Introduction
Meniscal fibrocartilage injury of the knee joint is
one of the most common sports injuries, and is the focus for
numerous specialists. If not treated in a timely manner, meniscal
fibrocartilage injury may lead to serious consequences for patients
(1). However, due to the low
content of chondrocytes, the poor self-repair capabilities and the
deficient local blood supply in the meniscal fibrocartilage,
current treatments, including the widely recognized arthroscopic
meniscal repair techniques, do not lead to satisfactory outcomes
(2). For example, it remains
unknown whether certain areas or types of meniscal injury repaired
using sutures are capable of healing (3). Yoon et al (4) presented a novel technique designed
for the reduction and repair of bucket-handle meniscal tears to
make an avenue to success of the surgery. Wang et al
(5) developed a novel arthroscopic
technique of direct repair for the radial tear of the posterior
root medial meniscus using a posterior trans-septal portal which
could avoid disturbing the normal meniscal movement during flexion
in a loaded condition. In spite of the different techniques used by
the aforementioned surgeons, the ultimate results of meniscal
healing are not certain. Our previous study showed that canine
myoblasts can be induced to transform into chondrocytes by
cartilage-derived morphogenetic protein-2 (CDMP-2) and transforming
growth factor β1 (TGF-β1) in vitro (6). The present study explores the effects
of canine myoblasts expressing human (h)CDMP-2 on the repair of
meniscal fibrocartilage injury in vivo.
Materials and methods
Animals and reagents
A total of 10 one-year-old male canines were
provided by the College of Agriculture at the Shanghai
Transportation University (Shanghai, China; permit no. 2007-0004).
Study approval was obtained from the Shanghai Animal Care and Use
Committee (Shanghai, China) prior to performing the study. The
following reagents were purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA): pcDNA™6.2-GW/EmGFP, plenti6/v5, one shot Stbl3
chemically competent Escherichia coli, BLOCK-iT polymerase
II (Pol II) expression vector kit with emerald green fluorescent
protein (EmGFP) and BLOCK-iT™ lentiviral Pol II expression system.
The following reagents were also used: AxyPrep plasmid purification
kit (Axygen, Union City, CA, USA), polylactic acid (PLA) and
polyglycolic acid (PGA) (Sigma, St. Louis, MO, USA), recombinant
human CDMP-2 (PeproTech, London, UK), 0.25% F-10 basic culture
media and Safranin-O dye (Shanghai Reagent Company, Shanghai,
China), toluidine blue dye (Shanghai Reagent Company),
anti-collagen-I antibodies (mouse and canine; Abcam, Cambridge,
UK), anti-collagen-II antibodies (mouse anti-canine; Neomarkers,
Fremont, CA, USA), anti-S-100 antibodies (rabbit anti-canine;
Neomarkers) and Alcian Blue staining buffer (Pierce Biotechnology,
Inc., Rockford, IL, USA).
Lentiviral infection of canine
myoblasts
Canine myoblasts were purified and cultured, as
previously described (7). The
cells were infected with lentiviruses containing the empty vector
or the hCDMP-2 gene for two days. GFP+ clones were
selected for further studies. The expression of GFP and hCDMP-2
genes in different clones were quantified by quantitative
polymerase chain reaction (qPCR) using total RNA. The sequences of
the primers were: GAPDH forward, 5′-GACAACTTTGGTATCGTGGAAGG-3′ and
reverse, 5′-CCAGTAGAGGCAGGGATGATGT-3′; GAPDH probe,
fam-5′-CTCATGACCACAGTCCATGCCATCACT-3′-tamra; GFP forward,
5′-AAGCAGCACGACTTCTTCAAGTC-3′ and reverse,
5′-TCGCCCTCGAACTTCACCTC-3′; GFP probe,
5′-CATGCCCGAAGGCTACGTCCAGGAG-3′; hCDMP-2 forward,
5′-CAGAAGTATTTGTTTGATGTGTCCA-3′ and reverse:
5′-AAAGGCAAGGGAAGAGCTGCA-3′; and hCDMP-2 probe,
5′-CAGACAAAGAAGAGCTGGTGG GCGC-3′.
Western blot analysis
Western blot analyses were performed with total
protein extracts prepared using T-PER® tissue protein
extraction reagent (Pierce). Filters were probed with anti-CDMP-2
(mouse anti-human; Sigma).
