Introduction
Lung cancer is currently the leading cause of
cancer-related mortality worldwide. In lung cancer patients, ~85%
are diagnosed with non-small cell lung cancer (1,2). The
prognosis for patients with non-small cell lung cancer is poor.
Surgical intervention, chemotherapy, target therapy and
radiotherapy are the treatments of choice for lung cancer (3). However, progression and recurrence of
disease often occur after treatment. In addition, lung cancer is
often resistant to the current treatment options available.
Therefore, there is an requirement for the identification of a
novel treatment for non-small cell lung cancer.
Apoptosis is key in numerous diseases, including
lung cancer (4). There are
intrinsic and extrinsic pathways that are involved in cell
apoptosis. In cancer cells, alternations of the upstream regulators
of intrinsic and extrinsic pathways are common, which may result in
an imbalance between cell proliferation and apoptotic cell death. A
number of chemotherapy-related drugs may induce apoptosis of cancer
cells (5). Fas is a receptor on
the cell surface, which may be activated by its ligand (Fas L) or
by a cross-linking antibody. Following activation, oligomerization
of the intracellular death domain of Fas is likely to occur
(6). Fas results in cleavage of
pro-caspase-8 and -3, then pro-caspase-3 turns into activated
caspase-3 and induces apoptosis (7).
Lindera aggregata (SIMS)
KOSTERM
The Lauraceae family is comprised of ~55 genera and
2,500 species. The majority of them are in the tropics and
subtropics (8). In China, it is
known as Wu Yao and in Japan, Uyaku. Lauraceae is used for treating
renal, cystic and rheumatic diseases (9). Lindera aggregata,
isolinderalactone, linderalactone and linderane are known
sesquiterpenes derived from root tubers. In the present study, the
antiproliferative activity of these compounds was determined
(Fig. 1) (10) and the effect of isolinderalactone
on the cell cycle distribution and apoptosis was examined in A549
human non-small cell lung cancer cells. Many anticancer drugs can
induce apoptosis of cancer cells. The Fas/FasL system is a key
regulator of apoptosis.
Materials and methods
Materials
Isolinderalactone, linderalactone and linderane were
purchased from ChemFaces (Wuhan, China). Fetal bovine serum (FBS),
penicillin G, streptomycin, amphotericin B and RPMI-1640 were
obtained from Gibco-BRL (Gaithersburg, MD, USA). Dimethylsulfoxide
(DMSO), ribonuclease and propidium iodide were purchased from Sigma
Chemical (St. Louis, MO, USA). p21 Waf1/Cip 1 and APO-1/Fas/CD95
ELISA kits as well as sFas Ligand Immunoassay kits were purchased
from Invitrogen Life Technologies (Camarillo, CA, USA). Anti-Fas
antibody (ZB4) was obtained from Upstate Biotechnology, Inc. (Lake
Placid, NY, USA). Caspase-8 assay kit, and caspase-8 inhibitor,
benzyloxy-carbonyl-Val-Ala-Asp-fluoromethylketone (Z-IETD-FMK) were
purchased from Calbiochem (Cambridge, MA, USA).
Cell culture
A549 human non-small cell lung cancer cells were
incubated at 37°C in a 5% CO2-containing incubator.
Minimum essential medium with 10% FBS, 100 U/ml penicillin G, 100
μg/ml streptomycin, 0.25 μg/ml amphotericin B, non-essential amino
acids and 0.1 mM sodium pyruvate was used. The medium was changed
every 2–3 days and we ensured the cells reached a distinct cell
density.
