Introduction
Claudins (CLDNs) are tetraspan membrane proteins
consisting of two extracellular loops protruding into the
intercellular space. The CLDN family consists of at least 27
members. Each type of CLDN has a different expression pattern and
function according to tissue type. In structure of CLDNs, the first
extracellular loop determines the ion selectivity of CLDNs;
sealing, anion- or cation-selective channel (1–6).
Certain CLDNs (CLDN1, 3, 5, 11 and 19) contain a non-charged first
loop and thus do not exhibit ion selectivity, therefore, they
function to seal intercellular junctions. These CLDNs are expressed
in tight epithelial cells, including in the skin, rather than in
leaky epithelial cells.
CLDNs in tight junctions accumulate within
epithelial or endothelial cells that are critical for paracellular
transport indicating a novel function of CLDNs as channel proteins.
These functions have been investigated by measuring ion
transportation in vitro and in vivo under
physiological or pathological conditions (7). CLDN2 and 15 are well known
cation-selective channel proteins (8,9).
Additionally, CLDN10a is anion-selective while 10b is
cation-selective (6). When CLDN10b
is more widely distributed in epithelial cells in various tissues,
CLDN10a is only detected in the uterus and kidney (10). A number of the CLDNs (CLDN4, 7, 8
and 16) have inconsistent ion selectivity, and they function as
ion-selective channels and sealing proteins (9). CLDN1 and 2 are relatively well
studied (2,11–13),
whereas, the roles of other CLDNs, including CLDN4, 7, 8 and 16,
remain to be defined due to inconsistent results (9). In particular, CLDN4 exhibits varying
functions according to the cell type observed. For example, it
functions as a sodium barrier when overexpressed in MDCK cells,
whereas it acts as a sodium pore during knockdown in M-1 and mIMCD3
cells (14,15). This inconsistency implies that the
function of CLDN4 is correlated with interactions with other tight
junction proteins.
Previous studies have analyzed the expression and
function of CLDNs in various tissues and species. In the present
study, the distribution of well-known CLDN family members was
examined in order to compare the expression and correlation with
other CLDN proteins.
The epithelial and endothelial cells are positive
regions and are a novel location in which ion permeability could be
more directly controlled by the action of a tight junction. In
order to investigate the expression of CLDNs in various tissues,
the quantification of CLDN genes, including CLDN3, 4,
5, 7, 8, 10a, 10b, 11,
14, 15 and 17, was performed. Notably, the
majority of CLDN transcripts were detected in various tissues.
Since these observations were noteworthy, the distribution and
expression of several CLDN proteins, CLDN1, 2, 12, and 16 that are
responsible for ion transport, in particular calcium, in the
intestine and kidney (16,17) were intensively investigated.
Materials and methods
Animals and tissues
Five age-matched (age, 9–10 weeks) male C57BL/6 mice
were obtained from Koatech (Pyeongtaek, Gyeonggi, Korea). All the
mice were euthanized by ether, and tissue samples from the
duodenum, ileum, colon, kidney, liver and lung were obtained. These
samples were used for quantitative polymerase chain reaction
(qPCR), western blotting and immunohistochemistry analysis. The
Ethics Committee of Chungbuk National University (Cheonju, Korea)
approved all the experimental procedures.
RNA extraction and qPCR
In order to extract the total RNA, TRIzol reagent
(Ambion, Austin, TX, USA) was used according to the manufacturer’s
instructions. The total RNA concentration was determined by
measuring the absorbance at 260 nm by EPOCH (BioTek Instruments,
Inc., Winooski, VT, USA). To synthesize the first-strand
complementary DNA (cDNA), 1 μg total RNA was reverse-transcribed
using moloney murine leukemia virus reverse transcriptase (1:1,000;
Invitrogen Life Technologies, Carlsbad, CA, USA) along with random
primers (9-mers; Takara Bio, Inc., Otsu, Shiga, Japan). Reverse
transcription-PCR was performed on a 7300 Real-Time PCR system
(Applied Biosystems, Foster City, CA, USA) according to the
manufacturer’s instructions. β-actin was used for normalization and
relative gene expression levels were quantified using RQ software
(Applied Biosystems). The primer sequences used are listed in
Table I.