In vivo repair of meniscal fibrocartilage
injury in canines
The PLA/PGA (Sigma) scaffold was made using a
specific mold. The canine model of meniscal injury was established
by cutting across the entire meniscus (0.5 cm from the front edge,
with a width of 2 mm, and a thickness of 2 mm at the
meniscocapsular junction and 1.5 mm at the other side) (Fig. 1A). The injury encompassed all three
zones (the red-red zone, red-white zone and white-white zone). The
animals were divided into four groups: Group A, suture only; group
B, suture with the addition of the recombinant hCDMP-2 on the
PLA/PGA scaffold; group C, PLA/PGA scaffold with canine myoblasts
carrying the empty vector; D, PLA/PGA scaffold with canine
myoblasts expressing hCDMP-2 (six menisci for each group) (Fig. 1B and C). Samples of repaired tissue
extracted at weeks 3, 8 and 12 post-repair were subjected to gross
morphological studies and hematoxylin and eosin (H&E) and
Safranin-O staining. Immunohistochemistry (IHC) studies were
performed to assess the expression of collagen I and II and S-100
protein. Quantitative assays were also performed to assess the
levels of collagen I and II (by ELISA), and the glycosaminoglycans
(GAGs) (by Alcian Blue staining) in the red-red and white-white
zones. The normal value was obtained by assessing the meniscal
tissue at the uninjured part using the same methods as those used
for the repaired tissue.
Statistical analysis
The data are expressed as the mean ± standard
deviation. Statistical significance was determined using one-way
analysis of variance and the level of statistical significance was
set at a probability value of P<0.05.
Results
Expression of hCDMP-2 in canine myoblasts
following lentiviral infection
The infection efficiency was determined to be
71±0.088% by qPCR, and the expression level of hCDMP-2 was
0.0015±0.0005. Western blot analysis confirmed the expression of
hCDMP-2 protein upon lentiviral infection of the canine
myoblasts.
H&E and Safranin-O staining for the
evaluation of in vivo meniscal fibrocartilage injury repair
H&E and Safranin-O staining showed partial
staining of the repaired tissue in group D at week 3 post-repair. A
few cartilage lacunae mixed with the scaffold tissue were observed
in the red-red zone, while no staining was observed in the
