Introduction
The pathogenesis of preeclampsia (PE) is complex,
involving immunological, genetic and nutritional factors (1), but the above factors can not fully
explain its clinical and pathophysiological behavior. However,
numerous studies have found that there are similar
pathophysiological characteristics in cases of preeclampsia:
namely, the disorders of uterine spiral artery remodeling (USAR)
cause placental ischemia and hypoxia, followed by the release of a
high number of vasoactive factors into the peripheral circulation.
The vascular endothelial functions are thus impaired, leading to
the appearance of various clinical PE symptoms, with the USAR
disorder being the central one (2). The mechanisms causing USAR disorder
are numerous, but none has been elucidated yet. Studies focusing on
the underlying mechanisms of USAR disorder have gained considerable
interest in the context of elucidating PE pathogenesis.
Spiral artery is the branch of the uterine artery
that mainly provides nutrients to the uterine basement membrane.
Since 1967, when Brosens et al (3) first proposed the concept of ‘spiral
artery remodeling’ (SAR), the roles of the spiral artery in
pregnancy have gradually attracted the scientific attention.
Gestational SAR is a highly complex process, mainly mediated by the
trophoblast mother cells (4). With
the progress of pregnancy, ectodermal trophoblast mother cells
differentiate into two types: The syncytiotrophoblasts and the
extravillous trophoblast cells (ETCs). ETCs invade the spiral
arteries via their extremity and against the blood flow direction,
as well as spiral arteries surrounding the decidual stroma
(5,6). The villous trophoblast cells inside
and outside the spiral arteries interact with endothelial and
smooth muscle cells, mediate their apoptosis, secrete the matrix
metalloproteinase (MMP) protein to degrade the elastic matrix of
the spiral arterial walls, and secrete an amorphous cellulose-like
substance (endotheliocyte/uterine smooth muscle cell/decidua) to
replace it at the same time, until the ETCs and the cellulose-like
substance become the main component of the spiral arterial wall
(5–7). The diameter of the remodeled spiral
arteries increases, and their sensitivity towards maternal
vasomotor regulatory factors is reduced, forming vessels with high
flow rate and low resistance to provide sufficient nutrient and
oxygen supply for fetus and placenta (6). Any alteration in the events of this
process can lead to SAR disorder, followed by pregnancy
complications such as PE, and restricted fetal growth.
A number of studies has found that
20-hydroxyeicosatetraenoic acid (20-HETE), one of the arachidonic
acid metabolites, is the upstream regulatory factor or the 2nd/3rd
messenger of multiple signal transduction systems (e.g., HIF-1α and
PI3K/AKT) involved in the regulation of cell proliferation,
differentiation, apoptosis and migration in vascular endothelial
and smooth muscle cells (8–10).
Therefore, 20-HETE can be considered as a key factor in functional
regulation, and abnormalities related to its metabolism are
expected to result in dysfunctions of endothelial and smooth muscle
cells, leading to vascular dysfunctional diseases, such as
hypertension (11). Llinás et
al (12) found that pregnant
rats with chronic uteroplacental ischemia exhibited certain PE
features. The authors confirmed that these rats have a reduced
20-HETE production due to the inhibition of the activity of the
cytochrome P450 that catalyzes its formation, and show reduced
hypertension, induced by reductions in placental perfusion
pressure. These results indicated that 20-HETE is involved in the
development of vascular disorders, and may be involved in the
regulation of placental vessels in pregnant rats (5).
In the present study, we hypothesized, based on the
relevant characteristics of PE and gestational USAR, that since
20-HETE is the upstream regulatory factor of multiple signal
transduction pathways involved in the functional maintenance of
vascular endothelial and smooth muscle cells, abnormalities in its
metabolism would result in altered transduction of the related
signal, thus leading to SAR and vascular dysfunction disorders, and
causing preeclampsia. In order to prove this hypothesis, mono- or
co-cultures of the main cells involved in SAR (human villous
trophoblasts, HVTs and human uterine vascular smooth muscle cells,
HUVSMCs) were treated with 20-HETE and its inhibitor (HET0016).
Using this model, we investigated the effect of 20-HETE on
apoptosis of HUVSMCs co-cultured with HVTs, which was caused by the
invasion of 20-HETE into the villous trophoblasts, as well as the
effect of 20-HETE on USAR, with the aim of gaining novel insights
on the pathogenesis of PE. To the best of our knowledge, this is
the first report on a similar study system.