 | Table IPrimer list for claudins (CLDNs) used
in the present study. |
Table I
Primer list for claudins (CLDNs) used
in the present study.
| Claudin | Forward | Reverse |
|---|
| CLDN1 |
5′-AGGTCTGGCGACATTAGTGG-3′ |
5′-CGTGGTGTTGGGTAAGAGGT-3′ |
| CLDN2 |
5′-TGGTTCCTGACAGCATGAAA-3′ |
5′-CTTTGGGCTGTTGAGCAGAT-3′ |
| CLDN3 |
5′-GAGATGGGAGCTGGGTTGTA-3′ |
5′-ACGTAGTCCTTGCGGTCGTA-3′ |
| CLDN4 |
5′-ATCGTTGTCCGCGAGTTCTA-3′ |
5′-GCTTGTCGTTGCTACGAGGT-3′ |
| CLDN5 |
5′-GCTGGTGGCACTCTTTGTTA-3′ |
5′-GCACCGTCGGATCATAGAAC-3′ |
| CLDN7 |
5′-TTTCATTGTGGCAGGTCTTG-3′ |
5′-TTGCTTTCACTGCCTGGAC-3′ |
| CLDN8 |
5′-ATGCAGTGCAAGGTCTACGA-3′ |
5′-AGCCGGTGATGAAGAAGATG-3′ |
| CLDN10a |
5′-TCCAACGAATGGAAAGTGACC-3′ |
5′-TCTCCTTCTCCGCCTTGATAC-3′ |
| CLDN10b |
5′-TCGCCTTCGTAGTCTCCATC-3′ |
5′TCTCCTTCTCCGCCTTGATAC-3′ |
| CLDN11 |
5′-CAGGCTTGTAGAGCCCTCAT-3′ |
5′-GTGGGCACATACAGGAAACC-3′ |
| CLDN12 |
5′-AGGAAGTTTGAGCCGGTCTT-3′ |
5′-CGTGATGAATAGGGCTGTGA-3′ |
| CLDN14 |
5′-CTGGGCTTCATCTCCTCATC-3′ |
5′-CATTCAGCCTGTACCCACTGT-3′ |
| CLDN15 |
5′-GCCTCTTTCTAGGCATGGTG-3′ |
5′-TCCAGCATACAGTGGGTTGA-3′ |
| CLDN16 |
5′-CTTGGCCATATTCTCCACTG-3′ |
5′-GAGTCGTACTCATCGCAGGT-3′ |
| CLDN17 |
5′-ATTCCAGTGTCCTGGACTGC-3′ |
5′-ATGCAGGGACTGGGTATCTG-3′ |
| CLDN19 |
5′-TCCTCTTGGCAGGTCTCTGT-3′ |
5′-GTGCAGCAGAGAAAGGAACC-3′ |
qPCR reactions contained 1 μl cDNA template with 10
μl of 2× SYBR Premix Ex Taq (Takara Bio, Inc.) and 10 pmol
specific primers. Each reaction was conducted over 40 cycles of
denaturation at 95°C for 15 sec, annealing at 62°C for 15 sec, and
extension at 72°C for 30 sec. The fluorescence intensity was
measured at the end of the extension phase of each cycle, and was
performed using an ABI Prism 7300 Sequece 10 detections system
(Applied Biosystem) equipped with a 96-well optical reaction plate.
Threshold values for the fluorescence intensity of the samples were
manually set. A threshold cycle was defined as the cycle when
sample fluorescence reached the threshold value. For normalization
purposes, β-actin was used as a control.
Western blotting
Protein extraction was performed using
radioimmunoprecipitation assay (RIPA) buffer [50 mM Tris-HCl
(Amresco Inc., Solon, OH, USA), 1% NP-40 (Calbiochem, Darmstadt,
Germany), 150 mM NaCl (Duchefa Biochemie, Haarlem, The
Netherlands), 1 mM EDTA (Boehringer, Mannheim, Germany) and 2
protease inhibitor cocktail tablets (Roche Diagnostics, Mannheim,
Germany) in 50 ml of total RIPA solution] and homogenized.
