Introduction
Osteosarcoma is an aggressive type of malignant
cancer, which develops from primitive transformed cells of
mesenchymal origin. Osteosarcoma is the most common histological
form of primary bone cancer (1).
Despite recent improvements in the long term prognosis of patients
with osteosarcoma, the identification of novel therapeutic
molecular targets and therapeutic strategies for the prevention and
treatment of osteosarcoma are required. The development of
osteosarcoma involves the accumulation of multiple genetic and
epigenetic changes in critical genes that control cell
proliferation and migration (2).
Understanding these mechanisms of proliferative alteration and
advanced metastasis in osteosarcoma is important for the treatment
of the disease.
Krüppel-like factor 8 (KLF8) is a ubiquitously
expressed Krüppel-like transcription factor. Members of the KLF
family contain a C-terminal DNA-binding domain with three
Krüppel-like zinc fingers (3,4).
Several members of the KLF family have been shown to have diverse
functions in various cell types (5). KLF8 has been reported to be a
critical mediator of oncogenic transformation, EMT and invasion
(6–9). Increased KLF8 expression has been
observed in several cancer tissues compared with that in normal
tissues (10,11). Furthermore, developments in
functional genomics have led to a greater focus on the biological
function of KLF8 and its interaction with genes and proteins.
Recent research has identified poly (ADP-ribose) polymerase 1 and
matrix metallopeptidase 9 as novel KLF8-interacting and -regulating
proteins (12,13). In addition, KLF8 has been
identified to be a novel Wnt/beta-catenin signaling target gene and
regulator (14).
The role of KLF8 in osteosarcoma is yet to be
elucidated. In the present study, lentivirus-mediated siRNA was
employed to knockdown KLF8 expression in the Saos-2 human
osteosarcoma cell line. The effects of KLF8 on osteosarcoma cell
growth and invasion were subsequently investigated.
Materials and methods
Materials
The Saos-2 cell line was purchased from the
Institute of Biochemistry and Cell Biology (Shanghai, China). KLF8
and GAPDH primers were synthesized by Applied Biosystems (Carlsbad,
CA, USA). All antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) unless stated otherwise.
Drugs and reagents
3-(4,5)-dimethylthiahiazo(-z-yl)-3,5-di-phenytetrazoliumromide
(MTT) was purchased from Shanghai Dingguo Biological Technology
Co., Ltd. (Shanghai, China). Dulbecco’s modified Eagle’s medium
(DMEM) and fetal bovine serum (FBS) were purchased from Thermo
Fisher Scientific Inc. (Waltham, MA, USA). TRIzol®
Reagent and Lipofectamine® 2000 were purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA). M-MLV Reverse
Transcriptase was purchased from Promega Corporation (Madison, WI,
USA) and SYBR®-Green PCR Master mix was purchased from
Takara Bio Inc. (Shiga, Japan). A Cell cycle analysis kit and an
apoptosis kit were purchased from Nanjing KeyGen Biotech., Co.,
Ltd. (Nanjing, China). An ECL-PLUS™ kit was purchased from GE
Healthcare (Piscataway, NJ, USA).
Cell culture
Saos-2 cells were cultured in DMEM supplemented with
10% heat-inactivated FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin. Cells were incubated in a humidified atmosphere
containing 5% CO2 at 3°C.
Lentivirus packaging and infection
Small interfering (si)RNA targeting the KLF8 gene
(CAGCACTGTTTAATGACAT) and negative control siRNA
(TTCTCCGAACGTGTCACGT) were cloned into a pGCSIL-green fluorescent
protein (GFP) vector (Shanghai GeneChem Co., Ltd., Shanghai,
China). The siRNA plasmids were transfected into 293T cells,
together with two lentiviral packaging plasmids (pHelper1.0 and
pHelper2.0; Shanghai Gene ChemCo., Ltd.) to generate a lentivirus.
