Introduction
It is estimated that 2–3% of the world’s population
are chronically infected with the hepatitis C virus (HCV), which is
considered to be a major risk factor for the development of
hepatocellular carcinoma (HCC) (1,2). HCV
belongs to the Flaviviridae family of enveloped RNA viruses and
contains a 9.6 kb single-stranded positive-sense RNA genome. This
genome is translated into a large polyprotein which is then cleaved
by viral and host proteases into structural (core, E1 and E2) and
nonstructural (p7, NS2, NS3, NS4A, NS4B, NS5A and NS5B) proteins
(3–5). In addition to their unique
involvement in the life cycle and assembly of the virus, these HCV
proteins also participate in processes, including transcriptional
activation, cell signaling, apoptosis and transformation by way of
interaction with host factors (6–10).
In particular, the core gene product has long been proposed as a
candidate protein implicated in liver oncogenesis.
Hypoxia-inducible factor 1 (HIF-1) is a heteroduplex
that contains a constitutively expressed HIF-1β subunit and an
oxygen- and growth factor-regulated HIF-1α subunit (the major
determinant for the activity of HIF-1). HIF-1 is the most important
regulator of oxygen homeostasis, which is required for cellular
metabolism (11). Under
physiological and normoxic conditions, HIF-1α is subjected to rapid
degradation by ubiquitin-proteasome pathways (12). However, under hypoxic induction or
non-hypoxic growth factor induction conditions, HIF-1α is
overexpressed and stabilized, resulting in the activation of genes
that stimulate angiogenesis, including vascular endothelial growth
factor (VEGF) (13). In human
cancers, HIF-1 mediated angiogenesis and metabolic adaptation are
important in tumor formation, progression and metastasis (14).
Extensive investigations have been conducted to
elucidate the inter-relationships between viral products and host
cellular factors. Previous studies have demonstrated that HCV
infection stabilizes HIF-1α and stimulates the synthesis of VEGF
(15). The present study
demonstrated that induction of HCV core protein expression in
Huh7.5.1 cells enhances the transcriptional level and protein
amount of HIF-1α, as well as VEGF, and also confirmed that the HCV
core protein increases the expression of VEGF directly via the
activation of HIF-1α. Thus, we propose a novel molecular mechanism
of the core protein in modulating gene expression that is
associated with HCC.
Materials and methods
Plasmids and siRNAs
The plasmid pCMV-Tag2B (Flag2B; Stratagene, La
Jolla, CA, USA) was used to construct the HCV core expression
plasmid pCMV-Tag2B-core (Flag2B-core). The selection of siRNAs
against HIF-1α (HIF-1α siRNA) and negative control siRNA (NC siRNA)
were based on the study by Gillespie et al (16).
Cell culture and transfection
The human hepatoma cell line, Huh7.5.1, was cultured
in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum, 100 U of penicillin/ml and 100 μg of
streptomycin sulfate/ml at 37°C in a humidified 5% CO2
incubator. Transient transfections of Huh7.5.1 cells with the
plasmids and siRNAs described above were conducted using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA), according to the manufacturer’s instructions.
Reverse transcriptase (RT)-PCR
Following 48 h of transfection, total cellular RNAs
were extracted using TRIzol (Invitrogen Life Technologies) and the
cDNA was reverse transcribed from 1 μg of total RNA using an oligo
(dT) primer. The resulting cDNA was PCR amplified with the
following gene-specific primers: HIF-1α, forward
5′-TAGTGCCACATCATCACC-3′ and reverse 5′-ACATGCTAAATCAGAGGG-3′;
VEGF, forward 5′-GGGCAGAATCATCACGAAGT-3′ and reverse
5′-GGCTCCAGGGCATTAGACA-3′. PCR amplification was performed under
the following conditions: 10 min at 95°C, followed by 35 cycles of
94°C for 15 sec, 55°C for 30 sec and 72°C for 30 sec and finishing
with a dissociation protocol. The PCR products were detected by 2%
agarose gel electrophoresis and visualized under UV light with
ethidium bromide staining.