Materials and methods
Establishment of the co-culture
model
The cultured HVT (ScienCell Research Laboratories,
Carlsbad, CA, USA) and HUVSMCs (PromoCell GmbH, Heidelberg,
Germany) were all adherent-growing cells, and the culture system
was Dulbecco’s Modified Eagle’s Medium/F12 (Gibco-BRL, Carlsbad,
CA, USA). The 3.0 μm aperture Transwell chamber was placed into the
24-well plate and the hydration was performed in a 5%
CO2 and 37°C incubator for 2 h (CO2
incubator; Forma Scientific Inc., Marietta, OH, USA). The HVT and
HUVSMC cells in the logarithmic growth phase were collected, then
the cell suspension density was 1×105 cells/ml and
1×105 cells/ml with the prepared optimized
concentrations of the corresponding drugs that could generate the
best culture environment. The HVT cell suspension (200 μl) was
added into the upper chamber, and the lower chamber was added with
500 μl HUVSMC cell suspension, which was then placed in the 5%
CO2 and 37°C incubator for 48 h cultivation, followed by
the corresponding detection. The co-culture model was based on that
of Stec et al (13).
Determination of optimal drug
concentration and treatment time
HVTs and HUVSMCs were treated with a gradient of
concentrations of 20-HETE and its inhibitor HET0016 (Cayman
Chemical Co., Ann Arbor, MI, USA) with 5 replicates for each
concentration. The optimal drug concentration and treatment time
were determined based on the results of the MTT assay
(Sigma-Aldrich, St. Louis, MO, USA), used to detect the
proliferation of cells after 24 and 48 h (Microplate reader;
Bio-Rad, Hercules, CA, USA).
Assessment of HVT invasive ability and
MMP-2 expression
HVTs in logarithmic growth phase were collected. The
cell suspension density was adjusted using media containing the
drug at the optimal concentration. Five wells were set for each
group. The experiment was conducted as follows: i) the cell
suspension was added to a Transwell chamber (Nunc A/S
Plastfabrikation, Roskilde, Denmark) having a well diameter of 8.0
μm. The cells were incubated at 37°C, under 5% CO2 for
48 h, followed by staining with 0.1% crystal violet staining
(Beyotime Institute of Biotechnology, Shanghai, China) and
determination of the HVT invasive ability according to a previously
described method (13). ii) The
cell suspension was added to a 24-well plate. Following incubation
(37°C, 5% CO2) for 48 h, the total RNA of cells was
extracted, followed by determination of the MMP-2 mRNA level using
a RT-PCR kit (TRIzol Reagent Power SYBR® Green PCR
Master mix; Invitrogen, Carlsbad, CA, USA). iii) The cell
suspension was added to a 24-well plate. Following incubation
(37°C, 5% CO2) for 48 h, the cell supernatant was
collected, and the protein concentration of MMP-2 was detected by
ELISA (SuperScript® Vilo™ cDNA Synthesis kit; R&D
Systems Inc., Minneapolis, MN, USA). Two-step immunohistostaining
was performed, using rat anti-human monoclonal anti-MMP-2 (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) as the primary
antibody and goat anti-rabbit HRP-polymer IgG (Beyotime Institute
of Biotechnology) as the second antibody. The scoring was conducted
using a double-blind method as in (14).
Determination of HUVSMC apoptotic
rate
HVTs/HUVSMCs in logarithmic growth phase were
collected. The cell suspension density was adjusted using media
containing the drug at the optimal concentration. Five wells were
set for each group. HVTs and HUVSMCs were added to the upper and
lower Transwell chamber (well diameter, 3.0 μm), respectively.
Following incubation (37°C, 5% CO2) for 48 h, HUVSMCs
was collected. The apoptotic rate of HUVSMCs was determined using
Annexin-V-fluorescein isothiocyanate (FITC), (Sigma-Aldrich), the
Annexin V-FITC Apoptosis Detection kit (Sigma-Aldrich) and a flow
cytometer (Beckman Coulter, Miami, FL, USA).
Statistical analysis
The SPSS 17.0 software package (IBM, Armonk, NY,
USA) was used to statistically analyze the experimental data.
Intergroup comparisons were performed with single- or multi-factor
analysis of variance, and LSD (when data met the condition of
homogeneity of variance) and Dunnett’s (when data did not meet the
condition of homogeneity of variance) tests were used to assess the
significance of differences, with P<0.05 indicating a
significant difference.