After three days of incubation, the culture medium containing the
recombinant virus was collected and concentrated using
Centricon®-plus-20 (Millipore, Billerica, CA, USA). For
lentiviral infection, 5×104/well Saos-2 cells were
incubated with the KLF8 siRNA-expressing lentivirus and
non-silencing control lentivirus [multiplicity of infection
(MOI)=20] for 24 h. The culture medium was then replaced.
Quantitative polymerase chain reaction
(qPCR) analysis
Lentiviral transduction efficiency was validated
using qPCR analysis after transduction for 72 h. Total RNA was
extracted using TRIzol reagent and reverse transcribed using M-MLV
Reverse Transcriptase according to the manufacturer’s instructions.
The resulting complementary (c)DNA was used for qPCR analysis using
SYBR-Green PCR Master mix. qPCR analysis was performed in
triplicate using the TP800 qPCR System (Takara Bio Inc.). Target
gene expression was normalized to that of the endogenous control
GAPDH. The relative quantitative expression of the target gene
compared with GAPDH was expressed as 2−(Ct-Cc) (Ct and
Cc represent the mean threshold cycle differences following
normalization to GAPDH). The qPCR primer sequences were as follows:
Forward: 5′-TTCAGAAGGTGGCTCAATGC-3′ and reverse:
5′-GGAGTGTTGGAGAAGTCATATTAC-3′ for KLF8; and forward:
5′-TGACTTCAACAGCGACACCCA-3′ and reverse:
5′-GGAGTGTTGGAGAAGTCATATTAC-3′ for GAPDH.
Western blot analysis
Lentiviral transduction efficiency was validated
using western blot analysis after transduction for four days. In
brief, cells were harvested following four days of infection and
treated with buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM
NaCl, 25 mM β-glycerophosphate, 50 mM NaF, 1 mM
Na3VO4, 1% Triton X-100, 10% glycerol and
protease inhibitors (1 mM phenylmethylsufonyl fluoride and 1 mg/ml
aprotinin, pepstatin A, and leupeptin). Cell lysates were separated
using 12% SDS-PAGE and transferred onto polyvinylidene fluoride
membranes (Millipore). Subsequent to blocking, membranes were
incubated in milk containing mouse anti-KLF8 monoclonal antibodies
(dilution, 1:1,000; Abcam, Cambridge, MA, USA). Western blots were
developed using horseradish peroxidase-conjugated goat anti-mouse
immunoglobulin G (dilution 1:5,000) and the immunoreactive bands
were detected using an enhanced chemiluminescence reagent
(Millipore). Endogenous GAPDH was used as an internal control.
Cell proliferation assay
Following confirmation of the transduction
efficiency, cells were seeded onto 96-well plates at a density of
2,000 cells/well on day zero for the MTT assay. Cell growth was
measured daily until day five. A volume of 20 μl MTT solution (5
mg/ml) was added into each well. Following incubation for 4 h at
3°C, 150 μl dimethylsulfoxide was added to dissolve the crystals.
After incubation for 10 min at room temperature, the absorbance was
read at 490 nm on the Shimadzu UV-1603 spectrophotometer (Shimadzu
Corp., Kyoto, Japan).
Colony forming assay
To determine the long-term inhibitory effect of the
lentivirus, Saos-2 cells were cultured in six-well plates at a
density of 200 cells/well and were treated with KLF8 siRNA
lentivirus (RNAi+) or non-silencing siRNA lentivirus
(RNAi−). Cells were incubated at 37°C in air with 5%
CO2 and the medium was replaced every three days. After
ten days, colonies were stained with Giemsa and the number of
colonies containing >50 cells were counted in each well.