Cell proliferation
Briefly, the cells were plated in 96-well culture
plates (1×104 cells/well), and after 24 h incubation,
treated with vehicle alone (0.1% DMSO) and various concentrations
of isolinderalactone, linderalactone and linderane for 48 h. A549
proliferation was determined by Premixed WST-1 Cell Proliferation
Reagent (Clontech Laboratories Inc., Mountain View, CA, USA)
according to the manufacturer’s instructions. In brief, the cells
were incubated with premixed WST-1 cell proliferation reagent for
0.5–4 h. Tetrazolium salt WST-1 was cleaved to a formazan-class dye
by mitochondrial succinate-tetrazolium reductase in viable and
metabolically active cells, thereby quantitating the formazan dye
by measuring the absorbance at 450 nm in a multiwell plate reader
(Multiskan EX, Labsystems, Helsinki, Finland) providing measurement
of cell proliferation. The percentage of inhibition was calculated
using the following formula: % inhibition = [100 - (ODt/ODs) × 100]
ODt and ODs indicate the optical density of the test substances and
the solvent control, respectively.
Cell cycle analysis
In order to determine cell cycle distribution,
5×105 cells were plated in 60-mm dishes and treated with
vehicle alone (0.1% DMSO) and isolinderalactone (40 μM) for 24 h.
Following treatment, the cells were collected by trypsinization,
fixed in 70% ethanol, washed in phosphate-buffered saline (PBS),
re-suspended in 1 ml PBS containing 1 mg/ml ribonuclease and 50
μg/ml propidium iodide, incubated in the dark for 30 min at room
temperature and analyzed by an EPICS flow cytometer (Beckman
Coulter, High Wycombe, UK). The data were analyzed using the
Multicycle software (Phoenix Flow Systems, San Diego, CA, USA).
Analysis of apoptosis
A quantitative analysis of apoptotic cells was
undertaken by the terminal deoxynucleotidyl transferase-mediated
deoxyuridine triphosphate nick end labeling (TUNEL) method, which
examines the DNA-strand breaks during apoptosis using the BD
ApoAlert™ DNA fragmentation assay kit (BD Biosciences Clontech,
Palo Alto, CA, USA). Briefly, the cells were incubated with vehicle
alone (0.1% DMSO) and isolinderalactone (40 μM) for the indicated
times, then trypsinized, fixed with 4% paraformaldehyde and
permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate.
Following washing, the cells were incubated with the reaction
mixture for 60 min at 37°C. The stained cells were then analyzed
with an EPICS flow cytometer and a fluorescence microscope (Nikon
Eclipse TE 300, Nikon, Tokyo, Japan) at magnification, ×20.
Assaying the levels of p21, Fas and
sFasL
p21 Waf1/Cip 1 and APO-1/Fas/CD95 ELISA kits and an
sFas ligand immunoassay kit were used to detect p21, Fas receptor
and sFasL, respectively. Briefly, the A549 cells were treated with
vehicle alone (0.1% DMSO) and isolinderalactone (40 μM) for 6, 12,
24, and 48 h. The samples of the cell lysate were placed in 96-well
microtiter plates that were coated with monoclonal detector
antibodies and incubated for 1 (Fas) or 2 h (p21 and sFasL) at room
temperature. Each sample is assessed in triplicate. Following
removal of the unbound material by washing with washing buffer (50
mM Tris, 200 mM NaCl and 0.2% Tween-20), the horseradish
peroxidase-conjugated streptavidin, which can bind to detector
antibody, was added and incubated at room temperature for 30 min.
Horseradish peroxidase catalyzed the conversion of a chromogenic
substrate (tetramethylbenzidine) to a colored solution with color
intensity proportional to the quantity of protein present in the
sample. The absorbance of each well was measured at 450 nm, and
concentrations of p21, Fas and sFasL were determined by
interpolating from standard curves obtained with known
concentrations of standard proteins.
Assay for caspase-8 activity
The assay is based on the ability of the active
enzyme to cleave the chromophore from the enzyme substrate,
Ac-IETD-pNA. The cell lysates were incubated with peptide substrate
in assay buffer [100 mM NaCl, 50 mM HEPES, 10 mM dithiothreitol, 1
mM EDTA, 10% glycerol, 0.1% CHAPS, (pH 7.4)] for 3 h at 37°C. Each
sample was assayed in triplicate. The release of
p-nitroaniline was monitored at 405 nm using a multiwell
plate reader (Multiskan EX). The results are presented as the
percentage change of the activity compared with the untreated
control.