Western blot analysis
Following 48 h of transfection, cell samples were
lysed with Nonidet P-40 lysis buffer [10 mM of Tris-HCl (pH 7.4),
10 mM of NaCl, 3 mM of MgCl2 and 0.5% Nonidet P-40]. The
cell lysates were then centrifuged at 3,000 × g for 10 min and the
supernatants were used in the assay. Protein samples were separated
by 12% SDS-polyacrylamide gel electrophoresis and then transferred
onto nitrocellulose membranes. Following the inhibition of
non-specific binding sites, western blot analysis was performed
using specific antibodies against HIF-1α (Santa Cruz Biotechnology
Inc., Santa Cruz, CA, USA), VEGF (Sigma, St. Louis, MO, USA), HCV
core protein (Affinity Bioreagents, Golden, CO, USA) and, as an
internal control, a monoclonal antibody against β-actin (Sigma).
Following washing, blots were developed with horseradish
peroxidase-labelled goat anti-rabbit IgG, using an enhanced
chemiluminescence kit (Amersham Life Sciences, Piscataway, NJ,
USA).
Enzyme-linked immunosorbent assay (ELISA)
analysis
Following 48 h of transfection, the VEGF
concentration in cell supernatants was measured by ELISA, which was
performed according to the manufacturer’s instructions (R&D
Systems, Minneapolis, MN, USA).
Statistical analysis
SPSS 13.0 software was used for statistical
analysis. Values are expressed as the means ± SD. The comparison of
two means was performed by t-tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
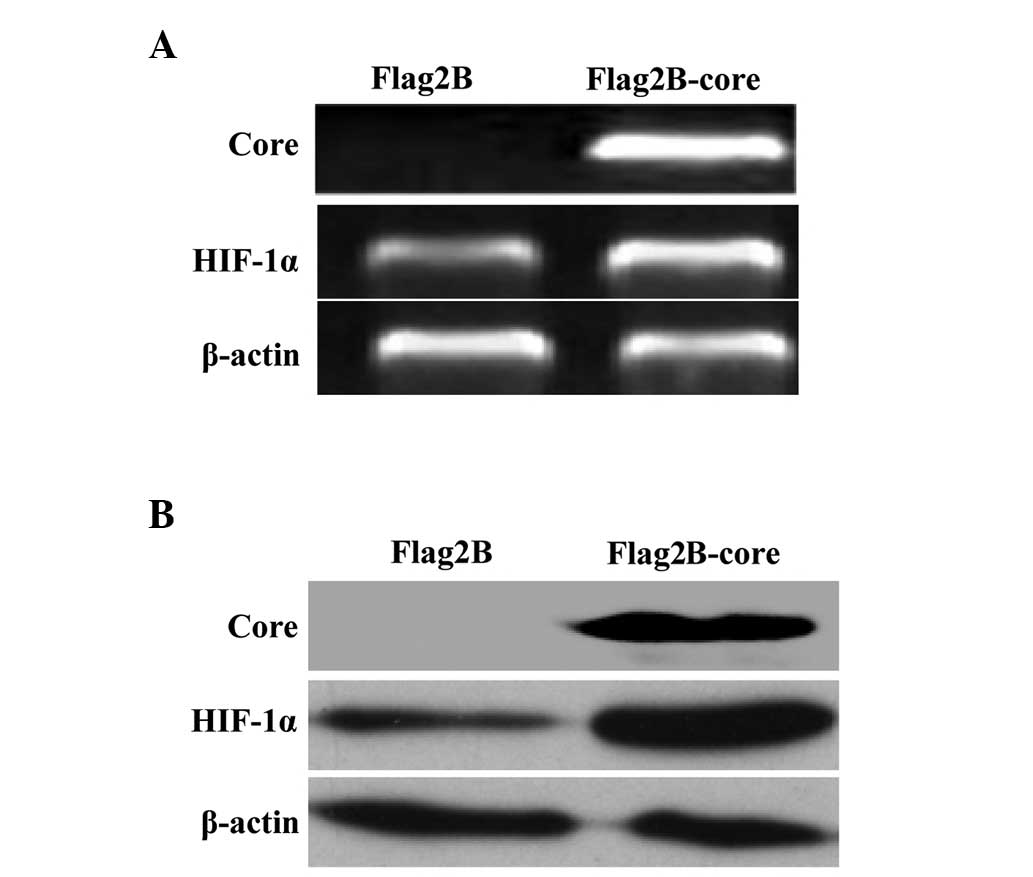
HCV core protein enhances the expression
levels of HIF-1α mRNA and protein in Huh7.5.1 cells
HIF-1 is a heterodimeric (HIF-1α coupled with
HIF-1β) protein that regulates oxygen homeostasis for cellular
metabolism and acts as an inducer of angiogenic factors. Under
physiological conditions, HIF-1α is constitutively expressed and
degraded, however, under hypoxia or other conditions, HIF-1α is
overexpressed and stabilized (11,13).
