Introduction
Dural arteriovenous fistulas (DAVFs) are
pathological arteriovenous shunts that involve any section of the
dura mater and its adjacent structures, and account for 10–15% of
all intracranial arteriovenous malformations (1). Although the etiopathogenesis remains
unclear, DAVFs are considered to be acquired lesions, in contrast
to arteriovenous malformations (1,2).
Venous sinus hypertension, which may have multiple
etiologies, is hypothesized to predispose patients to DAVFs
(3). DAVFs can be developed in
rats by surgically inducing venous hypertension via common carotid
artery (CCA)-external jugular vein (EJV) anastomosis, followed by
draining venous or dural sinus occlusion (4). Lawton et al (5) observed angiogenesis of the sclera and
corneal limbus following implantation of dura mater tissue
(obtained from rats with chronic venous hypertension at different
time periods) into rabbit corneas. In addition, high expression of
vascular endothelial growth factor (VEGF) and other angiogenic
growth factors has been detected in clinical DAVF samples (6,7).
These studies suggest that a high expression of angiogenic factors,
including VEGF, may be associated with the formation of DAVFs.
However, it remains unknown whether the high expression of VEGF is
pivotal in the formation of DAVFs or is only a secondary effect.
The purpose of the present study was to observe angiogenesis in the
dura mater and cerebral cortex, and the formation of DAVFs in
venous hypertension models when VEGF/VEGF receptor (VEGFR)
signaling pathways are modified. Activation and inhibition of the
VEGFR pathways was achieved using VEGF recombinant adenovirus and a
VEGFR inhibitor, respectively, in order to clarify the role of the
VEGF/VEGFR signaling pathways in the formation of venous
hypertension-induced DAVFs.
Materials and methods
Animal studies
All experiments involving animals were approved by
the Institutional Animal Care and Use Committee of Changhai
Hospital (Shanghai, China). To exclude any potential hormonal
effects on DAVF formation, only male Sprague-Dawley rats, weighing
350–400 g, were used. All rats were raised and maintained under
standard laboratory conditions.
Grouping and operation
All rats underwent surgical procedures following
intraperitoneal injection of 10% chloral hydrate at a dose of 0.5
ml/100 g body weight. The injection was supplemented as required
during the procedure. All surgical procedures were performed using
standard sterile techniques. A total of 192 rats underwent right
CCA-EJV anastomosis, followed by superior sagittal sinus ligation
and left transverse sinus (facial vein) occlusion. The other 24
rats received sham surgery.
A modification of the model described by Chen et
al (8) was used. A frontal
median incision was made and the skull was drilled through to
expose the superior sagittal sinus and this was ligated with the
use of a no. 10-0 polypropylene suture (Ethicon, Cincinnati, OH,
USA). Through a second incision below the left ear, the left
terminal end of the transverse sinus was exposed and destroyed
using bipolar electrocoagulation. Through a cervical median
incision, the right anterior facial vein was ligated with a no.
10-0 polypropylene suture and the proximal CCA was anastomosed to
the distal EJV in an end-to-end manner using no. 10-0 polypropylene
interrupted sutures. This resulted in retrograde flow through the
transverse sinus. The proximal segment of the EJV and the initial
portions of the right external and internal carotid arteries were
separately destroyed. Subsequent to surgery, the incision was
ligated with a no. 3-0 polyglactin suture (Ethicon). The rats that
received sham surgery only underwent frontal, post-aurem, and
cervical medial incision, and suture.
All rats were randomly divided into six groups
immediately prior to the procedures. Group A included 45 rats that
underwent superior sagittal sinus and left transverse sinus
occlusion, isolation and dissection of the right EJV. They were
then injected with VEGF recombinant adenovirus
(3×109/plaque-forming unit, diluted to 0.3 ml) in the
distal right EJV. The EJV was then anastomosed to the CCA in an
end-to-end manner following occlusion for 30 min. Group B comprised
12 rats that underwent the same surgical procedure as in group A,
but were injected with an equivalent quantity of control adenovirus
instead of VEGF recombinant adenovirus. Groups C, D and E (n=45
rats per group) underwent the same surgical procedure as group A,
but without injection of any adenovirus. Following surgery, the
rats in groups A, B and C were fed according to routine methods.
The group D rats were lavaged with the VEGFR inhibitor, vatalanib
(PTK787/ZK222584, 5 mg/100 g of body weight; LC Labs, Woburn, MA,
USA) and the group E rats were lavaged with an equal quantity of
saline weekly, prior to and subsequent to surgery. The 24 rats in
group F that received sham surgery served as a control group.
Construction of VEGF recombinant
adenoviral vectors
A VEGF165 gene fragment was amplified using
polymerase chain reaction (PCR), digested with EcoRI and
SalI (Invitrogen, Carlsbad, CA, USA), then connected onto
the plasmid carrier (pDC316-mCMV; Minghong Biological Engineering
Co., Ltd., Shanghai, China) for construction of a shuttle plasmid
(pDC316-VEGF165). A total of 293 cells were transfected with the
shuttle plasmid, skeleton plasmid (pBHGlox_E1,3Cre) and an
adenovirus packaging system (AdMax™, Microbix Biosystems
Inc., Mississauga, ON, Canada). The cells were transfected with the
first generation of virus (amplified by cell culture) to obtain the
second generation of the virus, which was identified using PCR.