Statistical analysis
All results are expressed as the mean ± standard
deviation and analyzed by one-way analysis of variance. The
differences between the experimental and control groups were
analyzed using Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Cell proliferation of A549 human
non-small-cell lung cancer cells is inhibited by
isolinderalactone
Isolinderalactone, linderalactone and linderane were
tested on A549 non-small-cell lung cancer cells. These compounds
are sesquiterpenes derived from the root tubers of Lindera
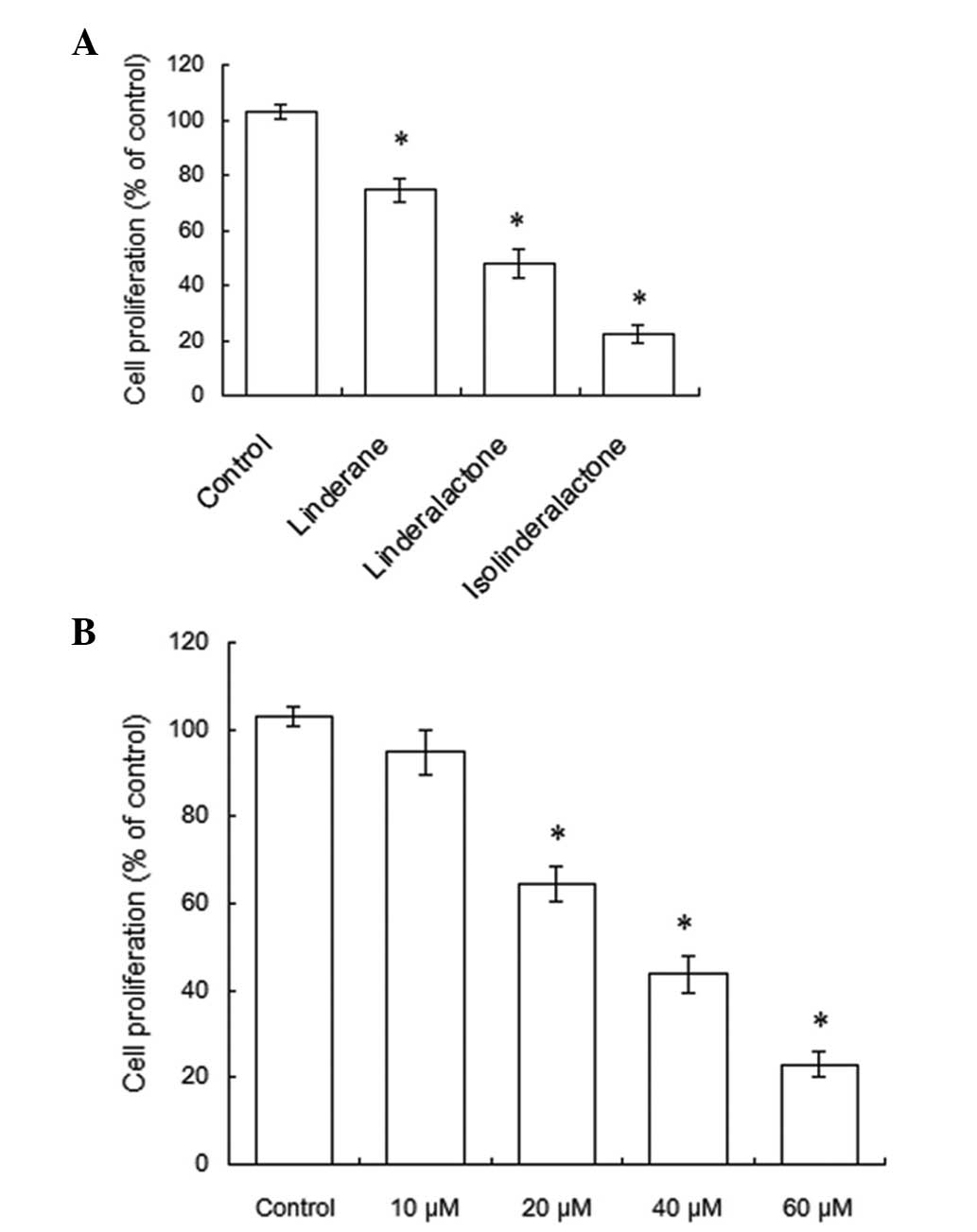
aggregates. Fig. 2A demonstrated
that after 48 h treatment, these three compounds (60 μM) inhibited
the proliferation of human non-small-cell lung cancer cells.