In the present study, the expression levels of HIF-1α mRNA and
protein were measured by RT-PCR and western blot analysis,
respectively, in Huh7.5.1 cells transfected with the HCV core gene
eukaryotic expression vector (Flag2B-core) or the empty vector
(Flag2B). The results in Fig. 1A
demonstrated a moderate increase of HIF-1α mRNA in HCV core induced
Huh7.5.1 cells relative to non-induced cells. The western blot
assay demonstrated a significant increase of HIF-1α protein in HCV
core induced Huh7.5.1 cells compared with the control (Fig. 1B).
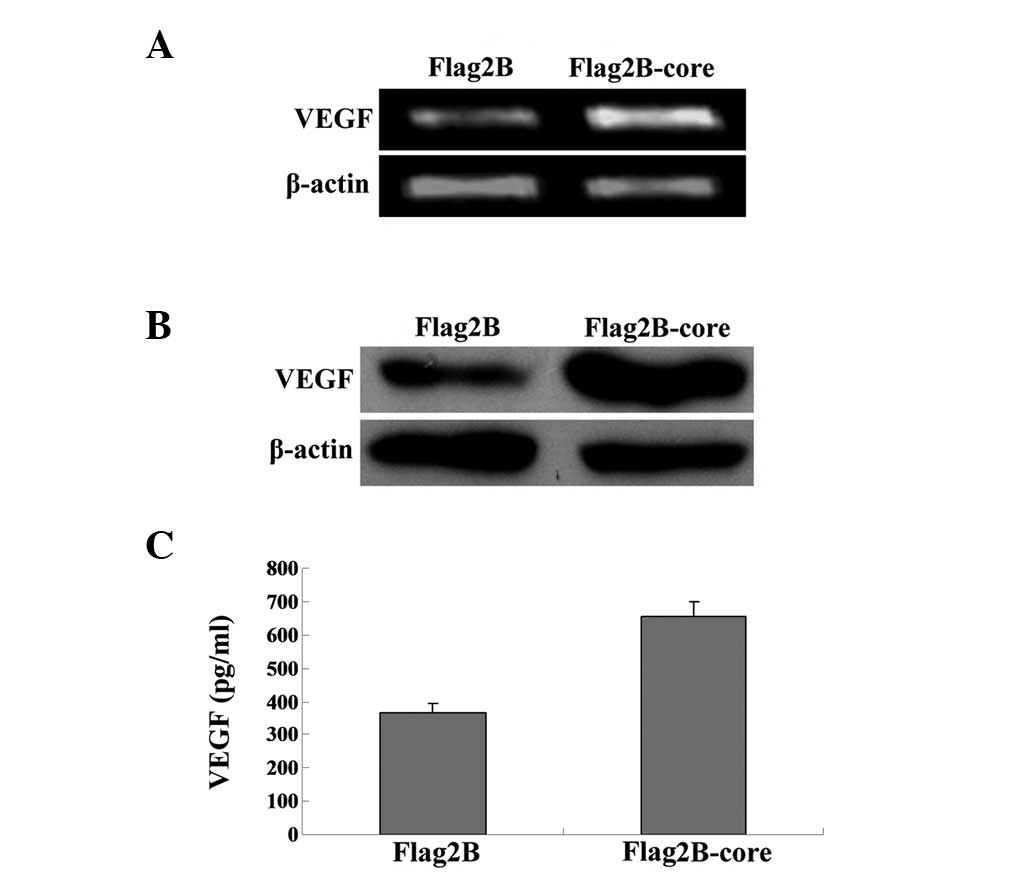
HCV core protein induces the expression
and secretion of VEGF in Huh7.5.1 cells
VEGF stimulates angiogenesis and vascular
permeability in neoplastic tissues, which means the expression and
secretion of VEGF are increased significantly in numerous types of
cancer (17,18). In order to investigate whether HCV
core gene expression alone can induce the expression and secretion
of VEGF, VEGF mRNA and protein levels in Huh7.5.1 cells were
measured according to the same instructions used to measure HIF-1α
expression levels. An increase in VEGF mRNA (Fig. 2A) and protein expression (Fig. 2B) was identified in HCV core
induced Huh7.5.1 cells, indicating that the HCV core protein
contributes to the biosynthesis of VEGF.