Fluorescence-activated cell sorting
(FACS) cell cycle analysis
Saos-2 cells infected with the KLF8 siRNA-expressing
lentivirus and negative control lentivirus were collected three
days following infection. For cell cycle analysis, cells were
collected and cultured in 6-cm dishes until they reached 80%
confluency. A total of 1×106 cells were harvested and
fixed in 70% ethanol for 1 h. Subsequent to three washes, cells
were treated with 50 μl/ml propidium iodide (PI) solution
(Sigma-Aldrich, St. Louis, MO, USA) and 100 μl/ml RNase in
phosphate-buffered saline for 15 min at room temperature in the
dark. Flow cytometric analysis was then performed using a BD
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Transwell® invasion assay
An in vitro cell invasion assay was performed
using a Transwell unit (8-μm pore size) with
polyvinylpyrrolidone-free polycarbonate filters coated with 500
μg/ml BD Matrigel™ Basement Membrane Matrix (BD Biosciences) placed
in 24-well Transwell chambers. Saos-2 cells were placed in the
upper compartment of the chamber and cells were allowed to attach
for 8 h. Cells were then incubated in FBS-free medium for 36 h at
37°C in 5% CO2. DMEM containing 10% FBS was placed in
the lower compartment of the chamber. Following incubation for 24
h, the filter inserts were removed from the wells and the cells on
the upper side of the filter were removed using cotton swabs. The
cells that had invaded the lower surface of the membrane were fixed
using methanol and stained with 0.5% crystal violet for 10 min.
Cells that had migrated to the lower side of the filter were scored
visually in five random fields using a light microscope (10×
objective lens; Nikon, Tokyo, Japan). The number of cells from
three filters was then averaged. In addition, the invaded cells