Compared with linderalactone and linderane, isolinderalactone
exhibited a greater effect on the inhibition of cell proliferation.
Due to the aforementioned results, isolinderalactone was selected
for the investigation of the mechanism of Lindera aggregates in
anticancer agent development. The A549 cells were treated with
isolinderalactone at varying concentrations. Following treatment
with isolinderalactone, the proliferation of A549 cells was
observed to decrease. Following treatment of the A549 cells with 60
μM isolinderalactone for 48 h, the proliferation of A549 cells was
inhibited by 78%. The IC50 values were 37.3 μM (Fig. 2B). Thus, a total concentration of
40 μM isolinderalactone was selected for detecting the mechanism of
the antiproliferative effect in A549 cells.
Isolinderalactone-mediated cell cycle
arrest may operate through the induction of p21 protein in A549
human non-small-cell lung cancer cells
The cell cycle distribution was assessed in order to
clarify the mechanism of the antiproliferative effect. Fig. 3 shows the results on the effects of
isolinderalactone on the cell cycle progression of the A549 cells.
As compared with the control group, the population of the
G0/G1 phase in A549 cells treated with 40 μM
isolinderalactone increased from 34.1–65.6% (Fig. 3A). The levels of the p21 protein
were assayed by a p21 Wafl/Cip 1 ELISA kit in order to investigate
whether p21 is involved in the mechanism of the
isolinderalactone-induced arrested cell cycle in the A549 cells.
Fig. 3B shows the p21 protein
level in A549 cells treated with 40 μM isolinderalactone increased
when the period of 40 μM isolinderalactone treatment was increased
(6, 12, 24 and 48 h). Therefore, it is hypothesized that the
induction of p21 is involved in isolinderalactone-mediated cell
cycle arrest.
Fas/sFasL apoptotic system may be a
possible pathway of isolinderalactone-mediated apoptosis
Due to the consideration that the mechanism of the
antiproliferative effect of isolinderalactone is associated with
apoptosis, TUNEL was used to detect DNA-strand breaks as a
quantitative evaluation. A concentration of 40 μM isolinderalactone
induced apoptosis in 35.5% of A549 cells at 48 h (Fig. 4A). The expression of the Fas
receptor and sFasL in A549 cells increased in a dose-dependent
manner after 6 h treatment with isolinderalactone. The
APO-1/Fas/CD95 ELISA and sFas ligand immunoassay kits were used in
this process (Fig. 4B and C). The
sFasL level reached the peak value after 24 h of treatment. It was
hypothesized that the Fas/sFasL system is involved in the
isolinderalactone-induced apoptosis of A549 cells, due to the time
correlation between the expression of Fas/sFasL at 6 h of treatment
and the initiation of apoptosis at 12 h of treatment. In order to
investigate this further, the A549 cells were pretreated with ZB4,
which is an antagonistic anti-Fas antibody for inhibiting the
antiproliferative and proapoptotic effects of isolinderalactone. At
48 h in A549 cells pretreated with ZB4 and treated with 40 μM
isolinderalactone, the isolinderalactone-induced inhibition of cell
proliferation inhibition decreased from 56.1 to 25.1% in the A549
cells (Fig. 5A). At the same time,
the induction of apoptosis by 40 μM of isolinderalactone decreased
from 35.1 to 13.9%.