As VEGF can be secreted into the extracellular
media, we further examined the concentrations of VEGF in cell
supernatants by ELISA. The supernatant was removed from all wells
and a human VEGF ELISA (R&D Systems) was performed on the cell
supernatants 48 h post-transfection, as described in the Quantikine
human VEGF ELISA instructions. Student’s t-test was used for
statistical analysis. The results in Fig. 2C demonstrated that VEGF
concentrations in the supernatants of HCV core induced Huh7.5.1
cells were significantly elevated compared with the controls
(654.5±43.7 vs 365.9±26.8 pg/ml).
RNA interference disrupts HIF-1α-induced
upregulation of VEGF
The present study utilized HIF-1α siRNA that, when
transfected into cells, targets HIF-1α mRNA for degradation, thus
reducing the expression of HIF-1α RNA and protein. The VEGF mRNA
(Fig. 3A) and protein levels
(Fig. 3B) in Huh7.5.1 cells
cotransfected with Flag2B-core and HIF-1α siRNA were significantly
reduced compared with the Flag2B-core plus NC siRNA-transfected
cells. The ELISA results in Fig.
3C (389.2±29.6 vs 768.8±47.3 pg/ml) were in line with the
results in Fig. 3A and B.
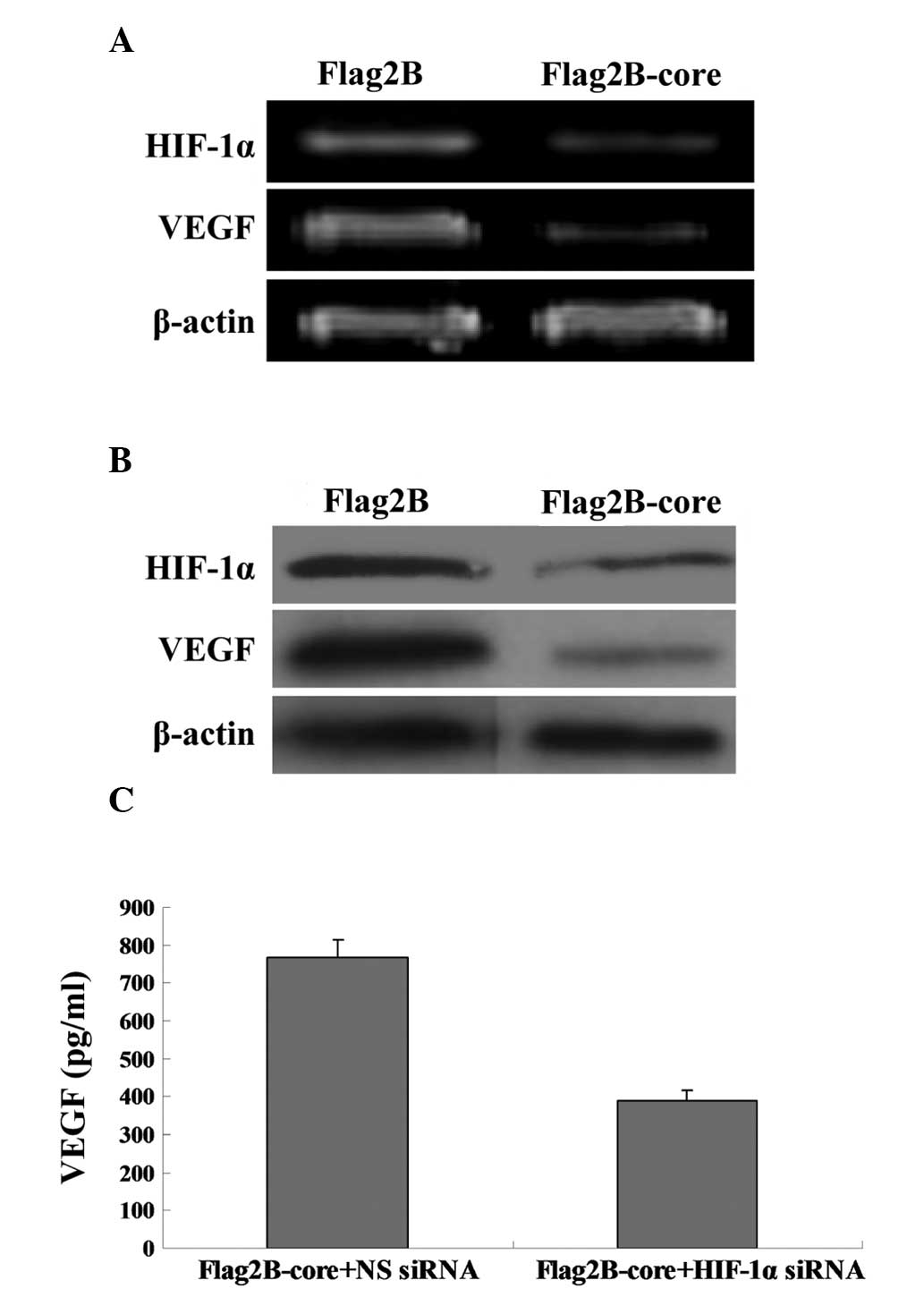 | Figure 3RNA interference disrupts
HIF-1α-induced upregulation of VEGF. (A) RT-PCR analysis was used
to compare the relative levels of VEGF mRNA in Huh7.5.1 cells
transfected with Flag2B-core plus NC siRNA (Lane 1) and Flag2B-core
plus HIF-1α siRNA (Lane 2). The β-actin gene was amplified as an
internal control. PCR products were detected by 2% agarose gel
electrophoresis with ethidium bromide staining. (B) Western blot
analysis of VEGF protein expression in Huh7.5.1 cells. Lane 1,
Huh7.5.1 cellular lysates transfected with Flag2B-core plus NC
siRNA; Lane 2, Huh7.5.1 cellular lysates transfected with
Flag2B-core plus HIF-1α siRNA. The middle panel represents the
expression of the HCV core protein and the bottom panel represents
the expression of β-actin as an internal control. (C) ELISA
analysis of VEGF concentrations in the supernatants of Flag2B-core
plus NC siRNA transfected Huh7.5.1 cells and Flag2B-core plus
HIF-1α siRNA transfected Huh7.5.1 cells. HCV, hepatitis C virus;
HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial
growth factor; RT-PCR, reverse transcriptase-PCR; Flag2B-core,
pCMV-Tag2B-core; Flag2B, pCMV-Tag2B; HIF-1α siRNA, siRNAs against