Following centrifugation at 13,000 × g, each protein (20 μg/lane)
was separated by 10% SDS-PAGE and transferred to nitrocellulose
membranes (Millipore, Bedford, MA, USA). The membranes were then
blocked with 5% skimmed milk (Merck, Darmstadt, Germany), resolved
in Tris-buffered saline with Tween-20 (TBS-T) for 2 h at room
temperature, and then incubated overnight with the following
primary antibodies at 4°C: Mouse anti-CLDN1, CLDN2 and CLDN5
(1:1,000; Invitrogen Life Technologies), rabbit anti-CLDN4, 12 and
16, (1:1,000; Invitrogen Life Technologies), rabbit anti-CLDN19
(1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), or
rabbit anti-β-actin (1:1,000; Santa Cruz Biotechnology, Inc.)
overnight at 4°C. Following the reaction with antibodies, the
membranes were washed with TBS-T for 1 h at room temperature and
incubated with anti-rabbit horseradish peroxidase-conjugated
secondary antibody (1:3,000; Santa Cruz Biotechnology, Inc.) for 2
h at room temperature. Subsequent to washing with TBS-T again,
antibody binding was detected using an enhanced chemiluminescence
reagent (Amersham Biosciences, Little Chalfont, UK), and was
recorded by exposure to Biomax™ Light film (Kodak Express, London,
UK) for 1–30 min.
Immunohistochemistry
Tissue-specific localizations of CLDNs were examined
by immunohistochemistry. The samples of the duodenum, ileum, colon,
kidney, liver and lung were embedded in paraffin, cut into 5-μm
sections, mounted onto slides glasses (Matunami, Ishikawa, Japan)
coated with amino silane, deparaffinized using xylene and then
hydrated in a descending graded ethanol series. The endogenous
peroxidase activity was blocked by incubation with 3% hydrogen
peroxidase in phosphate-buffered saline (PBS) for 30 min at room
temperature. Non-specific reaction of sections was blocked by
incubation with 10% goat serum (Vector Laboratories, Inc.
Burlingame, CA, USA) in PBS for 1 h at room temperature. Subsequent
to washing using TBS-T, the sections were reacted with biotinylated
secondary antibodies (1:500, rabbit or mouse IgG; Vector
Laboratories, Inc.) for 1 h at 37°C, followed by incubation with
ABC-Elite (Vector Laboratories, Inc.) for 30 min at 37°C.
Diaminobezidine (Sigma, St. Louis, MO, USA) was used as a
chromogen. The sections were then counterstained with hematoxylin
and mounted in CytoSeal™ 60 (Richard-Allan Scientific Co.,
Kalamazoo, MI, USA).
Results
Tissue-specific mRNA expression levels of
CLDNs
The tissue-specific mRNA expression levels of
CLDNs (1–5, 7–8, 10a and b,
11–12, 14–17 and 19) in the duodenum, ileum,
colon, kidney, liver and lung were analyzed by qPCR (Table II). CLDN1 expression was
highest in the liver and moderately higher in the kidney and lung
compared to other tissues (Fig.
1). CLDN2, 3, 4, 5 and 12
were highly expressed overall. Specifically, CLDN2
expression was highest in the kidney, similar in the duodenum,
ileum and kidney and relatively lower in the liver and lung
(Fig. 1). CLDN3 expression
was highest in the colon, similar in the duodenum, ileum and liver,
and relatively low in the kidney. CLDN4 expression was
highest in the ileum, and relatively lower in the liver and lung.