Another 293 cells were transfected with the second generation of
the virus in order to gain a large number of virus particles from
multiple cultures. SOURSE 15Q ion-exchange purification (GE
Healthcare Life Sciences, Piscataway, NJ, USA), molecular sieve
purification and desalination were performed. The purified virus
was filtered to remove bacteria.
Histological and microvascular density
(MVD) examination
At 1, 2, 4 and 12 weeks after surgery (only at 1 and
2 weeks in group B), six rats from groups A-E and one rate from
group F were anesthetized with an intraperitoneal injection of 7%
chloraldurate (0.5 ml/100 g body weight) and perfused
intracardially with 0.9% saline followed by fresh 4%
paraformaldehyde in 0.1 mol/l phosphate buffer (pH 7.2). The brains
of the rats, along with the dura mater, including the dural
sinuses, were carefully removed and postfixed for 48 h in 4%
paraformaldehyde. Sections of dura mater and brain tissue (1×1×0.5
cm) around the torcular herophili area were harvested. The dura
mater on the surface of the brains were fully unfolded and packed
with gauze, rinsed under running water, dehydrated with a set of
varying alcohol concentrations, immersed in a mixture of equal
quantities of xylene and dehydrated ethanol for 1 h, and hyalinized
in pure xylene for 20 min. The masses were embedded with paraffin
(melting point, 45–50°C) for 30 min. The paraffin blocks were
transected perpendicular to the superior sagittal sinus at a
thickness of 8 μm.
The tissue biopsy slides were immersed in
dimethylbenzene for dewaxing (subsequent to baking in a drying oven
at 56°C for 1.5 h) and then immersed in 100, 90 and 70% ethanol in
turn for 3 min each. The slides were washed in phosphate-buffered
saline (PBS; 0.01 mol/l, pH 7.2) for antigen retrieval, immersed in
boiling citric acid buffer (0.01 mol/l, pH 6.0) for 15 min, cooled
at room temperature following heat preservation, incubated in 0.3%
H2O2 for 20 min at room temperature and then
incubated in 10% normal fetal bovine serum for 30 min at room
temperature. The sections were rinsed with PBS and then incubated
overnight with either rabbit monoclonal primary antibody against
VEGF (Abcam, Cambridge, MA, USA, 1:200) or rabbit monoclonal
primary antibody against CD31 (Abcam, 1:150) in PBS containing 1%
normal goat serum and 0.3% Triton X-100 at 4°C. The sections were
washed three times in PBS for 3 min each and then incubated for 1.5
h at 37°C with horseradish peroxidase-conjugated goat anti-rabbit
antibody (Jackson ImmunoResearch Laboratories, Inc., Westgrove, MA,
USA), diluted to 1:200 in PBS containing 1% normal goat serum and
0.3% Triton X-100. The slides were rinsed with PBS three times for
3 min, stained with 3′3′-Diaminobenzidine for 3–5 min, restained
with hematoxylin for 30 sec and differentiated with alcohol
containing 1% hydrochloric acid for 1 sec. For dehydration, the
slides were immersed in 70, 90 and 100% ethanol in turn for 3 min
each. The slides were then dried, wax-sealed and mounted.
The immunohistochemical results were analyzed using
Image-Pro Plus 6.0 (Media Cybernetics, Bethesda, MD, USA). Yellow
or brown granules were regarded as positive signals. The expression
level of VEGF was presented as the positive area ratio. The high
expression areas in the occipital cortex and dura mater were
identified in the VEGF staining slices using adjacent sections of
the tissues from each time point at a low power (magnification,
×40) and then high power (magnification, ×200). Three high
expression areas in the occipital cortex and dura mater were
randomly selected for calculation of the VEGF expression positive
area ratio. The MVD was defined as the CD31-positive microvascular
number per unit area on the slice. Adjacent sections of the slices
with CD31 staining from groups A, C, D, E and F were observed at 4
and 12 weeks at high power (magnification, ×400), and the
microvascular number was recorded in three high-power fields in the
dura. The dural MVD was shown as the microvascular number per
square millimeter, which was converted from the mean of three
microvascular numbers.
Angiographic experiments
All angiography was conducted using a Innova 2100-IQ
(GE Healthcare, New York, NY, USA). Cerebral angiography was
performed 12 weeks after initial surgery on the remaining rats in
groups A, B, C, D, E and F. All surgical procedures were performed
using standard sterile techniques. Appropriate anesthesia was
induced in each animal via intraperitoneal injection of 7%
chloraldurate (0.5 ml/100 g body weight). The previous cervical
median incision was reopened and the right CCA and EJV were exposed
to confirm the patency of the right CCA-EJV anastomosis. The rats
with occluded anastomosis were excluded from angiographic analysis.
Following the ligation of the CCA-EJV bypasses with no. 3-0
polyglactin sutures, the abdominal center was opened, the abdominal
aorta was dissociated, the distal section was ligated and a 4
French arterial sheath (Terumo Corporation, Tokyo, Japan) was
placed into the proximal section. The left CCA was selected with a
3 French microcatheter guided by a microguide wire and 2 ml
iopromide (diluted at 1:1 with normal saline) was injected using a
high-pressure syringe at 0.6 ml/s. Anteroposterior and lateral
angiography was continuously performed at 6 frames/sec until the
venous sinus period was reached. Dynamic images were analyzed by
three neurointerventional physicians; DAVF formation was confirmed
when either the intracranial cortical vein or the venous sinus was
enhanced in the arterial phase.