were lysed and quantified at 570 nm using the Shimadzu UV-1603
spectrophotometer. The experiments were repeated three times with
three wells for each treatment.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5.0 software (GraphPad Software, Inc., San Diego, CA, USA).
Continuous variables were compared using Student’s t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
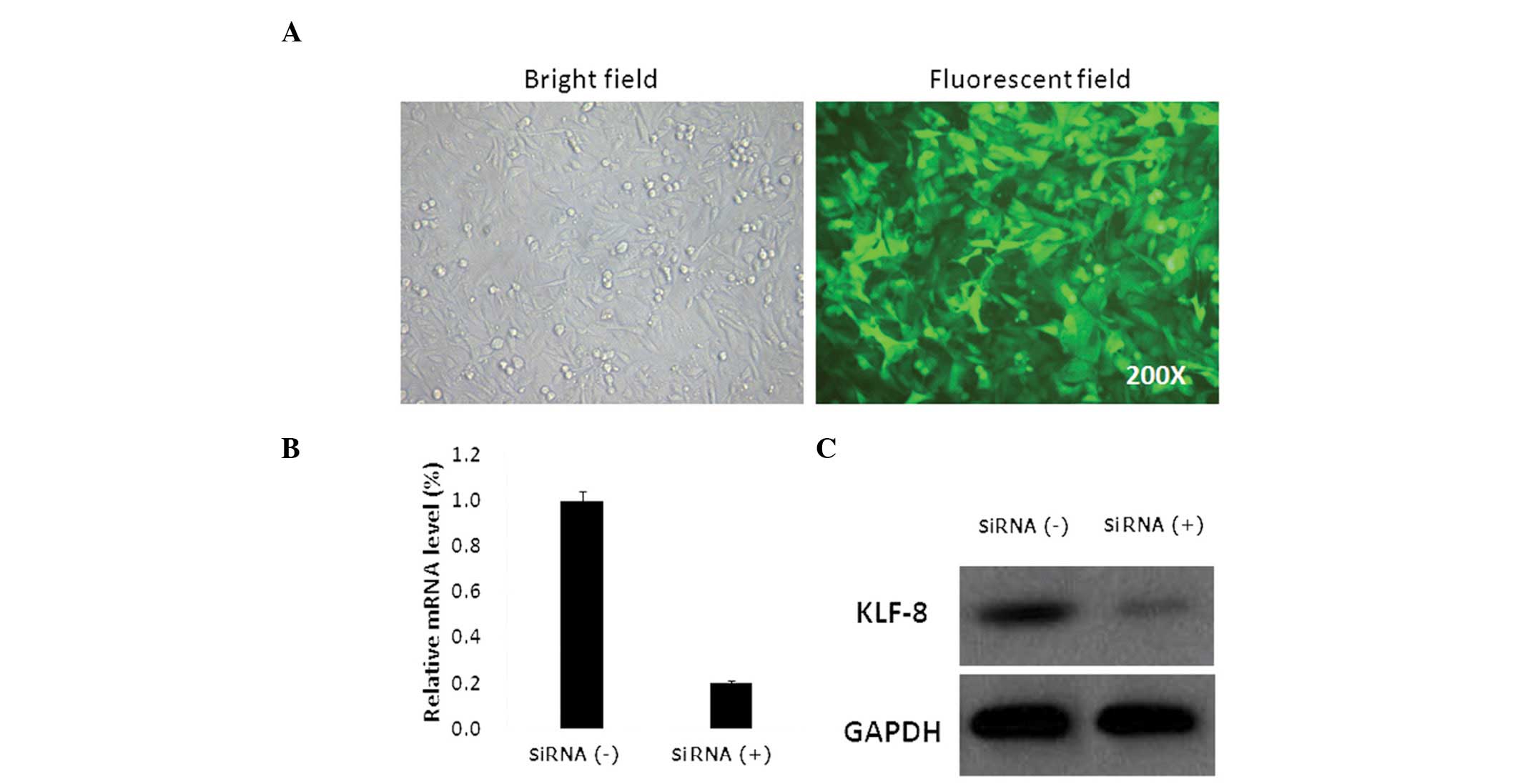
Lentivirus-mediated knockdown of KLF8 in
Saos-2 cells
To determine the effect of KLF8 expression on
osteosarcoma cell growth, lentiviruses expressing KLF8-specific
siRNA were generated. GFP expression was observed in >90% of
Saos-2 cells 72 h after lentivirus infection at an MOI=40 (Fig. 1A). qPCR analysis revealed that KLF8
mRNA expression in Saos-2 cells infected with KLF8 lentiviral siRNA
was significantly decreased. (P<0.05; Fig. 1B). Western blot analysis of cell
lysates extracted four days after lentiviral infection revealed
that KLF8 protein expression was also decreased (Fig. 1C). These findings demonstrate that
the lentivirus transduction system successfully downregulated KLF8
expression at the mRNA and protein levels compared with the Saos-2
cells infected with nonsense lentiviral siRNA.
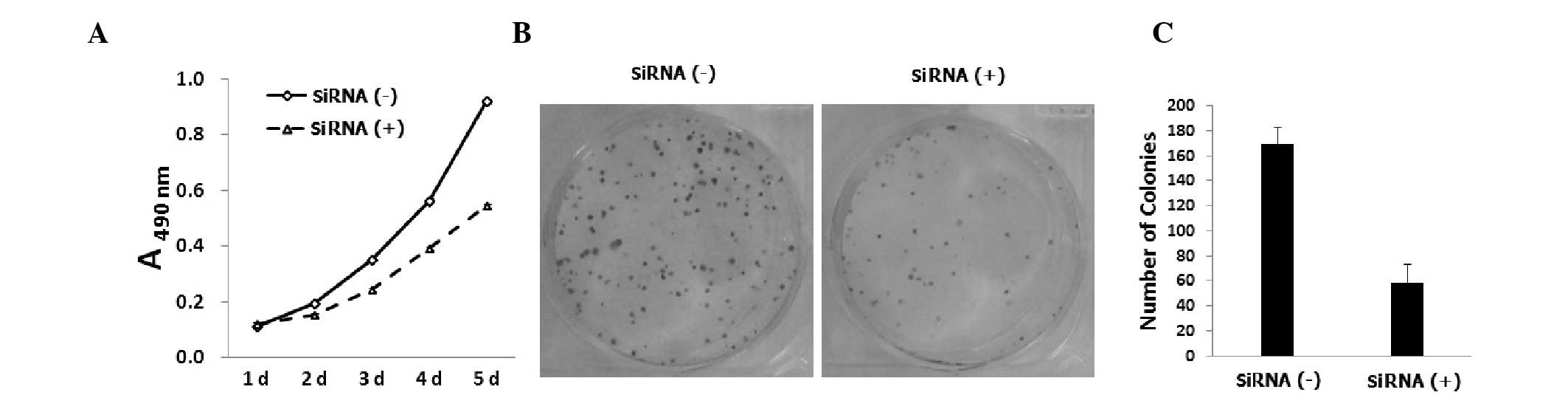
Saos-2 cell proliferation is inhibited by
KLF8 siRNA
To assess the effect of KLF8 knockdown on
osteosarcoma cell proliferation, Saos-2 cells were infected with
KLF8 siRNA-expressing lentiviral vectors and viable cells were
counted using an MTT assay five days post-infection. As shown in
Fig. 2A, KLF8 knockdown was found
to decreased the number of Saos-2 cells compared with the control
siRNA-infected cells (P<0.05). Lentiviral KLF8 siRNA-infected
Saos-2 cells were analyzed using a colony forming assay.