white-white zone (Fig. 2A and B).
At week 8, H&E staining showed increased cartilage lacunae in
the red-red zone of group D, and fibrous structures started to
appear (Fig. 2C), while a small
amount of cartilage lacunae without fibrous structures were
observed in the white-white zone (Fig.
2D). The red-red zone showed an increased intensity and volume
of cartilage lacunae (Fig. 2E),
while the white-white zone showed partial staining with Safranin-O
(Fig. 2F). At week 12 post-repair,
H&E staining showed obvious cartilage lacunae in the red-red
zone, with increased surrounding fibrous structures (Fig. 2G), while the presence of cartilage
lacunae was also increased in the white-white zone, with no fibrous
structures (Fig. 2H). Safranin-O
staining showed the increased intensity and volume of the
regenerated tissues in the red-red and the white-white zones at
week 12 (Fig. 2I and J).
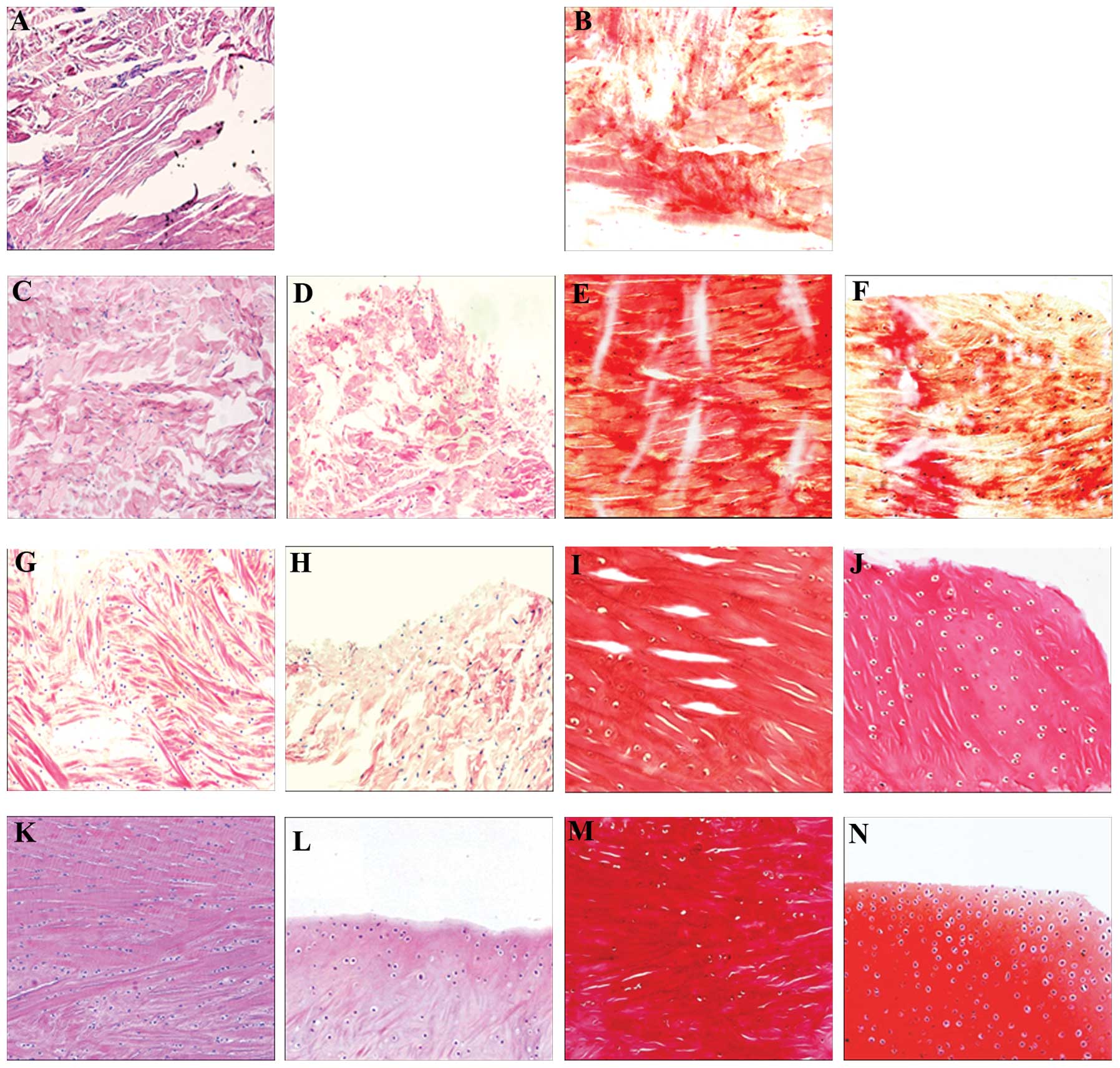 | Figure 2Morphology of the repaired tissue at
the red-red zone in treatment group D at (A and B) week 3, (C and
E) week 8 and (G and I) week 12 post-repair (A, C and G, H&E
staining; B, D and I, Safranin-O staining; magnification, ×100).
Morphology of the repaired tissue at the white-white zone in group
D at (D and F) week 8and (H and J) week 12 post-repair (D and H,
H&E staining; F and J, Safranin-O staining; magnification,
×100). (K and L) H&E staining of the normal meniscus at the
red-red and the white-white zones, respectively (magnification,
×100). (M and N) Safranin-O staining of the normal meniscus at the
red-red and the white-white zones, respectively. H&E,
hematoxylin and eosin. |
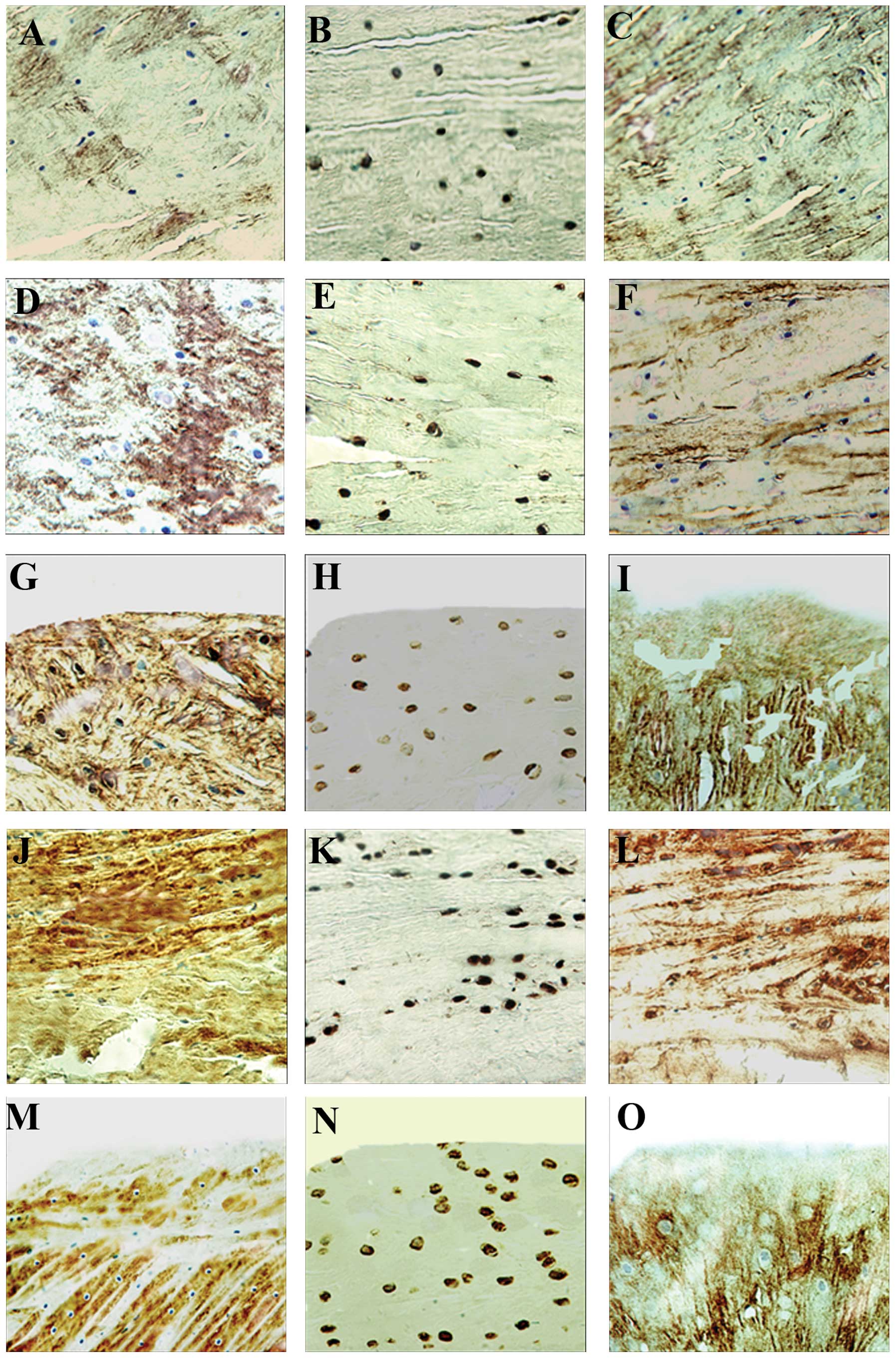
Examination of the protein expression of
collagen I, collagen II and S-100
IHC studies revealed low expression levels of the
cartilage-specific proteins, collagen II, collagen I and S-100, in
the red-red zone in group D at week 3 post-repair, while no
expression was observed in the white-white zone (Fig. 3A–C). At week 8 post-repair,
positive expression of these proteins was detected in the repaired
tissue in the red-red and white-white zones (Fig. 3D–I). At week 12, the expression of
these proteins remained positive in the two zones (Fig. 3J–O).
Quantitative assessment of collagen I,
collagen II and GAG proteins