HIF-1α; NC siRNA, negative control siRNA. |
Discussion
HCV infections are associated with the development
of HCC, however, the underlying mechanisms by which HCV induces HCC
are not well understood. Indeed, although accumulating studies have
implicated the specific roles of HCV proteins in the modulation of
cell proliferation and pathogenesis, it is unclear which viral gene
products are crucial for the establishment of HCC (19,20).
It has been demonstrated that the core protein of HCV can induce
HCC in transgenic mice by the modulation of cellular gene products,
which has brought the core protein to the attention of researchers
(21).
The core protein is located at the N-terminal
portion of the HCV polyprotein and is highly conserved among
various HCV subtypes. Apart from functioning as the building block
of the viral nucleocapsid, which is involved in binding and
packaging the viral RNA genome, the core protein exhibits
pleiotropic roles in numerous activities, including gene
transcription, cell proliferation and cell death through
interference with the normal functions of an extensive list of
cellular proteins (21). In this
regard, the present study was undertaken to investigate whether HCV
core gene expression is able to trigger angiogenesis, which is
pivotal in tumor formation and maintenance. Our results
demonstrated that the induction of HCV core protein expression
enhances the transcriptional level and amount of HIF-1α as well as
VEGF in Huh7.5.1 cells. HIF-1α and VEGF are regulators of
angiogenesis and are important in wound healing, the regeneration
of new vessels and reproductive functions. Therefore, these results
indicated that the HCV core protein is able to stimulate
angiogenesis.
The first study of HIF-1α overexpression in human
cancer was ~10 years ago. Since then, a large amount of data has
been collected demonstrating that HIF-1α overexpression is
associated with tumor angiogenesis and increased mortality in
cancer of the brain, breast, oropharynx, esophagus, colon, ovary
and uterine cervix (22–25). Notably, proteins encoded by
transforming viruses that cause tumors in humans, including EBV
latent membrane protein 1, hepatitis B virus × protein, human
papillomavirus E6/E7 proteins and human T-cell leukemia virus Tat
protein, also induce HIF-1α activity (26–29).