Subsequent to this, the downstream caspase of the
Fas/sFasL system was measured. Caspase-8 activity was observed to
increase at 10 h. The maximum induction effect appeared at 48 h in
40 μM isolinderalactone treated A549 cells (Fig. 6A). The activation of caspase-8 (at
10 h) was compared earlier with the production of DNA fragmentation
(at 12 h). This finding suggests that caspase-8 activation is
involved in isolinderalactone-induced apoptosis. To prove this
hypothesis, the A549 cells were pretreated with a caspase-8
inhibitor (Z-IETD-FMK) and isolinderalactone, compared the A549
cells that were only treated with isolinderalactone, there was
increased cell proliferation and fewer tunnel-positive cells. The
results indicated that inhibition of caspase-8 may decrease the
antiproliferative activity of isolinderalactone (Fig. 6B) and evidently decrease the
induction of apoptosis of the A549 cells (Fig. 6C).
Discussion
The protein p21 is classified as a member of the
cip/kip family, which function as cyclin-dependent kinase (CDK)
inhibitors (11). Upon stimulation
of the cells, the expression of p21 is regulated at the
transcriptional and post-translational levels (12). The p21 protein has the ability to
inhibit the cyclin-CDK2 and CDK1 complexes. Therefore, it may
regulate the cell cycle progression at the G1 phase. and
may also inhibit the phosphorylation of the retinoblastoma protein.
For this reason, p21 may inhibit the G1-S phase
transition. Following treatment of A549 cells with
isolinderalactone, the quantity of p21 was observed to increase. In
addition, flow cytometric analysis revealed that the A549 cells
were arrested in the G0/G1 phase by
isolinderalactone. Based on the aforementioned findings, we
hypothesize that the increase in the level of p21 protein is
involved in the blockade of cell cycle progression. However, p21
may also inhibit apoptosis (13).
The data of the present study may reveal that p21 has a greater
effect in inhibiting the G1-S phase transition compared with the
inhibition of apoptosis following treatment of the A549 cells with
isolinderalactone.
Apoptosis may be induced by intrinsic and extrinsic
pathways (14). In the extrinsic
pathway, the Fas/FasL system is a significant signal transduction
pathway of apoptosis in cancer cells. The Fas receptor is a cell
surface receptor and is significant in triggering the apoptotic
pathway. Following simulation with FasL, the Fas-associated death
domain (FADD) is recruited to the cytoplasmic domain of Fas
(15). FADD is an adaptor protein
and tumor necrosis factor receptor family-mediated apoptosis is
associated with FADD. FADD is involved in cell proliferation,
survival and embryonic death (16). Certain anticancer drugs activate
FasL, when FasL binds Fas in an auto-/paracrine manner, it triggers
the extrinsic pathway through activation of caspase-8 (17). Celastrol, a natural compound
derived from Chinese traditional herbs, induces apoptosis of the
A549 lung cancer cells by cleavage of caspase-9, -8, -3, and the
PARP protein, increasing the expression of Fas and FasL, and
reducing the mitochondrial membrane potential (17).
The present study demonstrates that sFasL increased
in the A549 cells that were treated with isolinderalactone. The
levels of Fas and the activity of caspase-8 increased in A549 cells
with upregulated sFasL. When the Fas/sFas ligand system was
inhibited with ZB4, the cell growth inhibition and the proapoptotic
effect of isolinderalactone were observed to decrease. The
apoptotic induction and cell growth inhibition of isolinderalactone
was observed to decrease in the A549 cells that were treated with
caspase-8 inhibitor.
