the mice were treated with activated carbon. |
Table I
Defecation status of mice during the
experiment when the mice were treated with activated carbon.
| | | | Pu-erh tea
(mg/kg) |
|---|
| | | |
|
|---|
| Content | Normal | Control | Bisacodyl | 200 | 400 | 800 |
|---|
| 1–6 day (dose with
samples) |
| Defecation weight
(g) | 0.89±0.12 | 0.86±0.08 | 1.05±0.10 | 0.90±0.08 | 0.92±0.07 | 0.94±0.05 |
| Particle counts of
defecation | 36±6 | 34±9 | 48±4 | 40±8 | 41±8 | 43±9 |
| Water content of
defecation (%) | 47±6 | 48±3 | 55±5 | 51±7 | 51±5 | 52±6 |
| 7–9 day (dose with
samples and activated carbon) |
| Defecation weight
(g) | 0.87±0.06 | 0.24±0.03 | 0.65±0.10 | 0.45±0.05 | 0.51±0.06 | 0.55±0.06 |
| Particle counts of
defecation | 38±4 | 16±6 | 42±3 | 28±3 | 31±4 | 35±3 |
| Water content of
defecation (%) | 48±5 | 13±2 | 42±5 | 30±3 | 33±4 | 37±4 |
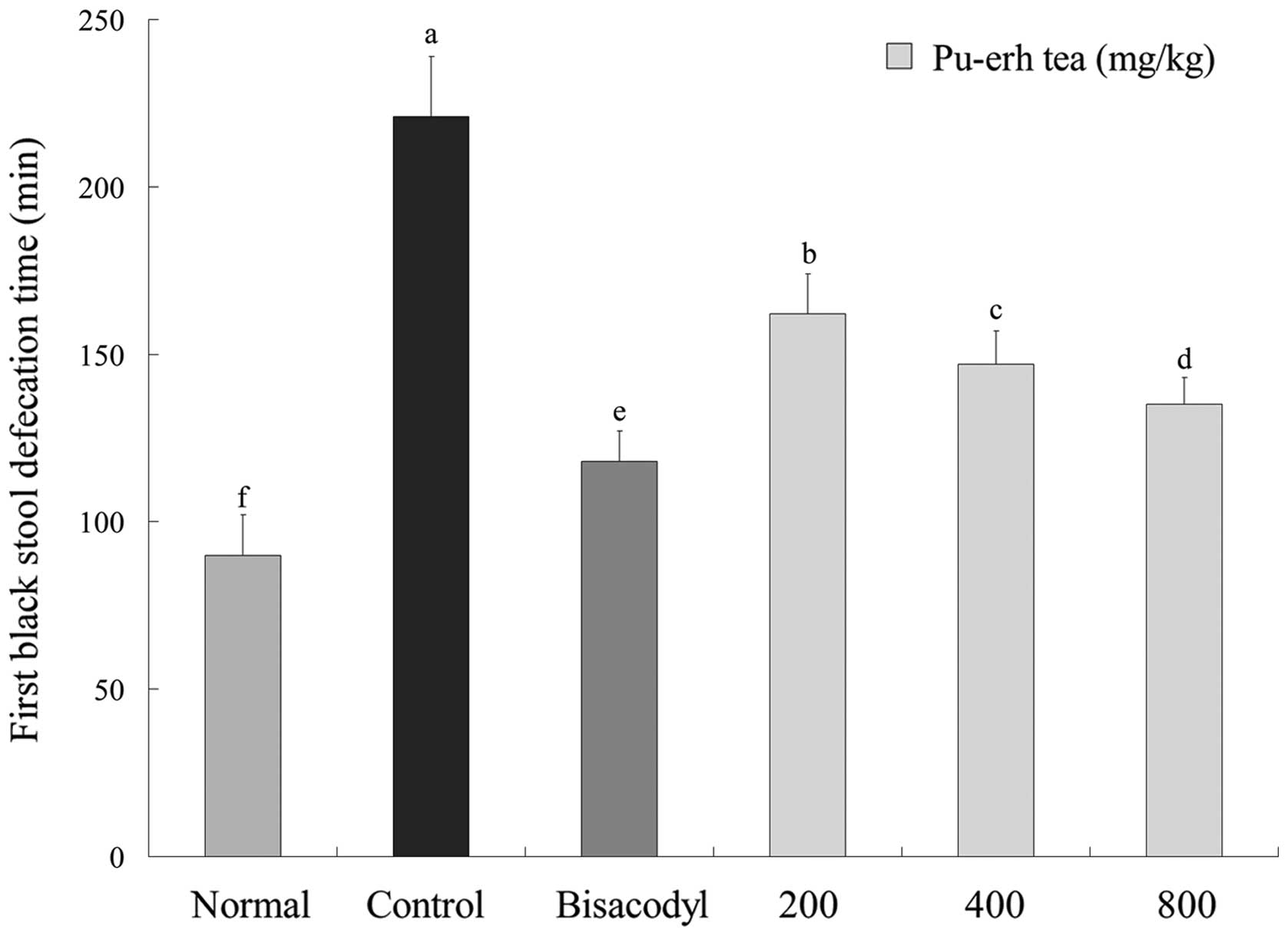
First black stool defecation time and
gastrointestinal transit of mice
As displayed in Fig.
4, the first black stool defecation time of each mice group
administered with activated carbon demonstrates the inhibitory
effect of the samples on constipation. Defecation time was the
shortest (90±12 min) in the normal group, with the longest (221±18
min) time in the control group. The time for the bisacodyl group
was 118±9 min, which was only slightly higher than the normal
group. Under 200, 400 and 800 mg/kg dosage concentrations, first
black stool defecation times were 162±12, 147±10 and 135±8 min,
respectively. According to the defecation time, it is evident that
Pu-erh tea had a stronger inhibitory effect on constipation.