Statistical analysis
All values in the text, tables and figures are
presented as the mean ± standard deviation. Analysis of variance
was used to examine the differences among groups. When the
difference was significant, the least significant difference and
Student-Newman-Keuls post hoc tests were used for multiple
comparisons to identify which group differences had significant
P-values. For cerebral angiography, the differences among groups
were analyzed by the Pearson’s χ2 test or Fisher’s exact
test, as appropriate. The statistical analyses were conducted using
SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA) and P<0.05 was
considered to indicate a statistically significant difference.
Results
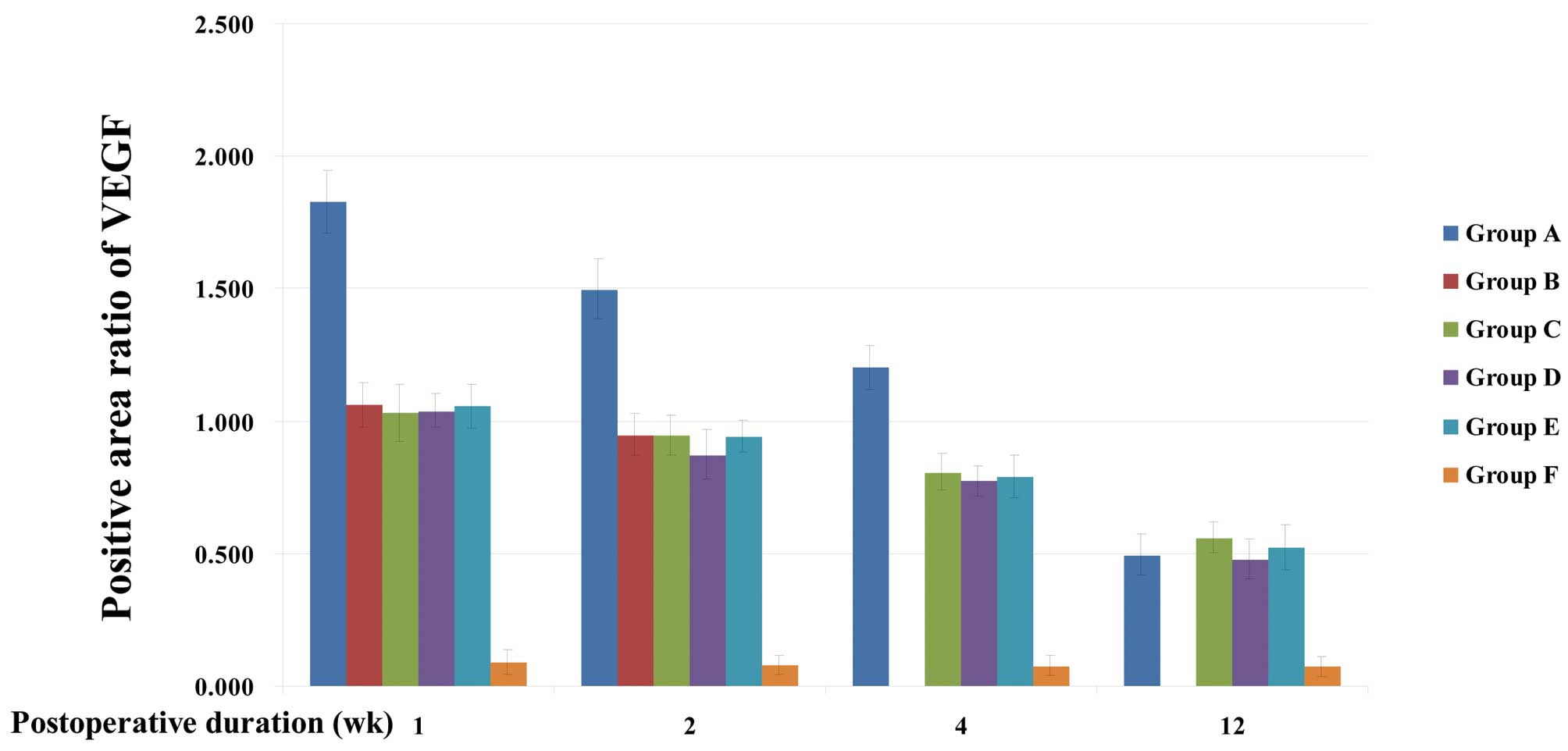
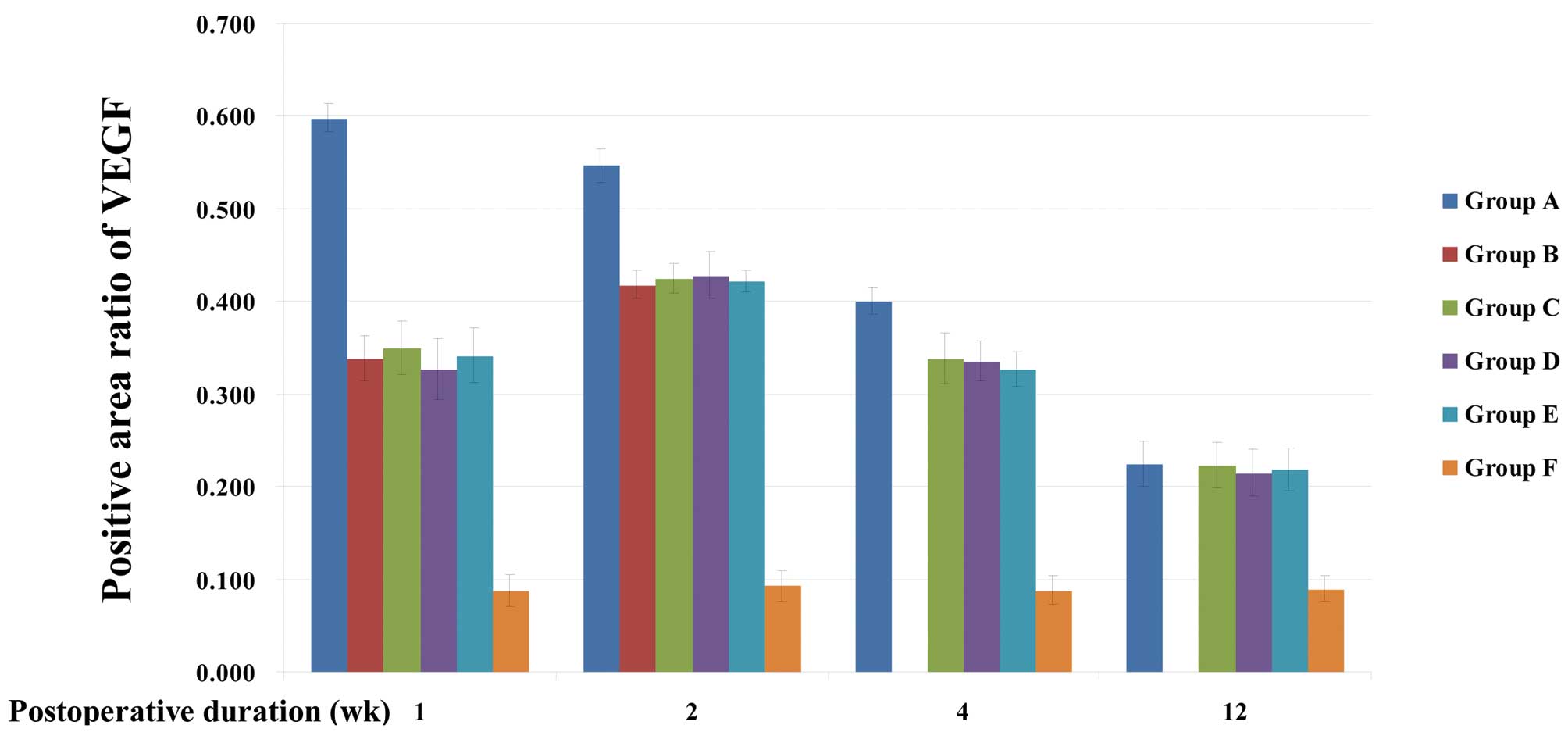
VEGF expression
VEGF was identified in all groups to be
predominantly expressed in the vascular matrix of small blood
vessels, endothelial cells, the vascular matrix of the dura mater,
and the neurons and glial cells of the occipital lobe cortex
(Fig. 1).
Group A, C, D and E rats exhibited continued high
expression of VEGF in the occipital cortex and dura mater from the
first week after surgery to the end of the study period. This was
significantly higher than the expression of VEGF in group F
(P<0.001), which was low. The expression levels of VEGF at 1, 2
and 4 weeks after surgery in group A rats were significantly higher
than those of groups B, C, D and E (P<0.001). In group A rats,