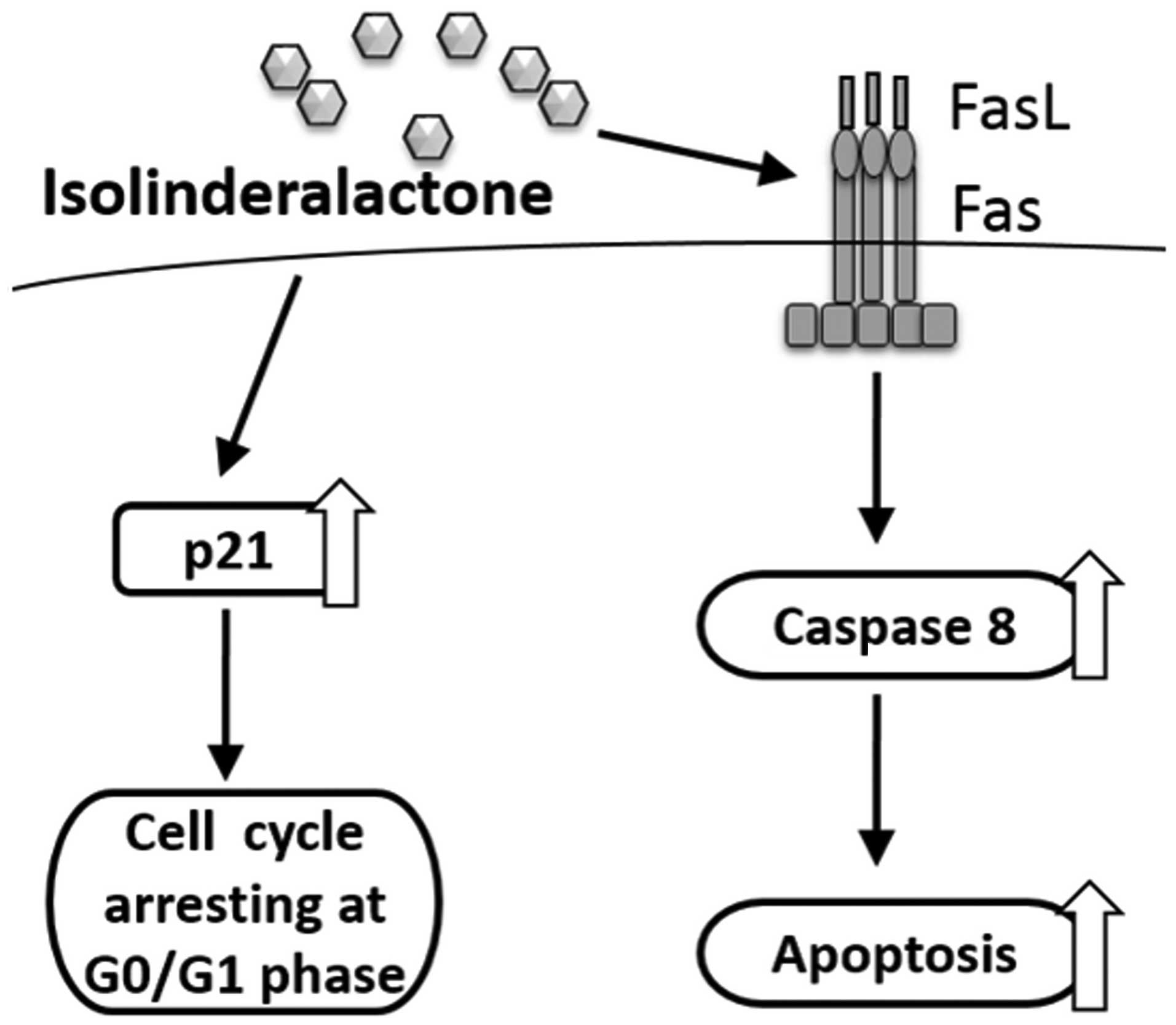
These findings indicate that the Fas/sFasL system
has a significant role in isolinderalactone-mediated A549 cellular
apoptosis. Finally, the present study demonstrated that
isolinderalactone induces cytotoxic activity in the A549 human
non-small cell lung cancer cells. To the best of our knowledge
these findings are the first to demonstrate that the Fas/sFasL
system is significant with regard to isolinderalactone-mediated
A549 cellular apoptosis and that isolinderalactone inhibits
proliferation of A549 human non-small cell lung cancer cells by
arresting the cell cycle at the G0/G1 phase
(Fig. 7). Thus, isolinderalactone
may have the potential to be a novel agent for future treatment of
non-small-cell lung cancer.