The constipation inhibitory effects of samples were
determined by gastrointestinal transits. The mice were administered
activated carbon (0.2 ml/mouse, 10% activated carbon). In the
bisacodyl treated group, GI transits were 87.3±5.2% which were more
than that of the control group (28.9±4.6%) (Fig. 5). At the 200, 400 and 800 mg/kg
body weight dose of Pu-erh tea extract, the GI transits were
83.3±4.1 and 79.3±4.1%, respectively. The Pu-erh tea was able to
increase the gastrointestinal transit more than the control and
higher doses were able to reduce constipation.
MTL, Gas, ET, SS, AchE, SP and VIP levels
in serum
The MTL level of normal mice was 170.2±12.6 pg/ml;
whereas that in the activated carbon-induced constipation control
mice decreased to 106.2±9.8 pg/ml (Fig. 6). The level of MTL in mice fed with
bisacodyl was 158.5±8.7 pg/ml. The levels of MTL in mice treated
with 200, 400 and 800 mg/kg of Pu-erh tea were 123.6±9.2, 134.2±9.5
and 142.2±8.1 pg/ml, respectively. The changes of Gas levels of
normal, control, 100 mg/kg dose bisacodyl, 200, 400 and 800 mg/kg
dose mice were 78.1±4.8, 48.9±2.7, 71.4±3.9, 55.3±4.4, 63.4±3.2 and
68.1±3.7, respectively. At the 200, 400 and 800 mg/kg body weight
doses, the levels of ET in the Pu-erh tea groups were 8.1±0.3,
8.6±0.3 and 9.4±0.4 pg/ml and the normal, control and bisacodyl
mice were 12.1±0.4, 7.8±0.2 and 11.5±0.4 pg/ml, respectively. The
SS levels of normal and control mice were 35.9±2.0 and 60.4±3.2
pg/ml, the sample dose mice were 39.8±2.2 (100 mg/kg of bisacodyl),
54.6±2.7 (200 mg/kg of Pu-erh tea), 48.7±2.4 (400 mg/kg of Pu-erh
tea) and 42.5±1.8 (800 mg/kg of Pu-erh tea) pg/ml, respectively.