Therefore, it is evident that HIF-1α activity represents a
fundamental common pathway in cancer pathogenesis.
What are the mechanisms by which the HCV core
protein activates HIF-1α? In various types of human cancer, the
increased expression of HIF-1α is induced either by intratumoral
hypoxia or by genetic alterations affecting key oncogenes and tumor
suppressor genes. For example, ras signaling has been
demonstrated to be instrumental in hypoxia-induced stabilization of
HIF-1α and inactivation of p53 in tumor cells, which
contributes to the activation of the angiogenic switch via
amplification of normal HIF-1 dependent responses to hypoxia
(30). Regarding HCV-induced
tumors, the HCV core protein is able to co-operate with the
ras oncogene in the transformation of rodent fibroblasts
under certain conditions and is able to exert transcriptional
repression of the p53 promoter (31). However, we were unable to draw a
conclusion regarding the mechanism of activation of HIF-1α by the
HCV core protein as there is not enough evidence that HCV can
directly activate any oncogene or deactivate any tumor suppressor
genes at present. As the complete cell culture systems of HCV are
now available, it may be useful to study more aspects of the HCV
core protein in order to elucidate the exact mechanisms underlying
HCV core protein induction of HIF-1α.
Our results confirmed that the core protein
activates HIF-1α, which, in turn, increases the expression of VEGF.
At present, VEGF inhibitors are undergoing clinical testing as a
strategy for the prevention and treatment of certain malignancies.
These findings may prompt worldwide study into the inhibition of
HIF-1α, which may be a novel approach to cancer therapy.
In conclusion, the role of HCV in inducing
oncogenesis is complicated and awaits further detailed
investigation. In the case of the prevention and control of the
virus induced tumors, the cellular response factors activated by
viral infection warrant further studies. Thus, a mixture of
antibodies or inhibitors may be required that target the virus
itself and such cellular factors.
Acknowledgements
This study was supported by research grants from the
Major State Basic Research Development Program (973 Program;
2012CB518900), the National Clinical Key Subject (no. 2010305), the
National Science Foundation of China (no. 81101485 to Zhu CL, no.
31270206 to Wu KL), the Open Research Program of the State Key
Laboratory of Virology of China (no. 2011009, 2012007, 2013004) and
the China Postdoctoral Foundation (no. 201104485).
References
|
1
|
Raimondi S, Bruno S, Mondelli MU and
Maisonneuve P: Hepatitis C virus genotype 1b as a risk factor for
hepatocellular carcinoma development: a meta-analysis. J Hepatol.
50:1142–1154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yotsuyanagi H, Koike K, Yasuda K, et al:
Hepatitis C virus genotypes and development of hepatocellular
carcinoma. Cancer. 76:1352–1355. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Francesco R: Molecular virology of the
hepatitis C virus. J Hepatol. 31(Suppl 1): 47–53. 1999.
|
|
4
|
Rosenberg S: Recent advances in the
molecular biology of hepatitis C virus. J Mol Biol. 313:451–464.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suzuki R, Suzuki T, Ishii K, Matsuura Y
and Miyamura T: Processing and functions of Hepatitis C virus
proteins. Intervirology. 42:145–152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duong FH, Filipowicz M, Tripodi M, La
Monica N and Heim MH: Hepatitis C virus inhibits interferon
signaling through up-regulation of protein phosphatase 2A.
Gastroenterology. 126:263–277. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones DM, Patel AH, Targett-Adams P and
McLauchlan J: The hepatitis C virus NS4B protein can
trans-complement viral RNA replication and modulates production of
infectious virus. J Virol. 83:2163–2177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park CY, Jun HJ, Wakita T, Cheong JH and
Hwang SB: Hepatitis C virus nonstructural 4B protein modulates
sterol regulatory element-binding protein signaling via the AKT
pathway. J Biol Chem. 284:9237–9246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wagoner J, Austin M, Green J, et al:
Regulation of CXCL-8 (interleukin-8) induction by double-stranded
RNA signaling pathways during hepatitis C virus infection. J Virol.
81:309–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yi M, Ma Y, Yates J and Lemon SM:
Trans-complementation of an NS2 defect in a late step in hepatitis
C virus (HCV) particle assembly and maturation. PLoS Pathog.
5:e10004032009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Semenza GL: Regulation of oxygen
homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda).