VEGF was most highly expressed in the occipital cortex and dura
mater the first week after surgery. This expression declined
gradually, and no significant difference compared with the
expression levels in groups C, D and E was identified 12 weeks
after surgery (P>0.05). In the dura mater and cerebral cortex,
no significant difference in VEGF expression levels was identified
among groups B, C, D and E at any time point. In group B, C, D and
E rats, peak expression of VEGF in the occipital cortex was
observed in the first week after surgery (P<0.001), while in the
dura mater, peak expression was observed 2 weeks after surgery
(P<0.001). The expression level decreased 4–12 weeks after
surgery (Tables I and II; Figs.
2 and 3).
 | Table IPositive area ratio of vascular
endothelial growth factor expression in the cortex. |
Table I
Positive area ratio of vascular
endothelial growth factor expression in the cortex.
| Group | 1 week | 2 weeks | 4 weeks | 12 weeks |
|---|
| A | 1.828±0.119a | 1.498±0.112a | 1.203±0.084a | 0.495±0.078 |
| B | 1.062±0.082 | 0.950±0.078 | | |
| C | 1.032±0.108 | 0.947±0.077 | 0.808±0.069 | 0.562±0.057 |
| D | 1.040±0.062 | 0.873±0.092 | 0.775±0.057 | 0.480±0.075 |
| E | 1.057±0.082 | 0.942±0.060 | 0.790±0.081 | 0.525±0.086 |
| F | 0.090±0.046 | 0.078±0.035 | 0.076±0.039 | 0.075±0.037 |
 | Table IIPositive area ratio of vascular
endothelial growth factor expression in the dura mater. |
Table II
Positive area ratio of vascular
endothelial growth factor expression in the dura mater.