The AchE, SP and VIP levels of normal mice were 30.1±1.4, 60.3±3.1
and 50.1±2.5 pg/ml, the bisacodyl dose mice were 28.1±1.3, 53.2±2.7
and 46.4±1.9 pg/ml, the 200 (19.4±1.2, 44.6±2.4 and 39.9±1.4
pg/ml), 400 (22.3±1.6, 47.8±1.8 and 43.5±1.4 pg/ml) and 800 mg/kg
(26.4±1.1, 50.9±1.9 and 45.1±0.9 pg/ml) dose Pu-erh tea mice were
higher than that in the control mice (14.0±1.1, 39.1±2.7 and
32.3±0.9 pg/ml).
Discussion
Pu-erh tea is known as a medicinal tea because of
its various health benefits. Long-standing consumers of Pu-erh tea
believe this beverage has anti-aging properties and is able to
prolong life. Pu-erh tea has been demonstrated to have antifungal,
antimicrobial, antioxidant, antimutagenic, antitumor and weight
reduction effects (11).
Pu-erh tea contained functional contents of
epigallocatechin, catechin, quercetin-3-galactoside,
kaempferol-3-rutinoside, kaempferol-3-glucoside, quercetin and
kaempferol. These contents may be useful for preventing
constipation. Green tea contains epigallocatechin and catechin and
may be effective in preventing constipation. There are also
numerous other epigallocatechin products for constipation that are
manufactured (12).
Quercetin-3-galactoside, kaempferol-3-rutinoside,
kaempferol-3-glucoside, quercetin and kaempferol are functional
contents that are able to alleviate indigestion, flatulence and
constipation (13–15).
Anorexia is an important symptom of constipation
(16). Through observing the
dietary intake and drinking water amount of mice, we may determine
constipation and the inhibitory effect of mice treated with
different samples. The definition of constipation includes
infrequent bowel movements and difficulty during defecation
(17,18).
Constipation most commonly occurs when the stool
that forms following food digestion moves too slowly (slow transit)
as it passes through the digestive tract. Dehydration, changes in
diet and activity and certain drugs are frequently to blame for the
slow transit of stool. When stool moves slowly, too much water is
absorbed from the stool and it becomes hard and dry (19). Defecation status, dietary intake,
drinking water amount, stool defecation time and gastrointestinal
transit are important standards for constipation inspection.
The serum levels of MTL, Gas, ET, AchE, SP and VIP
in patients with constipation are lower than those in healthy
patients and the SS level was higher than the general population
(20–22). The main function of MTL is to
increase the migrating myoelectric complex component of
gastrointestinal motility and stimulate the production of pepsin.
It is one of the intestinal hormones responsible for the proper
filling and emptying of the gastrointestinal system in response to
the intake of food and hunger stimuli (23). Gas is a polypeptide hormone
secreted by certain cells of the pyloric glands, which markedly
stimulates the secretion of gastric acid and pepsin and weakly
stimulates the secretion of pancreatic enzymes and contraction of
the gall bladder (24). Gas has
effects throughout the gastrointestinal tract. It is able to
promote gastrointestinal secretion, increase gastrointestinal
movement and at the same time it is able to promote pyloric
sphincter relaxation. ET is important in the stability of vascular
tension and maintains the basic cardiovascular system. Constipation
is able to cause diseases, including intestinal obstruction, or
colon cancer and is also able to induce or aggravate
cardio-cerebro-vascular diseases in the elderly (25). The somatostatin analogue may
stimulate intestinal motor complexes and this agent has been used
to treat sclerodermatous pseudo-obstruction. Stool is formed from
the non-digestible component of food after water is either absorbed
or secreted in the large intestine. Mucous is also produced in the
large intestine to provide viscosity. Thin segments of muscle line
the intestinal tract and contract and relax in concert to propel
the stool forward. Muscle contraction and mucous secretion are
regulated by acetylcholine (26).