CLDN5 was extremely highly expressed in the lung compared
with the other tissues. CLDN12 was relatively highly
expressed in the kidney and liver (Fig. 1). The mRNA expression of
CLDN7 was higher in the small and large intestines compared
with that in the other organs, although it was highest in the
colon. CLDN8 mRNA expression was highly expressed in the
colon and kidney compared with the tissues. The mRNA levels of
CLDN11 were the highest in the kidney, and CLDN14
transcripts were the highest in the liver. The relative expression
of CLDN10a and 16 mRNA in various tissues was
monitored, and they were shown to be highly expressed in the kidney
compared with that of any other tissues, less expressed in the
liver than that of the kidney, and not expressed in the others. The
CLDN10b mRNA distribution pattern was different from that of
CLDN10a; it was expressed in all the tested tissues and
highly expressed in the kidney compared with the others. The
CLDN17 mRNA expression in the lung was higher compared with
the other tissues. The CLDN19 mRNA expression was highest in
the kidney and relatively high in the liver compared with the lung
and the expression of CLDN19 mRNA was not detected in the
intestine.
 | Figure 1Tissue-specific mRNA expression of
CLDNs in mouse tissues. The mRNA expression of CLDN1, 2, 12 and 16
in the duodenum, ileum, colon, kidney, liver and lung was
investigated by qPCR. CLDN mRNA expression in each tissue was shown
as the percentage of relative expression compared with the that of
the tissue with the highest expression of each CLDNs (CLDN1: liver,
CLDN2, 12 and 16: kidney). CLDNs, claudins; qPCR, quantitative
polymerase chain reaction. |
 | Table IIRelative mRNA expression levels of
CLDNs. |
Table II
Relative mRNA expression levels of
CLDNs.
| Organ-specific
claudin expression |
|---|
|
|
|---|
| Claudin | Duodenum | Ileum | Colon | Kidney | Liver | Lung |
|---|
| CLDN1 | 100.00 | 110.00 | 240.00 | 8300.00 | 38670.00 | 2140.00 |
| CLDN2 | 100.00 | 100.00 | 90.00 | 1380.00 | 20.00 | 10.00 |
| CLDN3 | 100.00 | 100.00 | 290.00 | 20.00 | 100.00 | 80.00 |
| CLDN4 | 100.00 | 150.00 | 90.00 | 80.00 | 2.00 | 14.00 |
| CLDN5 | 100.00 | 310.00 | 350.00 | 1080.00 | 550.00 | 25060.00 |
| CLDN7 | 100.00 | 130.00 | 160.00 | 8.20 | 0.20 | 10.00 |
| CLDN8 | 100.00 | 370.00 | 12800.00 | 43220.00 | 160.00 | 680.00 |
| CLDN10a | - | - | - | 100.00 | 0.10 | - |
| CLDN10b | 100.00 | 210.00 | 680.00 | 401170.00 | 510.0 | 23750.00 |
| CLDN11 | 100.00 | 300.00 | 210.00 | 11140.00 | 20.00 | 630.00 |
| CLDN12 | 100.00 | 90.00 | 210.00 | 770.00 | 640.00 | 100.00 |
| CLDN14 | 100.00 | 120.00 | 2390.00 | 520.00 | 25730.00 | 460.00 |
| CLDN15 | 100.00 | 42.00 | 43.00 | 5.30 | 0.20 | 2.00 |
| CLDN16 | - | - | - | 100.00 | 0.01 | - |
| CLDN17 | 100.00 | 400.00 | 430.00 | 590.00 | 220.00 | 800.00 |
| CLDN19 | - | - | - | 100.00 | 0.07 | 0.04 |
Tissue-specific translational expression
of CLDNs
The protein levels of CLDN1, 2, 12 and 16 were
analyzed in the duodenum, ileum, colon, kidney, liver and lung by
western blotting (Fig. 2). The
CLDN1 protein expression was highest in the kidney and relatively
higher in the lung compared with the duodenum, ileum and liver. The
CLDN2 protein distribution was relatively high in the duodenum,
ileum and colon compared with the kidney, whereas it was absent
from the lung. The protein expression of CLDN12 was detected in all
tested tissues; it was higher in the kidney and colon compared with
the liver and lung. The protein distribution of CLDN16 was similar
to its mRNA distribution; it was only expressed in the kidney and
liver and was highest in the kidney. The expression of CLDN4 and 19
proteins was not detectable.