| Group | 1 week | 2 weeks | 4 weeks | 12 weeks |
|---|
| A | 0.598±0.015a | 0.547±0.018a | 0.400±0.014a | 0.225±0.024 |
| B | 0.338±0.025 | 0.418±0.015 | | |
| C | 0.350±0.029 | 0.425±0.016 | 0.338±0.027 | 0.223±0.024 |
| D | 0.327±0.033 | 0.428±0.025 | 0.335±0.022 | 0.215±0.025 |
| E | 0.342±0.029 | 0.422±0.012 | 0.327±0.019 | 0.218±0.023 |
| F | 0.088±0.017 | 0.093±0.016 | 0.088±0.015 | 0.090±0.014 |
MVD assay
Dural capillary hyperplasia was observed 4 weeks
after surgery in groups A, C, D and E (Fig. 1). The MVD of rats in groups A, C, D
and E was significantly higher than that of rats in group F
(P<0.01). The MVD in the dura mater was 349±24/mm2 in
group A 4 weeks after surgery, significantly higher than that of
group C (274±15/mm2, P<0.001), and the MVD in the
dura mater was 242±13/mm2 in group D 4 weeks after
surgery, significantly lower than the MVDs of group C (P<0.001)
and group E (264±20/mm2, P<0.05).
The MVDs in the dura mater 12 weeks after surgery
were 369±29/mm2, 291±13/mm2 and
280±22/mm2 in groups A, C and E, respectively,
indicating an increase when compared with those at 4 weeks.
However, there were no significant differences identified between
the MVDs at 4 weeks and 12 weeks (independent samples t-test,
P>0.05). The MVD in the dura mater at 12 weeks was
245±13/mm2 in group D, similar to that at 4 weeks. The
MVD of group A at 12 weeks was significantly higher than that of
group C (P<0.001), a similar result to that at 4 weeks, while
the MVD of group D at 12 weeks was significantly lower than those
of group C (P<0.001) and group E (P<0.01, Fig. 4).
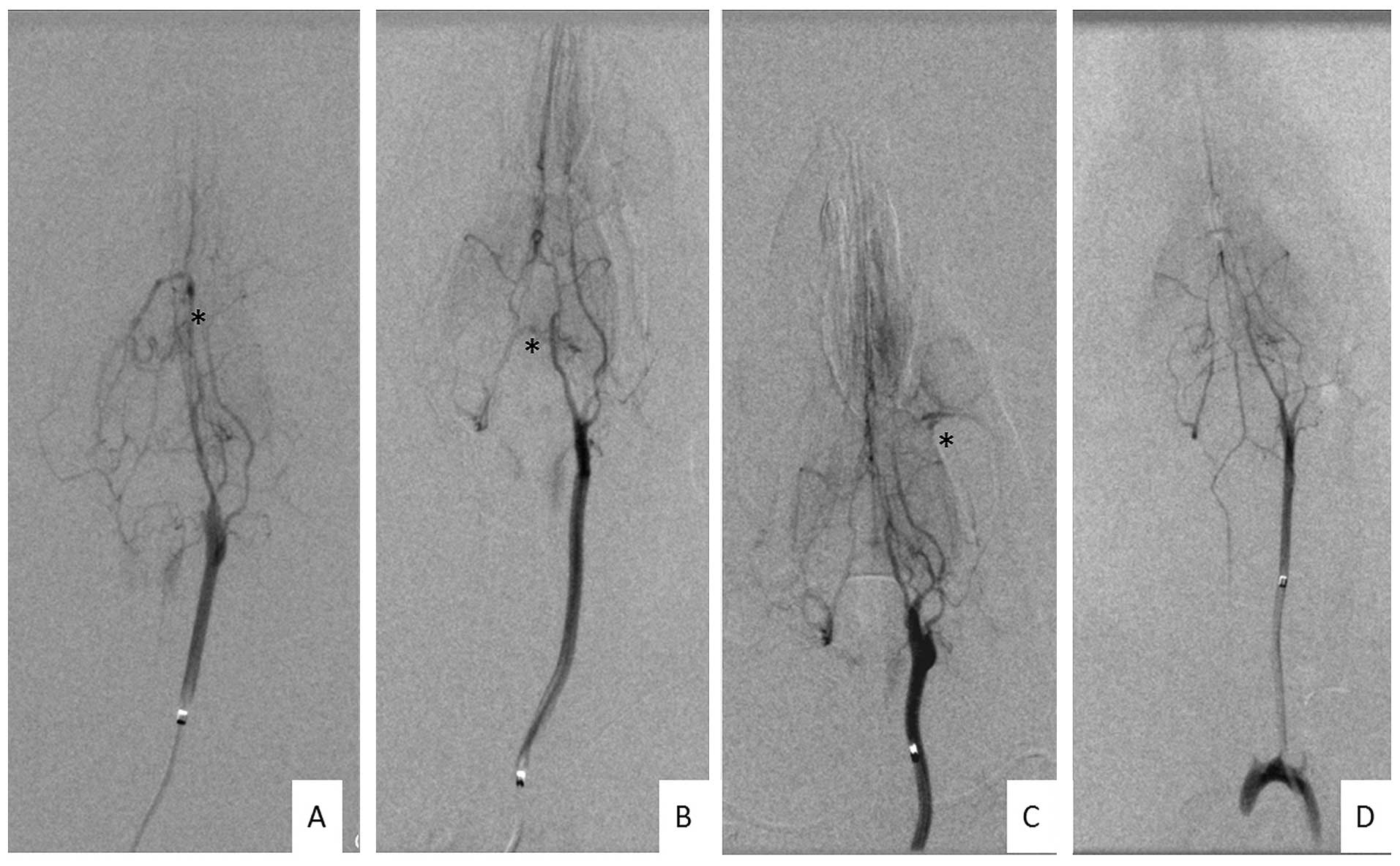
DAVF formation
Twelve weeks after surgery, the cervical incisions
of all groups were opened prior to radiography and CCA-EJV
anastomosis occlusion was identified in one rat in group A and one
in group E. Angiography was successfully performed on all the
remaining 102 rats and the images were clear enough for
identification.