Results
Optimal drug concentration and treatment
time
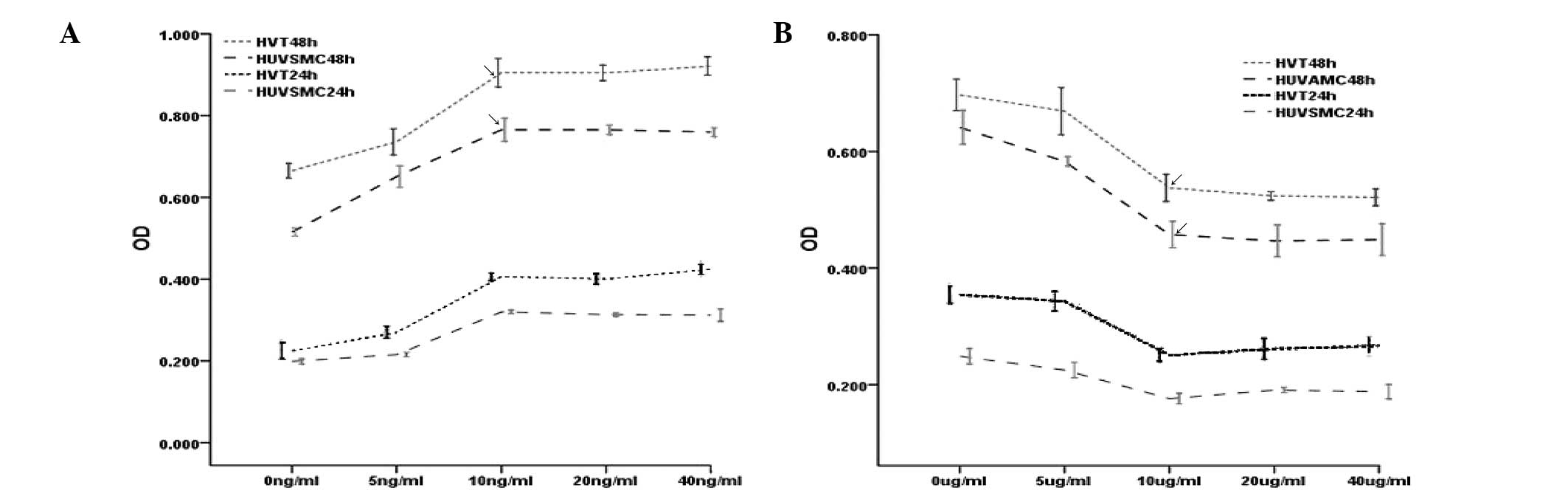
Following 24- and 48-h treatment of HVTs and HUVSMCs
with increasing concentrations of 20-HETE and/or HET0016, the MTT
assay was used to detect cell proliferation (by measuring OD
values).The multi-factor analysis of variance revealed that the
effect of 10 ng/ml of 20-HETE on HVT and HUVSMC proliferation was
most prominent after 48 h of incubation. This concentration of
20-HETE enhanced the proliferation of both cell types (Fig. 1A). As for HET0016, the most
prominent effect on HVT and HUVSMC proliferation was observed after
48 h of incubation with 10 μg/ml of the inhibitor; at this
concentration, the proliferation of both cell types was inhibited
(Fig. 1B).
Inhibitory effect of 20-HETE on the
invasive ability of HVTs
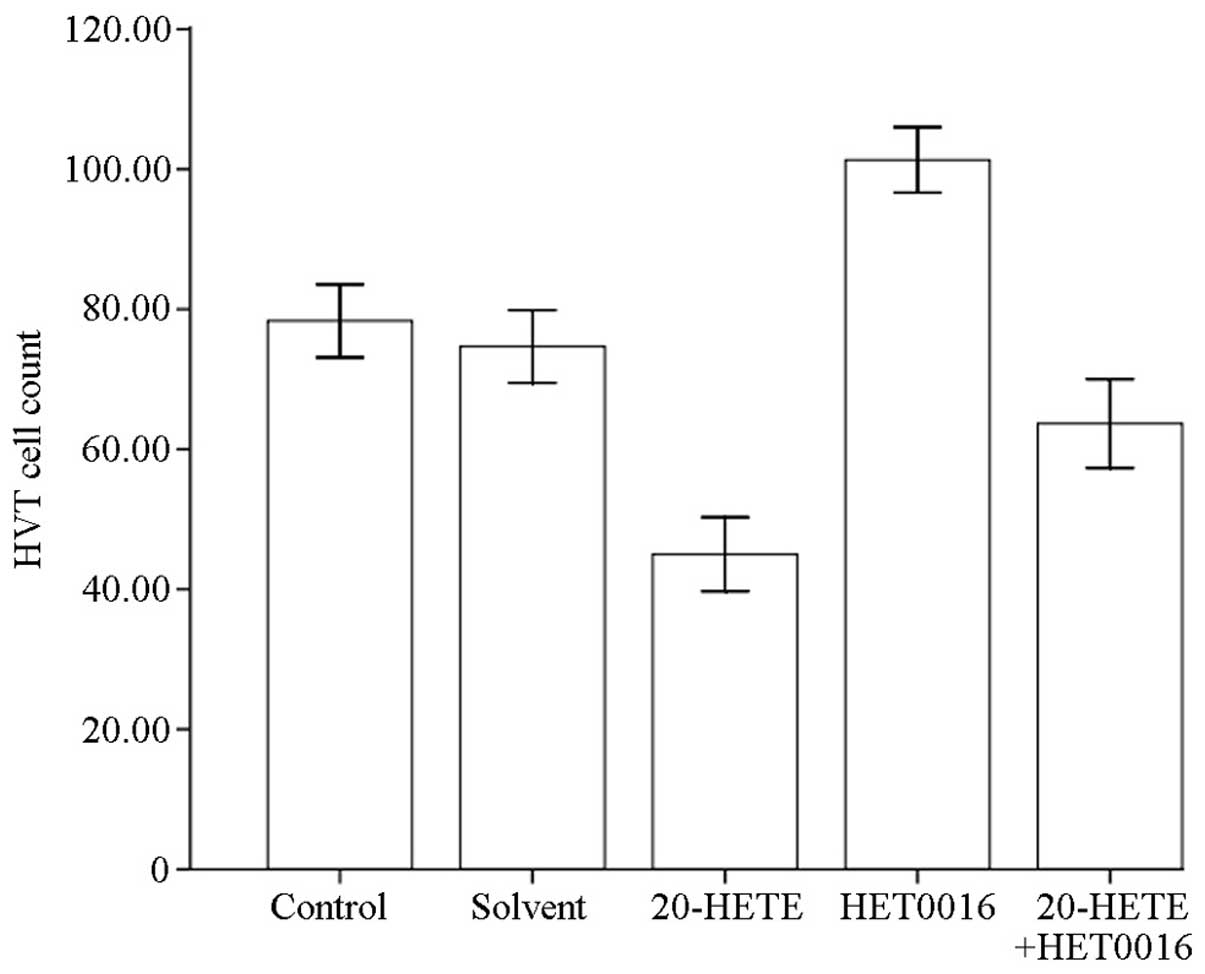
The Transwell chamber assay combined with trypan
blue staining was performed to study the invasive ability of HVTs
after 48 h of treatment with 20-HETE and HET0016. HVT cells were
counted in 5 non-overlapping fields of the optical microscope
(Olympus BX41, Tokyo, Japan; magnification, ×400). Compared to the
control group (normal group without drug and reagents), no
statistically significant difference was observed in the solvent
group (P>0.05), while the number of invading HVTs was
significantly reduced (P<0.05) after 20-HETE treatment, was
significantly increased after HET0016 treatment (P<0.05), and
was significantly reduced (P<0.05) after 20-HETE + HET0016