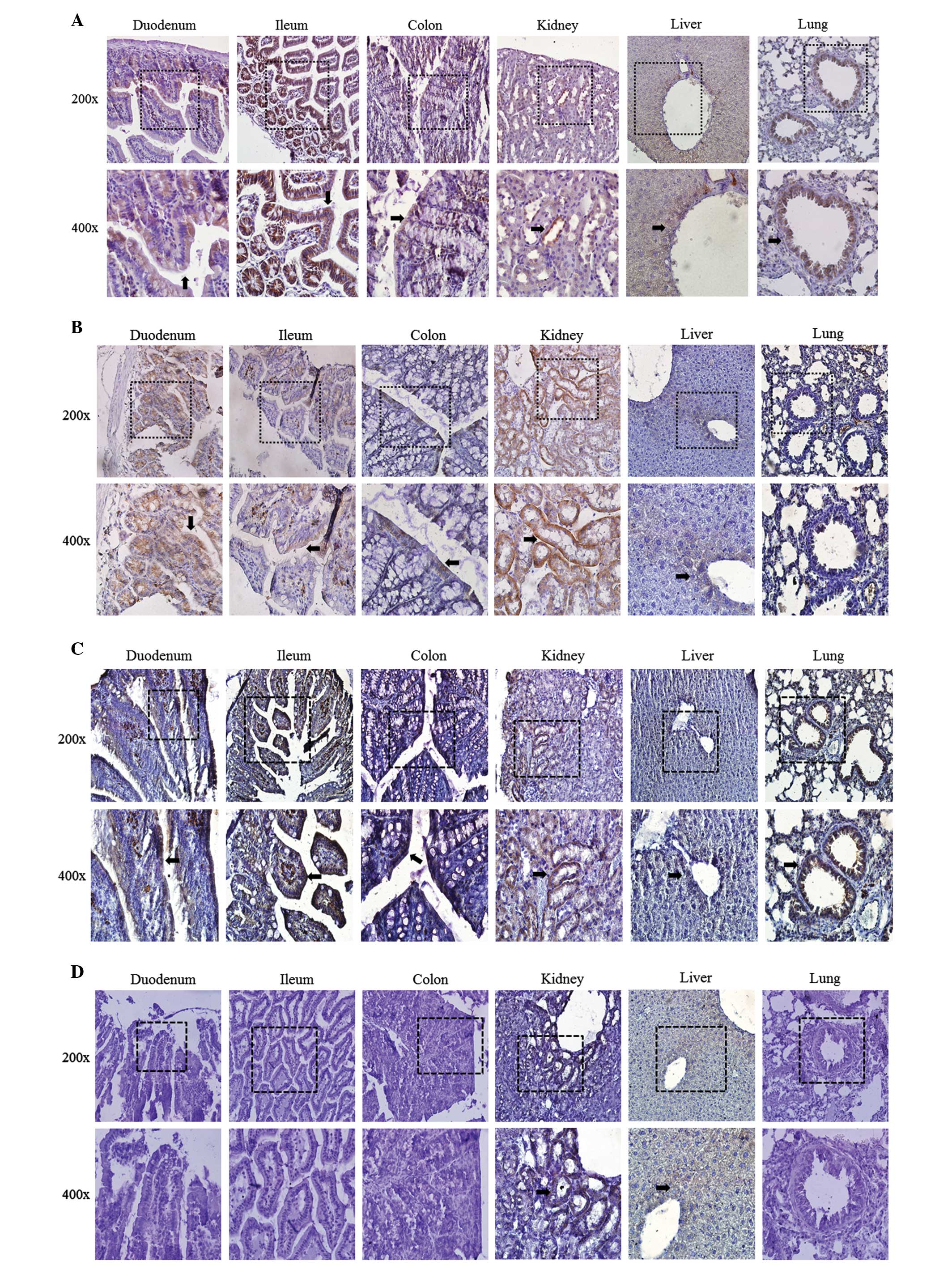
Tissue-specific localization of
CLDNs
CLDNs were commonly expressed in erythrocytes of the
small/large intestine, hepatocytes of the liver and epithelial
cells of teh bronchium. Tissue-specific localization of CLDN1, 2,
12 and 16 in the duodenum, ileum, colon, kidney, liver and lung
were investigated by immunohistochemistry (Fig. 3). Localization of CLDN1 and 12 were
detected in all the tested tissues (Fig. 3A and C). CLDN2 was localized to the
duodenum, ileum, colon, kidney and liver (Fig. 3B). However, the localization of
CLDN16 was observed in the kidney and liver only (Fig. 3D). In the kidney, each CLDN
localization pattern was different. CLDN1 was localized to Bowman’s
capsules, proximal tubules and the distal nephron. CLDN2 and CLDN12
were detected in the proximal tubules. CLDN16 was positioned in the
thin and thick ascending limbs of the loop of Henle.