Patients with slow transit constipation have abnormal
neurotransmitter levels in the muscular layer of their intestinal
walls. These abnormalities include a deficiency of SP peptide,
which is thought to contribute to peristalsis (27). The disturbances in the normal
neural content of VIP in the bowel wall in idiopathic constipation
and diverticular disease, may initiate or contribute to the
functional changes observed in these disorders (28).
The aim of the present study was to demonstrate that
Pu-erh tea has a preventive effect on activated carbon-induced
constipation in mice. According to the results, it was demonstrated
that Pu-erh tea has significant effects on body weight, dietary
intake, drinking water amount and feces status, including
defecation weight, particle counts of defecation and water content
of defecation in ICR mice. First black stool defecation time was
only a little longer than bisacodyl. Gastrointestinal transit was
longer than in the control and similar to bisacodyl, and various
serum levels, including MTL, Gas, ET, AchE, SP and VIP in Pu-erh
tea dose mice were higher than in the control mice, with the SS
levels demonstrating an opposite tendency. These results suggest
that Pu-erh tea has a significant preventive effect on activated
carbon-induced constipation in mice.
Acknowledgements
This study was supported by the Program for
Chongqing Innovative Research Team in University (KJTD201325).
References
|
1
|
Weisburger JH: Tea and health: the
underlying mechanisms. Proc Soc Exp Biol Med. 220:271–275. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gong ZQ, Watanabe N, Yagi A, Etoh H,
Sakata K, Ina K and Liu QJ: Compositional change of Pu-erh tea
during processing. Biosci Biotechnol Biochem. 57:1745–1746. 1993.
View Article : Google Scholar
|
|
3
|
Duh PD, Yen GC, Yen WJ, Wang BS and Chang
LW: Effects of pu-erh tea on oxidative damage and nitric oxide
scavenging. J Agric Food Chem. 52:8169–8176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ueki A and Otsuka M: Life style risks of
Parkinson’s disease: Association between decreased water intake and
constipation. J Neurol. 251:18–23. 2004.
|
|
5
|
Wexner SD, Beck DE, Baron TH, Fanelli RD,
Hyman N, Shen B and Wasco KE: A consensus document on bowel
preparation before colonoscopy: prepared by a task force from the
American society of colon and rectal surgeons (ASCRS), the American
society for gastrointestinal endoscopy (ASGE), and the society of
American gastrointestinal and endoscopic surgeons (SAGES). Dis
Colon Rectum. 49:792–809. 2006.
|
|
6
|
Farrugia G, Miller SM, Rich A, Liu X,
Maines MD, Rae JL and Szurszewski JH: Distribution of heme
oxygenase and effects of exogenous carbon monoxide in canine
jejunum. Am J Physiol. 274:G350–G358. 1998.PubMed/NCBI
|
|
7
|
Farrugia G, Lei S, Lin X, Miller SM, Nath
KA, Ferris CD, Levitt M and Szurszewski JH: A major role for carbon
monoxide as an endogenous hyperpolarizing factor in the
gastrointestinal tract. Proc Natl Acad Sci USA. 100:8567–8570.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller SM, Reed D, Sarr MG, Farrugia G and
Szurszewski JH: Haem oxygenase in enteric nervous system of human
stomach and jejunum and co-localization with nitric oxide synthase.
Neurogastroenterol Motil. 13:121–131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xue L, Farrugia G, Miller SM, Ferris CD,
Snyder SH and Szurszewski JH: Carbon monoxide and nitric oxide as
coneurotransmitters in the enteric nervous system: evidence from
genomic deletion of biosynthetic enzymes. Proc Natl Acad Sci USA.