24:97–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roos-Mattjus P and Sistonen L: The
ubiquitin-proteasome pathway. Ann Med. 36:285–295. 2004. View Article : Google Scholar
|
|
13
|
Shemirani B and Crowe DL: Hypoxic
induction of HIF-1alpha and VEGF expression in head and neck
squamous cell carcinoma lines is mediated by stress activated
protein kinases. Oral Oncol. 38:251–257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi YH and Fang WG: Hypoxia-inducible
factor-1 in tumour angiogenesis. World J Gastroenterol.
10:1082–1087. 2004.PubMed/NCBI
|
|
15
|
Nasimuzzaman M, Waris G, Mikolon D,
Stupack DG and Siddiqui A: Hepatitis C virus stabilizes
hypoxia-inducible factor 1alpha and stimulates the synthesis of
vascular endothelial growth factor. J Virol. 81:10249–10257. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gillespie DL, Flynn JR, Ragel BT, et al:
Silencing of HIF-1alpha by RNA interference in human glioma cells
in vitro and in vivo. Methods Mol Biol. 487:283–301.
2009.PubMed/NCBI
|
|
17
|
Katoh R, Miyagi E, Kawaoi A, et al:
Expression of vascular endothelial growth factor (VEGF) in human
thyroid neoplasms. Hum Pathol. 30:891–897. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Machein MR and Plate KH: VEGF in brain
tumors. J Neurooncol. 50:109–120. 2000. View Article : Google Scholar
|
|
19
|
Banerjee A, Ray RB and Ray R: Oncogenic
potential of hepatitis C virus proteins. Viruses. 2:2108–2133.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
McGivern DR and Lemon SM: Virus-specific
mechanisms of carcinogenesis in hepatitis C virus associated liver
cancer. Oncogene. 30:1969–1983. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moriya K, Fujie H, Shintani Y, et al: The
core protein of hepatitis C virus induces hepatocellular carcinoma
in transgenic mice. Nat Med. 4:1065–1067. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chun SY, Johnson C, Washburn JG,
Cruz-Correa MR, Dang DT and Dang LH: Oncogenic KRAS modulates
mitochondrial metabolism in human colon cancer cells by inducing
HIF-1alpha and HIF-2alpha target genes. Mol Cancer. 9:2932010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Doronkin S, Djagaeva I, Nagle ME, Reiter
LT and Seagroves TN: Dose-dependent modulation of HIF-1alpha/sima
controls the rate of cell migration and invasion in Drosophila
ovary border cells. Oncogene. 29:1123–1134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Talks KL, Turley H, Gatter KC, et al: The
expression and distribution of the hypoxia-inducible factors
HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and
tumor-associated macrophages. Am J Pathol. 157:411–421. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wong C, Wellman TL and Lounsbury KM: VEGF
and HIF-1alpha expression are increased in advanced stages of
epithelial ovarian cancer. Gynecol Oncol. 91:513–517. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moon EJ, Jeong CH, Jeong JW, et al:
Hepatitis B virus × protein induces angiogenesis by stabilizing
hypoxia-inducible factor-1alpha. FASEB J. 18:382–384. 2004.
|
|
27
|
Tomita M, Semenza GL, Michiels C, et al:
Activation of hypoxia-inducible factor 1 in human T-cell leukaemia
virus type 1-infected cell lines and primary adult T-cell leukaemia
cells. Biochem J. 406:317–323. 2007. View Article : Google Scholar
|
|
28
|
Wakisaka N, Kondo S, Yoshizaki T, Murono
S, Furukawa M and Pagano JS: Epstein-Barr virus latent membrane
protein 1 induces synthesis of hypoxia-inducible factor 1 alpha.
Mol Cell Biol. 24:5223–5234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang EY and Tang XD: Human papillomavirus
type 16/18 oncoproteins: potential therapeutic targets in
non-smoking associated lung cancer. Asian Pac J Cancer Prev.
13:5363–5369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Markert EK, Levine AJ and Vazquez A:
Proliferation and tissue remodeling in cancer: the hallmarks
revisited. Cell Death Dis. 3:e3972012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smirnova IS, Aksenov ND, Kashuba EV, et
al: Hepatitis C virus core protein transforms murine fibroblasts by
promoting genomic instability. Cell Oncol. 28:177–190.
2006.PubMed/NCBI
|