Immunohistochemistry was also performed for CLDN4 and CLDN19, but
immune-positive staining was not detected.
Discussion
Tight junctions consist of transmembrane proteins,
of which CLDNs are a major type. CLDNs are representative
transmembrane proteins, and are responsible for barrier and
selective ion transport functions of tight junctions. In the
present study, the expression and distribution of CLDNs in various
tissues, including the duodenum, ileum, colon, kidney, liver and
lung, was examined. The mRNA expression of 16 CLDNs (1–5, 7–8, 10a
and b, 11–12, 14–17 and 19) was tested. Among the tested CLDNs,
CLDN1, CLDN2, CLDN12 and CLDN16 were notably expressed in a
tissue-dependent manner, and therefore the protein expression and
localization of those CLDNs was analyzed further.
The mRNA expression of CLDN1 was highest in
the liver and relatively high in the kidney, lung and muscle.
However, CLDN1 protein was highest in the kidney and moderate in
the colon and lung. Unlike its mRNA distribution, the CLDN1 protein
expression was low in the liver. The localization of CLDN1 was
confirmed as previously reported. CLDN1 is known to function in
sealing junctions without ion selectivity (12). The presence of sealing CLDNs render
tight junctions more ‘tight’, thus they are highly expressed in
tight rather than leaky epithelial cells, which are distinguished
by their electrical resistance (9). CLDN1 was investigated as it was shown
to be correlated with skin defects in a knockout study in mice, and
was also shown to be capable of enhancing the ability of the tight
junction in MDCK cells in an overexpression study (12). CLDN1 has also been shown to
act as a tumor suppressor gene in breast tumor (11,18).
CLDN2 is known as a cation-selective channel-forming
protein (2). Expression of CLDN2
has been reported in various tissues, including the intestine,
kidney, human ovarian surface epithelium and inner ear (19,20).
In the present study, CLDN2 mRNA was generally distributed
in all the tested tissues. Specifically, expression of CLDN2
was highest in the kidney, relatively high in the duodenum, ileum,
colon and liver, and relatively low in the lung. However, CLDN2
protein was not detected in the lung, whereas its expression was
low in the liver and relatively high in the kidney, duodenum, ileum
and colon. CLDN2 is responsible for calcium absorption in the
enterocytes of the intestine, and it increases paracellular cation
permeability in the kidney (2,16,21).
In contrast to CLDN1, CLDN2 expression has been reported in leaky
epithelial cells, including those of the renal proximal tubules,
intestinal crypts and choroid plexus, rather than in tight
epithelial cells (22–24). It is therefore of note that CLDN2
has cation selectivity by the charged amino acids in the first
extracellular loop in its structure. It has been revealed that
CLDN2 and 12 are critically correlated with vitamin D-dependent
calcium absorption between enterocytes (12), and that CLDN2 knockout mice are
defective in the leaky- and cation-selective paracellular
permeability properties of the renal proximal tubules (16,25).
Although the CLDN2 transcript was highly expressed in the liver,
its protein levels were lower compared with that in other somatic
tissues.
Among all the tested CLDN mRNAs, CLDN12 mRNA
was most equally distributed, although it was highest in the kidney
and moderately high in the liver. The levels of CLDN12 mRNA
were well expressed in all the tested tissues, whereas its proteins
were differentially expressed. CLDN12 expression was found in the
blood-brain barrier with CLDN3 and 5 (26). Another study has reported that
CLDN12 is suppressed by hyper-ammonemia in brain capillary
endothelial cells (27), and it
was detected in various tissues, including the intestine, urinary
bladder and epidermis (28,29).