Acknowledgements
This study was supported by grants from the National
Science Council of Taiwan (grant nos. 101-2628-B-037-001-MY3,
101-2221-E-025-008 and 100-2221-E-025-017); the Excellence for
Cancer Research Center Grant, the Ministry of Health and Welfare,
Executive Yuan, Taipei, Taiwan (grant no. MOHW103-TD-B-111-05); the
Kaohsiung Medical University Hospital (grant no. KMUH101-1M11); and
the Kaohsiung Medical University Research Foundation (grant no.
KMUER011). The authors would like to thank the Center for
Resources, Research, and Development of Kaohsiung Medical
University for their support with the instrumentation.
References
|
1
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gottschling S, Schnabel PA, Herth FJ and
Herpel E: Are we missing the target? Cancer stem cells and drug
resistance in non-small cell lung cancer. Cancer Genomics
Proteomics. 9:275–286. 2012.PubMed/NCBI
|
|
3
|
Spira A and Ettinger DS: Multidisciplinary
management of lung cancer. N Engl J Med. 350:379–392. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Favaloro B, Allocati N, Graziano V, Di
Ilio C and De Laurenzi V: Role of apoptosis in disease. Aging
(Albany NY). 4:330–349. 2012.
|
|
5
|
Ramakrishnan R and Gabrilovich DI: Novel
mechanism of synergistic effects of conventional chemotherapy and
immune therapy of cancer. Cancer Immunol Immunother. 62:405–410.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chopin V, Toillon RA, Jouy N and Le
Bourhis X: Sodium butyrate induces P53-independent, Fas-mediated
apoptosis in MCF-7 human breast cancer cells. Br J Pharmacol.
135:79–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ostrand-Rosenberg S, Sinha P, Chornoguz O
and Ecker C: Regulating the suppressors: apoptosis and inflammation
govern the survival of tumor-induced myeloid-derived suppressor
cells (MDSC). Cancer Immunol Immunother. 61:1319–1325. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller RE and Tuck KL: Reports on the
distribution of aromatic cyanogenic glycosides in Australian
tropical rainforest tree species of the Lauraceae and Sapindaceae.
Phytochemistry. 92:146–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gan LS, Zheng YL, Mo JX, Liu X, Li XH and
Zhou CX: Sesquiterpene lactones from the root tubers of Lindera
aggregata. J Nat Prod. 72:1497–1501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobayashi W, Miyase T, Sano M, Umehara K,
Warashina T and Noguchi H: Prolyl endopeptidase inhibitors from the
roots of Lindera strychnifolia F. Vill. Biol Pharm Bull.
25:1049–1052. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wettersten HI, Hee Hwang S, Li C, et al: A
novel p21 attenuator which is structurally related to sorafenib.
Cancer Biol Ther. 14:278–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Warfel NA and El-Deiry WS: p21WAF1 and
tumourigenesis: 20 years after. Curr Opin Oncol. 25:52–58.
2013.PubMed/NCBI
|
|
13
|
Gartel AL and Tyner AL: The role of the
cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer
Ther. 1:639–649. 2002.PubMed/NCBI
|
|
14
|
Peter ME: Programmed cell death: Apoptosis
meets necrosis. Nature. 471:310–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu YQ, Mu ZQ, You S, Tashiro S, Onodera S
and Ikejima T: Fas/FasL signaling allows extracelluar-signal
regulated kinase to regulate cytochrome c release in
oridonin-induced apoptotic U937 cells. Biol Pharm Bull.
29:1873–1879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhuang H, Gan Z, Jiang W, Zhang X and Hua
ZC: Functional specific roles of FADD: comparative proteomic
analyses from knockout cell lines. Mol Biosyst. 9:2063–2078. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mou H, Zheng Y, Zhao P, Bao H, Fang W and
Xu N: Celastrol induces apoptosis in non-small-cell lung cancer
A549 cells through activation of mitochondria- and
Fas/FasL-mediated pathways. Toxicol In Vitro. 25:1027–1032. 2011.
View Article : Google Scholar : PubMed/NCBI
|