treatment (Fig. 2).
Effect of 20-HETE on MMP-2
expression
Following treatment of HVTs with 20-HETE and HET0016
for 48 h, cDNA was synthesized from total cellular RNA, the MMP-2
gene was amplified by PCR and the product was run on an agarose
gel, in order to detect its relative expression using a gel imaging
system. No significant difference between the solvent and the
control group (P>0.05) was observed, while the expression of
MMP-2 was significantly reduced in the 20-HETE group (P<0.05),
significantly increased in the HET0016 group (P<0.05), and
significantly reduced (P<0.05) in the 20-HETE + HET0016 group
compared with the control (Fig.
3A).
Following HVT treatment with 20-HETE and HET0016 for
48 h, the supernatant was collected for the detection of the MMP-2
protein using an ELISA assay. No significant difference between the
solvent and the control group (P>0.05) was observed, while the
protein levels of MMP-2 were significantly reduced in the 20-HETE
group (P<0.05), significantly increased in the HET0016 group
(P<0.05), and significantly reduced (P<0.05) in the 20-HETE +
HET0016 group compared with the control (Fig. 3B). MMP-2 was also detected with
immunohistostaining following 20-HETE and HET0016 treatment of HVTs
for 48 h. No difference in detected MMP-2 was observed in the
comparison between the 20-HETE, the 20-HETE + HET0016 and the
solvent group to the control, while the expression of MMP-2 was
prominently detected in the HET0016 group (Fig. 3C).