DAVF formation occurred in 13 of the 20 rats in
group A, 9 of the 21 rats in group C, 2 of the 21 rats in group D,
10 of the 20 rats in group E and none of the 20 rats in group F. Of
the 34 DAVFs, 9 were located in the superior sagittal sinus area,
15 in the transverse-sigmoid sinus area and 10 in the basis cranii
(Fig. 5).
The DAVF induction rate in group A (65.0%) was not
identified to be significantly different from that of group C
(42.9%, P=0.155). However, the DAVF induction rate of group D
(9.5%) was significantly lower than those of groups C (P=0.035) and
E (50.0%, P=0.012), with no significant differences identified
compared with that of group F (P=0.488). According to these
results, venous hypertensive rats with VEGF recombinant adenovirus
transfection exhibited higher levels of VEGF expression and MVD.
However, the DAVF induction rates were not significantly higher
than the rates of venous hypertensive rats without adenovirus
transfection. The DAVF formation rates decreased significantly in
rats treated with VEGFR antagonists.
Discussion
Clinical studies suggest that DAVFs result from
intracranial venous sinus thrombosis and stenosis (9,10).
Certain studies have reported that 39–80% of DAVF patients had
intracranial venous sinus thrombosis and some other DAVF patients
had venous sinus dysplasia, stenosis or separation, instead of
thrombosis. (9,10). However, numerous cases of DAVFs
without venous sinus abnormalities have also been reported.
DAVFs have been established in a venous hypertensive
model, which has confirmed that venous sinus hypertension is one of
the main causes of DAVFs. In 1994, Terada et al (4) focused on DAVF etiology in the chronic
sinus hypertensive rat model. Anastomosis was performed between the
right CCA and the EJV, and successfully induced DAVFs. One year
later, Herman et al (11)
improved this method through embolization of the lateral transverse
sinus and superior sagittal sinus, which further increased venous
hypertension, and the DAVF induction rate reached 40%.
In the present study, all the extracranial lateral
branches, including the anterior facial veins in rats of groups A,
B, C, D and E, were ligated prior to suturing the right EJV with
the CCA, which reduced venous collateral circulation and further
increased postoperative venous hypertension. According to Chen
et al (8), the
postoperative venous pressure can reach >20 mmHg in >80% of
rats. Although venous collateral circulation can be established
with time and can return to baseline levels within 2–4 weeks, the
cerebral angiogram in groups C and E at 12 weeks revealed that the
DAVF induction rate was as high as 50%.
The mechanism underlying how venous hypertension
induces DAVFs remains unknown. In 1997, Lawton et al
(5) first hypothesized that
angiogenesis was involved. The dura mater of venous hypertensive
rats was implanted into rabbit corneas, which exhibit extremely
active angiogenesis, and DAVF formation was found to positively
correlate with sinus hypertension and rabbit corneal angiogenic
activity. Based on these results, it was hypothesized that venous
hypertension decreases cerebral perfusion and produces brain
ischemia. Tissue hypoxia subsequently stimulated angiogenesis in
order to reverse the ischemia, while the increased angiogenesis of
the dura mater led to the formation of arteriovenous shunts and the
formation of DAVFs.
VEGF is one of the most important angiogenic
factors, which can promote angiogenesis and proliferation of
vascular endothelial and vascular smooth muscle. The overexpression
of VEGF and other factors associated with angiogenesis has been
detected in clinical DAVF specimens by Tirakotai et al
(6) and Uranishi et al
(7). Similar results were also
observed in venous hypertensive rat models (8), suggesting that VEGF may be important
in the process of venous hypertension-induced DAVF formation.
Chen et al (8) investigated the cerebral blood flow in
venous hypertensive rats using Doppler ultrasound and found that
chronic hypoperfusion around the right occipital lobe occurred in
rats that had undergone right CCA-EJV anastomosis and occlusion of
the contralateral transverse sinus and the superior sagittal sinus.
The cerebral blood flow in the right occipital lobe immediately
declined following surgery; although it continuously improved
during the postoperative period, it remained 11.44% lower than
preoperative levels at 12 weeks, suggesting that venous
hypertension can lead to chronic, stable, local hypoperfusion
(8).
Hypoxia inducible factor (HIF) responds rapidly to
the venous sinus hypertension-induced ischemic state. Following
induction of venous hypertension, endothelial cells in the venules
beside the sagittal sinus were identified to immediately exhibit
high expression of HIF-1, up to 5 times greater than the control
group, with peak levels one day after surgery (12,13).