The expression of CLDN12 has predominantly been reported in tight
junctions, in which it functions as a barrier between cells
(9). Nevertheless, CLDN12 is
responsible for calcium absorption in the intestine since it forms
a calcium-selective channel in intestinal enterocytes (16).
CLDN16 mRNA expression was expressed only in
the kidney and liver, and it was highest in the kidney. The level
of CLDN16 protein was parallel with that of its transcript, and it
was localized in the renal distal convoluted tubule. Previous
reports have revealed that renal hypomagnesemia was linked with a
mutation in CLDN16 in the kidney (30,31).
CLDN16 has been reported to form cation channels; however, the
latter could not be confirmed in another study (9). Moreover, attempts to explain the
pathophysiology of magnesium transport and to define the role of
CLDN16 have been unsuccessful (17). Although previous studies are
insufficient to define the functions of CLDN16, it is certain that
the interactions of CLDN16 with other proteins are critical for
calcium and magnesium homeostasis in the body (17). In conclusion, the present study on
mouse tissue indicated that CLDNs were expressed in the intestine,
kidney, liver and lung. An immunohistochemical study also revealed
that the majority of the CLDN subtypes were located in the
epithelium of certain tissues. These results indicated that CLDNs
are expressed and localized in a tissue-specific manner, which may
suggest functional roles of CLDNs in the tissues. Furthermore, the
results of this study may aid in the investigation of physiological
disorders linked with mutations or the overexpression of CLDNs.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korea government
(MEST) (grant no. 2013R1A2A2A05004582).
References
|
1
|
Wen H, Watry DD, Marcondes MC and Fox HS:
Selective decrease in paracellular conductance of tight junctions:
role of the first extracellular domain of claudin-5. Mol Cell Biol.
24:8408–8417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amasheh S, Meiri N, Gitter AH, et al:
Claudin-2 expression induces cation-selective channels in tight
junctions of epithelial cells. J Cell Sci. 115:4969–4976. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hou J, Paul DL and Goodenough DA:
Paracellin-1 and the modulation of ion selectivity of tight
junctions. J Cell Sci. 118:5109–5118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colegio OR, Van Itallie C, Rahner C and
Anderson JM: Claudin extracellular domains determine paracellular
charge selectivity and resistance but not tight junction fibril
architecture. Am J Physiol Cell Physiol. 284:C1346–C1354. 2003.
View Article : Google Scholar
|
|
5
|
Alexandre MD, Jeansonne BG, Renegar RH,
Tatum R and Chen YH: The first extracellular domain of claudin-7
affects paracellular Cl- permeability. Biochem Biophys Res Commun.
357:87–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Itallie CM, Rogan S, Yu A, Vidal LS,
Holmes J and Anderson JM: Two splice variants of claudin-10 in the
kidney create paracellular pores with different ion selectivities.
Am J Physiol Renal Physiol. 291:F1288–F1299. 2006.PubMed/NCBI
|
|
7
|
Van Itallie CM and Anderson JM: Claudins
and epithelial paracellular transport. Annu Rev Physiol.
68:403–429. 2006.PubMed/NCBI
|
|
8
|
Krug SM, Günzel D, Conrad MP, et al:
Charge-selective claudin channels. Ann NY Acad Sci. 1257:20–28.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schulzke JD, Günzel D, John LJ and Fromm
M: Perspectives on tight junction research. Ann NY Acad Sci.
1257:1–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Günzel D, Stuiver M, Kausalya PJ, et al:
Claudin-10 exists in six alternatively spliced isoforms that
exhibit distinct localization and function. J Cell Sci.
122:1507–1517. 2009.PubMed/NCBI
|
|
11
|
Myal Y, Leygue E and Blanchard AA: Claudin
1 in breast tumorigenesis: revelation of a possible novel ‘claudin
high’ subset of breast cancers. J Biomed Biotechnol.