Inhibition of HUVSMC apoptosis by
20-HETE
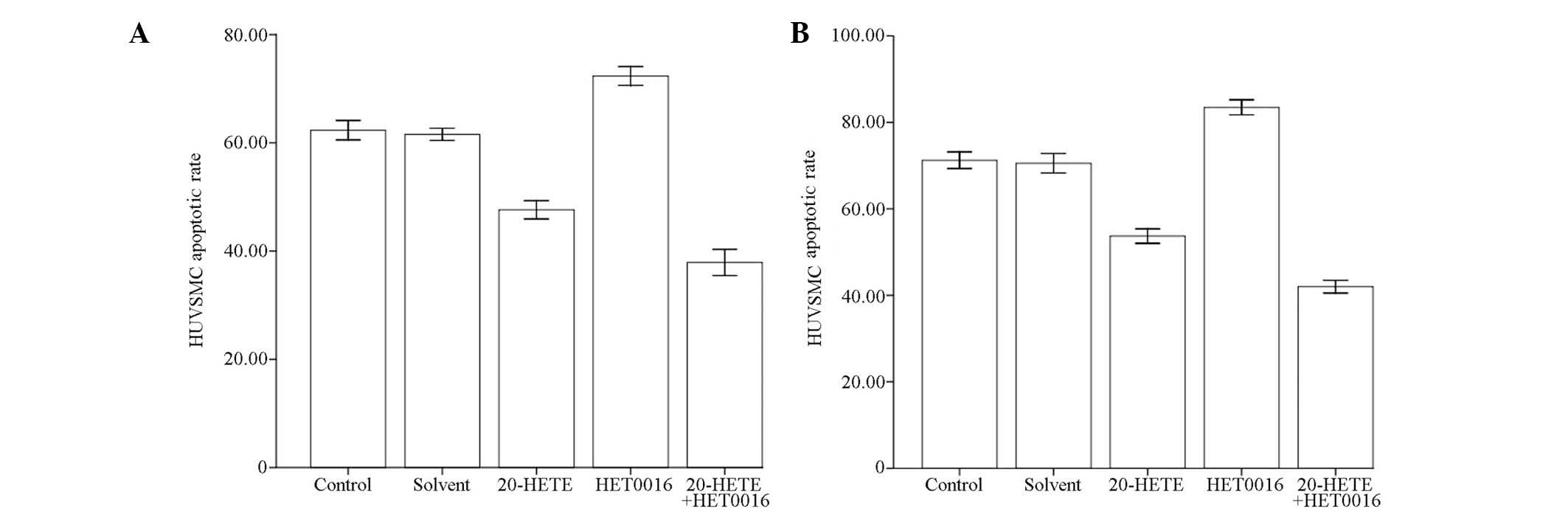
Following HUVSMC treatment with 20-HETE and HET0016
for 48 h, Annexin-V FITC staining and flow cytometric analysis were
performed to estimate the apoptotic rate (%) of HUVSMCs. No
statistically significant difference was observed between the
solvent and the control group (P>0.05), while the apoptotic rate
was significantly reduced in the 20-HETE group (P<0.05),
significantly increased in the HET0016 group (P<0.05), and
significantly reduced (P<0.05) in the 20-HETE + HET0016 group
compared to the control (Fig. 4A).
The same analyses were performed to detect the apoptotic rate of
HUVSMCs following treatment of the HVT-HUVSMC co-culture with
20-HETE and HET0016 for 48 h. No statistically significant
difference was observed between the solvent and the control group
(P>0.05), while the apoptotic rate was significantly reduced in
the 20-HETE group (P<0.05), significantly increased in the
HET0016 group (P<0.05), and significantly reduced (P<0.05) in
the 20-HETE + HET0016 group compared to the control (Fig. 4B).
Discussion
Arachidonic acid (AA) is metabolized into
hydroxyeicosatetraenoic acids (HETEs) and epoxy eicosatriene acids
(EETs) by cytochrome P450 (CYP450) proteins (15). In the human body, the two main
CYP450 isoenzymes are the CYP 4A and 4F, metabolizing AA into
20-HETE (16), while the other two
isoenzymes, CYP 2C and 2J, metabolize AA into EETs (17). HET0016 is a specific inhibitor of
20-HETE, which can selectively inhibit the in vivo
activities of CYP 4A and 4F, while increased doses of HET0016 can
also inhibit CYP 2C and 2J (18).
The active concentrations of 20-HETE and HET0016 depend on the type
of tissue and cell. There is currently no report on the impact of
20-HETE on the main cells involved in USAR, HVTs and HUVSMCs. In
the present study, 20-HETE and its inhibitor HET0016 were used to
treat HVTs and HUVSMCs, and the resulting changes in the biological
behavior of these cells are discussed below.