Downregulation of KLF8 was found to reduce the number of viable
Saos-2 cell colonies (Fig. 2B),
suggesting that the KLF8 siRNA-treated cells had a lower colony
formation ability compared with the control siRNA-infected cells
(P<0.05; Fig. 2C). In summary,
KLF8 knockdown was observed to inhibit cell growth and colony
formation in Saos-2 cells.
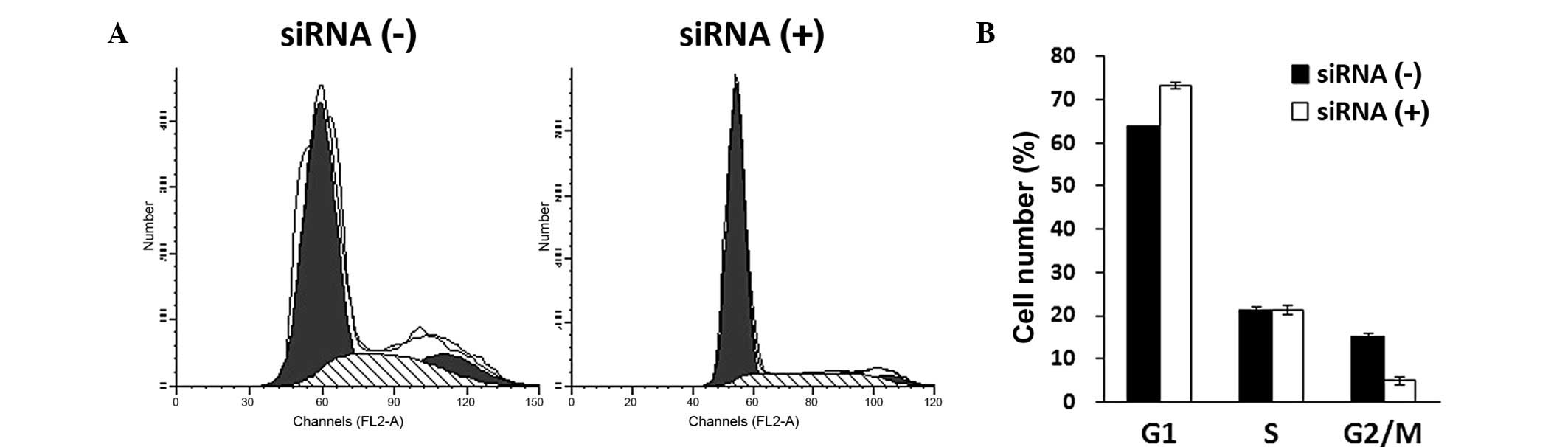
KLF8 knockdown arrests Saos-2 cells in
G0/G1-phase
Cell cycle distribution was assessed in
KLF8-knockdown cells using PI staining and FACS analysis five days
after lentiviral infection. Lentivirus-mediated KLF8-siRNA
infection affected cell cycle distribution in Saos-2 cells, as
shown in Fig. 3A. Statistical
analysis revealed that KLF8 siRNA treatment arrested Saos-2 cells
in G0/G1-phase of interphase of the cell
cycle. Furthermore, the number of Saos-2 cells in G2/M
phase was observed to be significantly reduced (P<0.05; Fig. 3B), indicating that DNA replication
was impaired following KLF8 knockdown.