97:1851–1855. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JS, Kim DH, Liu KH, Oh TK and Lee CH:
Identification of flavonoids using liquid chromatography with
electrospray ionization and ion trap tandem mass spectrometry with
an MS/MS library. Rapid Commun Mass Spectrom. 19:3539–3548. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu SC, Yen GC, Wang BS, Chiu CK, Yen WJ,
Chang LW and Du PD: Antimutagenic and antimicrobial activities of
pu-erh tea. LWT-Food Sci Technol. 40:506–512. 2007. View Article : Google Scholar
|
|
12
|
Chrubasik C, Maier T, Dawid C, Torda T,
Schieber A, Hofmann T and Chrubasik S: An observational study and
quantification of the actives in a supplement with Sambucus
nigra and Asparagus officinalis used for weight
reduction. Phytother Res. 22:913–918. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghoneim AI and Eldahshan OA:
Anti-apoptotic effects of tamarind leaves against ethanol-induced
rat liver injury. J Pharm Pharmacol. 64:430–438. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin MC, Lin JH, Chen SK, Cheng YW and
Cheng HW: Simultaneous determination of podophyllotoxin, quercetin
and kaempferol in podophyllin by liquid chromatography tandem mass
spectrometry. J Food Drug Anal. 16:29–40. 2008.
|
|
15
|
Maki KC, Reeves MS, Farmer M, et al: Green
tea catechin consumption enhances exercise-induced abdominal fat
loss in overweight and obese adults. J Nutr. 139:264–270. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dolk A, Brodén G, Holmström B, Johansson C
and Schultzberg M: Slow transit chronic constipation (Arbuthnot
Lane’s disease). An immunohistochemical study of
neuropeptide-containing nerves in resected specimens from the large
bowel. Int J Colorectal Dis. 5:181–187. 1990.
|
|
17
|
Walia R, Mahajan L and Steffen R: Recent
advances in chronic constipation. Curr Opin Pediatr. 21:661–666.
2009. View Article : Google Scholar
|
|
18
|
Emmanuel AV, Tack J, Quigley EM and Talley
NJ: Pharmacological management of constipation. Neurogastroenterol
Motil. 21:41–54. 2009. View Article : Google Scholar
|
|
19
|
Lubowski DZ, Chen FC, Kennedy ML and King
DW: Results of colectomy for severe slow transit constipation. Dis
Colon Rectum. 39:23–29. 1996. View Article : Google Scholar
|
|
20
|
Sjölund K, Ekman R, Akre F and Lindner P:
Motilin in chronic idiopathic constipation. Scand J Gastroenterol.
21:914–918. 1986.
|
|
21
|
El-Salhy M and Norrgård O: Colonic
neuroendocrine peptide levels in patients with chronic idiopathic
slow transit constipation. Ups J Med Sci. 103:223–230. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Silkoff P, Karmeli F, Goldin E, Ewenson A,
Gilon C, Chorev M, Laufer R, Selinger Z and Rachmilewitz D: Effect
of substance P on rat gastrointestinal transit. Digest Dis Sci.
33:74–77. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feighner SD, Tan CP, McKee KK, et al:
Receptor for motilin identified in the human gastrointestinal
system. Science. 284:2184–2188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Preston DM, Adrian TE, Christofides ND,
Lennard-Jones JE and Bloom SR: Positive correlation between
symptoms and circulating motilin, pancreatic polypeptide and
gastrin concentrations in functional bowel disorders. Gut.
26:1059–1064. 1985. View Article : Google Scholar
|
|
25
|
Soudah HC, Hasler WL and Owyang C: Effect
of octreotide on intestinal motility and bacterial overgrowth in
scleroderma. N Engl J Med. 325:1461–1467. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Furchgott RF and Zawadzki JV: The
obligatory role of endothelial cells in the relaxation of arterial
smooth muscle by acetylcholine. Nature. 288:373–376. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tzavella K, Riepl RL, Klauser AG,
Voderholzer WA, Schindlbeck NE and Müller-Lissner SA: Decreased
substance P levels in rectal biopsies from patients with slow
transit constipation. Eur J Gastroenterol Hepatol. 8:1207–1211.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Milner P, Crowe R, Kamm MA, Lennard-Jones
JE and Burnstock G: Vasoactive intestinal polypeptide levels in
sigmoid colon in idiopathic constipation and diverticular disease.
Gastroenterology. 99:666–675. 1990.PubMed/NCBI
|