In order to identify the most potent effects of
20-HETE and HET0016 on HVTs and HUVSMCs, we referred to the
reported active concentrations of 20-HETE and HET0016 in the
endothelial cells and the smooth muscle cells, and studied the
effects of 20-HETE and HET0016 on cell proliferation using the MTT
assay (19,20). We found that 10 ng/ml of 20-HETE
and 10 μg/ml of HET0016 exerted the most prominent effect on the
proliferation of HVTs and HUVSMCs after 48 h of treatment.
Our study found that 20-HETE can inhibit HVT
invasion, while HET0016 can promote it. Numerous signal
transduction pathways are involved in the regulation of HVT
migration, among which the most important are HIF-1α and
PI3K/AKT/FOSL1 (8,9,21).
HIF-1α can inhibit the migration of HVTs through promoting the
expression of TGF-β3, while PI3K/AKT/FOSL1 can enhance their
ability to migrate through regulating the expression of downstream
invasion-related proteins. Recent studies have found that 20-HETE
is the upstream regulatory factor of signal transduction cascades,
including HIF-1α and PI3K/AKT/FOSL1, and through these signaling
cascades, 20-HETE is able to regulate biological behaviors such as
vascular cell proliferation and migration (9). These studies overall suggest that
20-HETE can inhibit the invasion of HVTs through a number of signal
transduction pathways. The invasion of HVTs comprises two
processes, namely migration and infiltration. To the best of our
knowledge, there is no study reporting the effect of 20-HETE on the
invasive ability of HVTs to date.
The degradation of the extracellular matrix of
uterine spiral arteries is the main step in trophoblast invasion
and spiral artery remodeling. A recent study showed that MMP-2 is
an important molecule involved in the degradation of the
extracellular matrix of uterine spiral arteries (14). In order to further explore the
effect of 20-HETE on trophoblast invasion, 20-HETE and HET0016 were
here used to treat HVTs in vitro. The results showed that
20-HETE has inhibitory effects on the transcription and the
translation of the protein MMP-2 in HVTs, while HET0016 exhibited
an enhancing effect. Under normal physiological conditions, the
activation or inhibition of the transcription of endogenous MMPs
allows to adjust their relative activities (22), while the decreased expression of
placenta MMPs (MMP-2) leads to the relatively shallow invasion of
HVTs and to spiral artery remodeling disorder (23). Therefore, the inhibition of the
expression of MMP-2 by 20-HETE in HVTs might affect their ability
to invade the uterine spiral arteries. In patients with early PE,
the expression of AA metabolites (20-HETE) in the decidua was found
to be significantly enhanced, and the MMP-2 levels in plasma and
amniotic fluid were significantly increased (23,24),
indicating that metabolic abnormalities of 20-HETE and MMP-2 may
underlie the remodeling disorders associated with PE. The present
study is the first to report that 20-HETE can inhibit the invasion
of HVTs, which might provide a new target in the investigation of
therapies for spiral artery remodeling disorders and related
pregnancy complications.
Our study also found that 20-HETE can inhibit the
apoptosis of HUVSMCs, and strongly inhibit their apoptosis when
they are co-cultured with HVTs, while HET0016 showed a promoting
effect on apoptosis. To the best of our knowledge, this is the
first time that an inhibitory role on HUVSMC apoptosis is
demonstrated for 20-HETE. The extracellular death receptor pathway
is the mediator of HVT-induced apoptosis of spiral arterial
endothelial cells and smooth muscle cells (25–27).
HVTs can express certain factors (TNF-α, FasL and TRAIL) that bind
to their relative receptors and activate caspase-3, thereby
inducing apoptosis (6). A recent
study found that 20-HETE is involved in cell apoptosis by adjusting
the activity of caspase-3 (10).
It was hypothesized that 20-HETE may inhibit apoptosis of HUVSMCs
via regulating caspase-3, but this hypothesis on the underlying
mechanism was not confirmed.
In addition, our study revealed that the combination
of 20-HETE with HET0016 surprisingly exerts similar, and even
stronger, effects compared to treatment with 20-HETE alone. A
comprehensive review of the current literature suggested that:
HET0016 selectively inhibits the de novo synthesis of
20-HETE by targeting CYP 4A and 4F, while it can not inhibit the
activity of total free 20-HETE in vivo, and polymorphisms in
the CYP 4A and CYP 4F genes may result in related
functional changes (28–30). Another type of AA metabolite, EETs,
was also found to be involved in vascular protection,
anti-inflammatory and renal excretion, and in endogenous
compression. Under normal physiological conditions, HVTs, the
placenta, amnion, decidua and gravid myometrium cells all express
EETs (31). The in vivo
secretion of 20-HETE and EETs is regulated by independent isozymes,
while their biological activities intertwine, so that 20-HETE and
EETs can both regulate the fluid balance through inhibiting
relative ion channels and the PI3K/AKT signaling pathway (12,15,31,32).