KLF8 knockdown suppresses Saos-2 cell
invasion
To investigate the effect of KLF8 knockdown on
osteosarcoma cell invasion, Saos-2 cells were analyzed using
a Transwell assay and crystal violet staining. As shown in Fig. 4, the invasive potential of Saos-2
cells was significantly reduced with lentivirus-mediated KLF8 siRNA
treatment compared with the control group (P<0.05), indicating
that KLF8 may have a role in promoting osteosarcoma cell
invasion.
Discussion
KLF8 exhibits conserved C2H2 zinc finger domains at
its C-terminus through which it binds DNA, as well as a PVALS/T
motif at its N-terminus through which it interacts with
co-repressor C-terminal binding protein. KLF8 inhibits the
expression of genes containing a CACCC element and KLF8 is
overexpressed in several types of tumor cells, including gliomas
(15), ovarian (7), renal (16), hepatocellular (10), gastric (6) and breast carcinoma (13) cells.
The mechanism underlying KLF8 activation in cancer
cells is yet to be elucidated. Overexpression of KLF8 has been
reported to be highly correlated with decreased E-cadherin
expression, which is associated with cancer cell invasion (17). In a previous study, KLF8 expression
was found to be regulated by focal adhesion kinase signaling
(18). However, the function of
KLF8 in human osteosarcoma remains unknown.
Cancer cells undergo malignant proliferation and
have the capacity to migrate into the surrounding tissue, which is
known as metastasis. In the present study, downregulation of KLF8
was found to inhibit Saos-2 cell proliferation and colony
formation. It was hypothesized that alterations in cell cycle
progression may be the primary mechanism involved in this
inhibition of cancer cell growth. Therefore, the present study
investigated the effect of KLF8-siRNA lentiviral infection on
Saos-2 cell cycle arrest and its association with Saos-2 cell
growth inhibition. A significant increase in
G0/G1-phase cell cycle arrest was observed in
the KLF8 knockdown cells and this was found to account for the
inhibitory effect of KLF8 siRNA on Saos-2 cell proliferation.
Moreover, Saos-2 cell invasion was observed to be suppressed
following KLF8 knockdown. These findings show that KLF8 knockdown
reduced survival and invasion in Saos-2 osteosarcoma cells.
Therefore, lentivirus-mediated KLF8 siRNA treatment may be an
effective therapeutic strategy for osteosarcoma.
Studies have revealed that numerous genes, including
oncogenes and tumor suppressor genes are involved in the complex,
multi-step process of tumorigenesis (19,20).
Advances in bioscience have enhanced the understanding of the
molecular mechanisms underlying osteosarcoma progression. There are
now promising research opportunities to screen and identify
molecular targets for anticancer applications. The present study
identified the important role of KLF8 in osteosarcoma and its
potential as a biomarker for diagnosis and therapy. The present
study provides evidence that lentivirus-mediated KLF8 knockdown
inhibits growth and invasion in osteosarcoma cells, suggesting that
KLF8 may be a potential therapeutic biomarker for osteosarcoma.
Further investigations into the molecular mechanisms underlying the
regulation of cell proliferation and invasion by KLF8 are required
in the future.
Acknowledgements
This study was supported by grants from the Research
Projects of Shanghai Health Bureau (grant no. 2011171) and the
National Natural Science Foundation of China (grant no.
81001192).