2010:9568972010.PubMed/NCBI
|
|
12
|
Inai T, Kobayashi J and Shibata Y:
Claudin-1 contributes to the epithelial barrier function in MDCK
cells. Eur J Cell Biol. 78:849–855. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weber CR, Nalle SC, Tretiakova M, Rubin DT
and Turner JR: Claudin-1 and claudin-2 expression is elevated in
inflammatory bowel disease and may contribute to early neoplastic
transformation. Lab Invest. 88:1110–1120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van Itallie C, Rahner C and Anderson JM:
Regulated expression of claudin-4 decreases paracellular
conductance through a selective decrease in sodium permeability. J
Clin Invest. 107:1319–1327. 2001.PubMed/NCBI
|
|
15
|
Hou J, Renigunta A, Yang J and Waldegger
S: Claudin-4 forms paracellular chloride channel in the kidney and
requires claudin-8 for tight junction localization. Proc Natl Acad
Sci USA. 107:18010–18015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujita H, Sugimoto K, Inatomi S, et al:
Tight junction proteins claudin-2 and -12 are critical for vitamin
D-dependent Ca2+ absorption between enterocytes. Mol
Biol Cell. 19:1912–1921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hou J, Renigunta A, Konrad M, et al:
Claudin-16 and claudin-19 interact and form a cation-selective
tight junction complex. J Clin Invest. 118:619–628. 2008.PubMed/NCBI
|
|
18
|
Chao YC, Pan SH, Yang SC, et al: Claudin-1
is a metastasis suppressor and correlates with clinical outcome in
lung adenocarcinoma. Am J Respir Crit Care Med. 179:123–133. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kitajiri SI, Furuse M, Morita K, et al:
Expression patterns of claudins, tight junction adhesion molecules,
in the inner ear. Hear Res. 187:25–34. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu Y, Brännström M, Janson PO and
Sundfeldt K: Differences in expression patterns of the tight
junction proteins, claudin 1, 3, 4 and 5, in human ovarian surface
epithelium as compared to epithelia in inclusion cysts and
epithelial ovarian tumours. Int J Cancer. 118:1884–1891. 2006.
View Article : Google Scholar
|
|
21
|
Furuse M, Furuse K, Sasaki H and Tsukita
S: Conversion of zonulae occludentes from tight to leaky strand
type by introducing claudin-2 into Madin-Darby canine kidney I
cells. J Cell Biol. 153:263–272. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reyes JL, Lamas M, Martin D, et al: The
renal segmental distribution of claudins changes with development.
Kidney Int. 62:476–487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rahner C, Mitic LL and Anderson JM:
Heterogeneity in expression and subcellular localization of
claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut.
Gastroenterology. 120:411–422. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wolburg H, Wolburg-Buchholz K, Liebner S
and Engelhardt B: Claudin-1, claudin-2 and claudin-11 are present
in tight junctions of choroid plexus epithelium of the mouse.
Neurosci Lett. 307:77–80. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Muto S, Hata M, Taniguchi J, et al:
Claudin-2-deficient mice are defective in the leaky and
cation-selective paracellular permeability properties of renal
proximal tubules. Proc Natl Acad Sci USA. 107:8011–8016. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hawkins BT and Davis TP: The blood-brain
barrier/neurovascular unit in health and disease. Pharmacol Rev.
57:173–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Belanger M, Asashima T, Ohtsuki S,
Yamaguchi H, Ito S and Terasaki T: Hyperammonemia induces transport
of taurine and creatine and suppresses claudin-12 gene expression
in brain capillary endothelial cells in vitro. Neurochem Int.
50:95–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fujita H, Chiba H, Yokozaki H, et al:
Differential expression and subcellular localization of claudin-7,
-8, -12, -13 and -15 along the mouse intestine. J Histochem
Cytochem. 54:933–944. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brandner JM, McIntyre M, Kief S,
Wladykowski E and Moll I: Expression and localization of tight
junction-associated proteins in human hair follicles. Arch Dermatol
Res. 295:211–221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Simon DB, Lu Y, Choate KA, et al:
Paracellin-1, a renal tight junction protein required for
paracellular Mg2+ resorption. Science. 285:103–106.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Konrad M, Schaller A, Seelow D, et al:
Mutations in the tight-junction gene claudin 19 (CLDN19) are
associated with renal magnesium wasting, renal failure, and severe
ocular involvement. Am J Hum Genet. 79:949–957. 2006.PubMed/NCBI
|