In our experiments, a potential feedback effect of 20-HETE and
HET0016 on EETs was not explored. HET0016 could not inhibit the
activity of total free 20-HETE in vivo; the high
concentration HET0016 could not only inhibit the synthesis of
20-HETE, but also inhibit the synthesis of consecutive products of
arachidonic acid (29). Along the
course of development of 20-HETE inhibitors, which started with
17-ODYA, and eventually led to 1-ABT and HET0016, the specificity
of the inhibition gradually increased (13,17,30).
Since the metabolites of AA are mainly isomers, the development of
inhibitors has presented certain difficulties, and thus the
specificity of HET0016 still requires further verification.
The results from the present study confirmed that
20-HETE can inhibit the invasion and apoptosis of HVTs, and
strongly inhibit the apoptosis of HUVSMCs when co-cultured with
HVTs. HVT and HUVSMCs are the main cells involved in USAR. During
the remodeling process, the invasive ability of HVTs and the
apoptosis of HUVSMCs are altered, which leads to the remodeling
disorder, and indicates that abnormalities in the 20-HETE
metabolism in the placenta uterina may lead to the spiral arteries
remodeling disorder. At the early stages of pregnancy, USAR
disorders are centrally associated with the occurrence of PE.
Further investigation on the mechanism of action of 20-HETE with
regards to the reported effects on the biological behavior of HVTs
and HUVSMCs, may provide new clues for the etiology and
pathophysiology of PE.
It should be noted that, due to sampling
difficulties, research on USAR has its limitations (5). Here, in order to study spiral artery
remodeling, we used uterine vascular smooth muscle cells instead of
spiral arterial smooth muscle cells. It is generally established
from previous studies that the biological functions of uterine
arterial and spiral arterial smooth muscle cells are similar
(5,6).
In conclusion, as an upstream regulatory factor in
multiple signal transduction pathways involved in the remodeling
process and in vascular maintenance, 20-HETE can effectively
inhibit the invasion of HVTs and strongly inhibit the apoptosis of
uterine smooth muscle cells co-cultured with HVTs. This indicates
that abnormalities in the metabolism of 20-HETE cause inaccurate
transduction of the associated signals, which may lead to USAR and
vascular functional disorders, and eventually cause PE.
References
|
1
|
Shennan AH, Redman C, Cooper C and Milne
F: Are most maternal deaths from pre-eclampsia avoidable? Lancet.
379:1686–1687. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Young BC, Levine RJ and Karumanchi SA:
Pathogenesis of preeclampsia. Annu Rev Pathol. 5:173–192. 2010.
View Article : Google Scholar
|
|
3
|
Brosens I, Robertson WB and Dixon HG: The
physiological response of the vessels of the placental bed to
normal pregnancy. J Pathol Bacteriol. 93:569–579. 1967. View Article : Google Scholar
|
|
4
|
Harris LK: Review: trophoblast-vascular
cell interations in early pregnancy: how to remodel a vessel.
Placenta. 31:S93–S98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pijnenborg R, Vercruysse L and Hanssens M:
The uterine spiral arteries in human pregnancy: facts and
controversies. Placenta. 27:939–958. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Whitley GS and Cartwright JE: Cellular and
molecular regulation of spiral artery remodelling: lessons from the
cardiovascular field. Placenta. 31:465–474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bulmer JN, Innes BA, Levey J, et al: The
role of vascular smooth muscle cell apoptosis and migration during
uterine spiral artery remodeling in normal human pregnancy. FASEB
J. 26:2975–2985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo AM, Scicli G, Sheng J, Falck JC,
Edwards PA and Scicli AG: 20-HETE can act as a nonhypoxic regulator
of HIF-1alpha in human microvascular endothelial cells. Am J
Physiol Heart Circ Physiol. 297:H602–H613. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, Ackerman R and Guo AM: 20-HETE in
neovascularization. Prostaglandins Other Lipid Mediat. 98:63–68.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dhanasekaran A, Bodiga S, Gruenloh S, et
al: 20-HETE increases survival and decreases apoptosis in pulmonary
arteries and pulmonary artery endothelial cells. Am J Physiol Heart
Circ Physiol. 296:H777–H786. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pat Kunert M and Drenjancević I:
20-hydroxyeicosatetraenoic acid, endothelial dysfunction and
hypertension. Med Glas (Zenica). 8:170–180. 2011.PubMed/NCBI
|
|
12
|
Llinás MT, Alexander BT, Capparelli MF,
Carroll MA and Granger JP: Cytochrome P-450 inhibition attenuates
hypertension induced by reductions in uterine perfusion pressure in
pregnant rats. Hypertension. 43:623–628. 2004.PubMed/NCBI
|
|
13
|
Stec DE, Gannon KP, Beaird JS and Drummond
HA: 20-Hydroxyeicosatetraenoic acid (20-HETE) stimulates migration
of vascular smooth muscle cells. Cell Physiol Biochem. 19:121–128.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hazan AD, Smith SD, Jones RL, Whittle W,
Lye SJ and Dunk CE: Vascular-leukocyte interactions: mechanisms of
human decidual spiral artery remodeling in vitro. Am J Pathol.