References
|
1
|
Raymond AK and Jaffe N: Osteosarcoma
multidisciplinary approach to the management from the pathologist’s
perspective. Cancer Treat Res. 152:63–84. 2009.PubMed/NCBI
|
|
2
|
Pogribny IP and Rusyn I: Environmental
toxicants, epigenetics, and cancer. Adv Exp Med Biol. 754:215–232.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eaton SA, Funnell AP, Sue N, Nicholas H,
Pearson RC and Crossley M: A network of Krüppel-like Factors
(Klfs). Klf8 is repressed by Klf3 and activated by Klf1 in vivo. J
Biol Chem. 283:26937–26947. 2008.
|
|
4
|
van Vliet J, Turner J and Crossley M:
Human Krüppel-like factor 8: a CACCC-box binding protein that
associates with CtBP and represses transcription. Nucleic Acids
Res. 28:1955–1962. 2000.
|
|
5
|
Li L and Davie JR: The role of Sp1 and Sp3
in normal and cancer cell biology. Ann Anat. 192:275–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang H, Liu L, Wang Y, et al: KLF8
involves in TGF-beta-induced EMT and promotes invasion and
migration in gastric cancer cells. J Cancer Res Clin Oncol.
139:1033–1042. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu H, Wang X, Urvalek AM, et al:
Transformation of human ovarian surface epithelial cells by
Krüppel-like factor 8. Oncogene. 33:10–18. 2014.PubMed/NCBI
|
|
8
|
Wang X and Zhao J: KLF8 transcription
factor participates in oncogenic transformation. Oncogene.
26:456–461. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu H, Hu L, Li T, et al: A novel role of
Krüppel-like factor 8 in DNA repair in breast cancer cells. J Biol
Chem. 287:43720–43729. 2012.
|
|
10
|
Li JC, Yang XR, Sun HX, et al:
Up-regulation of Krüppel-like factor 8 promotes tumor invasion and
indicates poor prognosis for hepatocellular carcinoma.
Gastroenterology. 139:2146–2157. 2010.
|
|
11
|
Chen ZY, Shie J and Tseng C: Up-regulation
of gut-enriched krüppel-like factor by interferon-gamma in human
colon carcinoma cells. FEBS Lett. 477:67–72. 2000.
|
|
12
|
Lu H, Wang X, Li T, et al: Identification
of poly (ADP-ribose) polymerase-1 (PARP-1) as a novel Kruppel-like
factor 8-interacting and -regulating protein. J Biol Chem.
286:20335–20344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Lu H, Urvalek AM, et al: KLF8
promotes human breast cancer cell invasion and metastasis by
transcriptional activation of MMP9. Oncogene. 30:1901–1911. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang T, Cai SY, Zhang J, et al:
Krüppel-like factor 8 is a new Wnt/beta-catenin signaling target
gene and regulator in hepatocellular carcinoma. PLoS One.
7:e396682012.
|
|
15
|
Schnell O, Romagna A, Jaehnert I, et al:
Krüppel-like factor 8 (KLF8) is expressed in gliomas of different
WHO grades and is essential for tumor cell proliferation. PLoS One.
7:e304292012.
|
|
16
|
Fu WJ, Li JC, Wu XY, et al: Small
interference RNA targeting Krüppel-like factor 8 inhibits the renal
carcinoma 786-0 cells growth in vitro and in vivo. J Cancer Res
Clin Oncol. 136:1255–1265. 2010.
|
|
17
|
Wang X, Zheng M, Liu G, et al:
Krüppel-like factor 8 induces epithelial to mesenchymal transition
and epithelial cell invasion. Cancer Res. 67:7184–7193. 2007.
|
|
18
|
Wang X, Urvalek AM, Liu J and Zhao J:
Activation of KLF8 transcription by focal adhesion kinase in human
ovarian epithelial and cancer cells. J Biol Chem. 283:13934–13942.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto T: Molecular basis of cancer:
oncogenes and tumor suppressor genes. Microbiol Immunol. 37:11–22.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weinberg RA: The molecular basis of
oncogenes and tumor suppressor genes. Ann NY Acad Sci. 758:331–338.
1995. View Article : Google Scholar : PubMed/NCBI
|