Hypoxia is one of the most important factors
promoting VEGF expression. High VEGF expression occurs in
intracranial tissues with cerebral hypoperfusion or hypoxia. Shin
et al (14) observed high
VEGF expression in vascular endothelial cells and connective
tissues in one-third of rats 1 week after venous hypertension
establishment. Zhu et al (12) also found that VEGF expression
peaked in the basal ganglia region and the glial cells of the
cerebral cortex 1 week after the venous hypertensive model was
established, similar to the results of Chen et al (8). Chen et al (8) also observed that following
establishment of venous hypertension, VEGF was highly expressed in
the cytoplasm and vascular matrix of epidural vascular endothelial
cells, peaking in the second week, and remaining strongly positive
at 12 weeks. In accordance with the results of these previous
studies, the present study observed that rats with venous
hypertension (group C) exhibited high expression of VEGF in the
occipital cortex 1 week after model induction. The VEGF levels
gradually declined, but remained significantly higher than those of
the control group at 12 weeks after surgery. VEGF expression in the
dura mater near the sinus reached its peak at 2 weeks after
surgery.
It remains unclear whether the high expression of
VEGF around the DAVF lesions is key to DAVF formation or whether it
is only an accompanying phenomenon. A study revealed that
physiological arteriovenous shunts are in conformity with respect
to the location and structure of the fistula formation (15). These results may suggest that DAVFs
are induced by the opening of physiological arteriovenous shunts,
but not by angiogenesis.
The aim of the present study was to clarify the role
of VEGF/VEGFR. Analyses were conducted to identify whether
increased local levels of VEGF improved the induction of DAVFs.
Studies on ischemic cerebrovascular diseases revealed that the
expression of VEGF in a target region can be effectively increased
by VEGF165 adenovirus and plasmid transfection; the expression
peaks ~1 week after transfection, similar to that of endogenous
VEGF expression in venous hypertensive rats (16). In the current study, VEGF was
overexpressed in venous hypertensive rats using venous sinus
perfusion of VEGF recombinant adenovirus. VEGF was highly expressed
in the brain cortex and dura mater of rats in group A, a similar
location to the expression in groups B and C. Moreover, the VEGF
expression levels at 1 and 2 weeks after surgery in group A rats
were significantly higher than those of rats in groups B and C.
These levels gradually declined 4 weeks later, and eventually
declined to a level similar to that observed in venous hypertensive
rats without VEGF recombinant adenovirus transfection (group C) at
12 weeks. These results suggest that VEGF can be successfully
increased in the highly expressed VEGF regions of venous
hypertensive rats by recombinant adenovirus, resulting in a
short-term increase in VEGF levels.
At 4 and 12 weeks after surgery, active vascular
proliferation in the dura was observed in the rats of the venous
hypertension group (group C) and the MVD levels were significantly
higher than those of the control group (group F). Markedly greater
active angiogenesis was identified in the group A rats compared
with those in group C, which demonstrated that VEGF levels further
increase with recombinant adenovirus transfection. Although
angiography suggested that the rate of DAVF induction in venous
hypertensive rats with adenovirus transfection was higher than that
of the simple venous hypertension group (groups C and E), no
significant difference was identified, which may be due to the
relatively small sample size. Conversely, this also suggests that
the angiogenesis mediated by VEGF and its receptor is not the only
factor that affects DAVF formation. Certain new blood vessels
retrograde following formation, while others mature since they
arise from the vascular basement membrane, a process termed vessel
remodeling (17). In the process
of DAVF formation, whether abnormal blood vessel hyperplasia is
complicated by vascular remodeling requires further validation. In
addition, other factors associated with angiogenesis, such as
Ephrin (18) and metal matrix
proteases (8), are also important
in the process of angiogenesis and vascular remodeling. As a
result, improving VEGF expression levels alone may not be able to
significantly increase the rate of DAVF induction.
However, the current study has identified that
vascular hyperplasia of the dura and brain cortex in venous
hypertensive rats markedly declined using the VEGF receptor
antagonist Vatalanib, a type of tyrosine kinase inhibitor of VEGFR
that inhibits VEGF/VEGFR signaling pathways by competitively
interfering with VEGF binding sites on VEGFR and subsequently
affecting angiogenesis (19–20).
The DAVF induction rates were also significantly decreased
following vatalanib administration, suggesting that the effect of
venous hypertension on DAVF induction can be inhibited by VEGFR
antagonism. Therefore, the results of the current study illustrate
that VEGF/VEGFR is key in the process of venous
hypertension-induced DAVF formation. However, DAVFs were still
observed in certain rats. Further studies are required to determine
whether this result was observed due to Vatalanib drug
concentrations or as a result of other factors in venous
hypertension-induced DAVF formation, including the opening of
physiological arteriovenous shunts and the series of changes in the
biological characteristics of the vascular endothelial cells in
cerebral veins and venous sinuses due to venous hypertension
(21).
The sample size of the current study was limited due
to the difficulty in establishing a venous hypertensive rat model;
therefore, this study was preliminary research. The
adenovirus-transfected region may have been more clearly identified
if the adenovirus injected in the control group (group B) had been
replaced with adenovirus with green fluorescence labeling. In
addition, all the VEGF expression data were obtained by
semi-quantitative immunohistochemical analysis, but western
blotting may have been more accurate for the determination of
cytokines. However, in order to obtain brain tissues with dura, the
tissues were fixed with paraformaldehyde prior to euthenizing the
rats, rendering the samples unsuitable for western blotting.
Finally, due to the small sample size, the optimum dosage of
vatalanib was not investigated. Therefore, the efficiency of
vatalanib-induced inhibition of the VEGF/VEGFR pathway activated by
venous hypertension may not have reached its maximum.