177:1017–1030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McGiff JC and Quilley J: 20-HETE and the
kidney: resolution of old problems and new beginnings. Am J
Physiol. 277:R607–R623. 1999.PubMed/NCBI
|
|
16
|
Alexanian A and Sorokin A: Targeting
20-HETE producing enzymes in cancer - rationale, pharmacology, and
clinical potential. Onco Targets Ther. 6:243–255. 2013.PubMed/NCBI
|
|
17
|
Guengerich FP and Rendic S: Update
information on drug metabolism systems-2009, part I. Curr Drug
Metab. 11:1–3. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyata N, Taniguchi K, Seki T, et al:
HET0016, a potent and selective inhibitor of 20-HETE synthesizing
enzyme. Br J Pharmacol. 133:325–329. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z, Tang X, Li Y, et al:
20-Hydroxyeicosatetraenoic acid inhibits the apoptotic responses in
pulmonary artery smooth muscle cells. Eur J Pharmacol. 588:9–17.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bodiga S, Gruenloh SK, Gao Y, et al:
20-HETE-induced nitric oxide production in pulmonary artery
endothelial cells is mediated by NADPH oxidase,
H2O2, and PI3-kinase/Akt. Am J Physiol Lung
Cell and Mol Physiol. 298:L564–L574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soares MJ, Chakraborty D, Renaud SJ, et
al: Regulatory pathways controlling the endovascular invasive
trophoblast cell lineage. J Reprod Dev. 58:283–287. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lambert E, Dassé E, Haye B and Petitfrère
E: TIMPs as multifacial proteins. Crit Rev Oncol Hematol.
49:187–198. 2004. View Article : Google Scholar
|
|
23
|
Palei AC, Granger JP and Tanus-Santos JE:
Matrix metalloproteinases as drug targets in preeclampsia. Curr
Drug Targets. 14:325–334. 2013.PubMed/NCBI
|
|
24
|
Lavee M, Goldman S, Daniel-Spiegel E and
Shalev E: Matrix metalloproteinase-2 is elevated in midtrimester
amniotic fluid prior to the development of preeclampsia. Reprod
Biol Endocrinol. 7:852009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ashton SV, Whitley GS, Dash PR, et al:
Uterine spiral artery remodeling involves endothelial apoptosis
induced by extravillous trophoblasts through Fas/FasL interactions.
Arterioscler Thromb Vasc Biol. 25:102–108. 2005.
|
|
26
|
Harris LK, Keogh RJ, Wareing M, et al:
Invasive trophoblasts stimulate vascular smooth muscle cell
apoptosis by a fas ligand-dependent mechanism. Am J Pathol.
169:1863–1874. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stoneman VE and Bennett MR: Role of
Fas/Fas-L in vascular cell apoptosis. J Cardiovasc Pharmacol.
53:100–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peppiatt C and Attwell D: Neurobiology:
feeding the brain. Nature. 431:137–138. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hoff U, Lukitsch I, Chaykovska L, et al:
Inhibition of 20-HETE synthesis and action protects the kidney from
ischemia/reperfusion injury. Kidney Int. 79:57–65. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu CC and Schwartzman ML: The role of
20-HETE in androgen-mediated hypertension. Prostaglandins Other
Lipid Mediat. 96:45–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang H, McGiff JC, Fava C, et al:
Maternal and fetal epoxyeicosatrienoic acids in normotensive and
preeclamptic pregnancies. Am J Hypertens. 26:271–278. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Attwell D, Buchan AM, Charpak S, Lauritzen
M, Macvicar BA and Newman EA: Glial and neuronal control of brain
blood flow. Nature. 468:232–243. 2010. View Article : Google Scholar : PubMed/NCBI
|