In conclusion, angiogenesis in the dura mater of
venous hypertensive rats was increased by transfection with VEGF
recombinant adenovirus, subsequent to increases in the VEGF
expression levels in the brain and dura mater. However, the
induction rate of DAVF induced by venous hypertension was
significantly reduced using a VEGFR antagonist due to reduced
angiogenesis in the dura mater. These results suggest that
activation of the VEGF/VEGFR signaling pathway is important in the
formation of venous hypertension-induced DAVFs.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China [grant no. 30872677 (Xu) and grant no.
81200906 (Li)] and the Young Scholars Foundation of Shanghai Public
Health Bureau (grant no. 2010068).
References
|
1
|
Gandhi D, Chen J, Pearl M, et al:
Intracranial dural arteriovenous fistulas: classification, imaging
findings, and treatment. AJNR Am J Neuroradiol. 33:1007–1013. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaudhary MY, Sachdev VP, Cho SH, et al:
Dural arteriovenous malformation of the major venous sinuses: an
acquired lesion. AJNR Am J Neuroradiol. 3:13–19. 1982.PubMed/NCBI
|
|
3
|
Yeh PS, Wu TC, Tzeng WS and Lin HJ:
Endovascular angioplasty and stent placement in venous hypertension
related to dural arteriovenous fistulas and venous sinus
thrombosis. Clin Neurol Neurosurg. 112:167–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Terada T, Higashida RT, Halbach VV, et al:
Development of acquired arteriovenous fistulas in rats due to
venous hypertension. J Neurosurg. 80:884–889. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lawton MT, Jacobowitz R and Spetzler RF:
Redefined role of angiogenesis in the pathogenesis of dural
arteriovenous malformations. J Neurosurg. 87:267–274. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tirakotai W, Bertalanffy H, Liu-Guan B,
Farhoud A and Sure U: Immunohistochemical study in dural
arteriovenous fistulas and possible role of local hypoxia for the
de novo formation of dural arteriovenous fistulas. Clin Neurol
Neurosurg. 107:455–460. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uranishi R, Nakase H and Sakaki T:
Expression of angiogenic growth factors in dural arteriovenous
fistula. J Neurosurg. 91:781–786. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen L, Mao Y and Zhou LF: Local chronic
hypoperfusion secondary to sinus high pressure seems to be mainly
responsible for the formation of intracranial dural arteriovenous
fistula. Neurosurgery. 64:973–983. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsai LK, Jeng JS, Liu HM, Wang HJ and Yip
PK: Intracranial dural arteriovenous fistulas with or without
cerebral sinus thrombosis: analysis of 69 patients. J Neurol
Neurosurg Psychiatry. 75:1639–1641. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cognard C, Casasco A, Toevi M, et al:
Dural arteriovenous fistulas as a cause of intracranial
hypertension due to impairment of cranial venous outflow. J Neurol
Neurosurg Psychiatry. 65:308–316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herman JM, Spetzler RF, Bederson JB,
Kurbat JM and Zabramski JM: Genesis of a dural arteriovenous
malformation in a rat model. J Neurosurg. 83:539–545. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu Y, Lawton MT, Du R, et al: Expression
of hypoxia-inducible factor-1 and vascular endothelial growth
factor in response to venous hypertension. Neurosurgery.
59:687–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao P, Zhu Y, Ling F, et al: Nonischemic
cerebral venous hypertension promotes a pro-angiogenic stage
through HIF-1 downstream genes and leukocyte-derived MMP-9. J Cereb
Blood Flow Metab. 29:1482–1490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shin Y, Nakase H, Nakamura M, et al:
Expression of angiogenic growth factor in the rat DAVF model.
Neurol Res. 29:727–733. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamada Y, Goto K, Inoue T, et al:
Histopathological aspects of dural arteriovenous fistulas in the
transverse-sigmoid sinus region in nine patients. Neurosurgery.
40:452–458. 1997.PubMed/NCBI
|
|
16
|
Vogel J, Hörner C, Haller C and Kuschinsky
W: Heterologous expression of human VEGF165 in rat brain:
dose-dependent, heterogeneous effects on CBF in relation to
vascular density and cross-sectional area. J Cereb Blood Flow
Metab. 23:423–431. 2003. View Article : Google Scholar
|
|
17
|
Salvucci O and Tosato G: Essential roles
of EphB receptors and EphrinB ligands in endothelial cell function
and angiogenesis. Adv Cancer Res. 114:21–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salvucci O, Maric D, Economopoulou M, et
al: EphrinB reverse signaling contributes to endothelial and mural
cell assembly into vascular structures. Blood. 114:1707–1716. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldbrunner RH, Bendszus M, Wood J, et al:
PTK787/ZK222584, an inhibitor of vascular endothelial growth factor
receptor tyrosine kinases, decreases glioma growth and
vascularization. Neurosurgery. 55:426–432. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giatromanolaki A, Koukourakis MI, Sivridis
E, et al; Tumour and Angiogenesis Research Group. Vascular density
analysis in colorectal cancer patients treated with vatalanib
(PTK787/ZK222584) in the randomised CONFIRM trials. Br J Cancer.
107:1044–1050. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zaragoza C, Márquez S and Saura M:
Endothelial mechanosensors of shear stress as regulators of
atherogenesis. Curr Opin Lipidol. 23:446–452. 2012. View Article : Google Scholar : PubMed/NCBI
|