The expression of collagen I and II was further
quantified by ELISA, while the GAGs were assessed by Alcian blue
staining. At week 3 post-repair, the expression of collagen I,
collagen II and the GAGs was detected in the repaired tissue of the
red-red zone, while no expression of these proteins was observed in
the white-white zone (Tables
I–III). At week 8, the
expression of these proteins was detected in the red-red and
white-white zones (Fig. 4).
Similar results were obtained at week 12 (Fig. 4). Statistical analyses indicated
that the expression of these three proteins increased with
increasing time, and the differences between each pair of
time-points were significant (P<0.01). In addition, significant
differences were revealed between the red-red and white-white zones
at the same time-point (P<0.01).
 | Table IExpression of collagen I (ng/ml) at
the red-red and white-white zones in group D. |
Table I
Expression of collagen I (ng/ml) at
the red-red and white-white zones in group D.
| Time post-repair | |
|---|
|
| |
|---|
| Zone | 3 weeks | 8 weeks | 12 weeks | Normal |
|---|
| Red-red | 91.238±4.623a,b | 205.719±3.180a,b | 592.213±7.979a,b | 996.228±2.691a,b |
| White-white | 0.000±0.000 | 75.125±3.098b | 105.300±3.105b | 501.077±4.768b |
 | Table IIIExpression of GAGs (μg/ml) at the
red-red and white-white zones in group D. |
Table III
Expression of GAGs (μg/ml) at the
red-red and white-white zones in group D.
| Time post-repair | |
|---|
|
| |
|---|
| Zone | 3 weeks | 8 weeks | 12 weeks | Normal |
|---|
| Red-red | 1.758±0.125a,b | 2.626±0.067a,b | 3.365±0.121a,b | 5.019±0.178a,b |
| White-white | 0.000±0.000 | 1.696±0.106b | 2.807±0.070b | 9.688±0.320b |
Discussion
To facilitate the repair of injury to meniscal
fibrocartilage, which lacks a sufficient blood supply, numerous
methods have been assessed in the clinic, including the
establishment of a blood supply channel, blood clots and
implantation of pedunculated synovial flaps, isolated synovia,
porous polymers and fibrin clots (8,9). In
recent years, certain studies have employed purified growth
factors, including epidermal growth factor, TGF, platelet-derived
growth factor, basic fibroblast growth factor, hepatocyte growth
factor, bone morphogenetic protein 2 and interleukin 1 to
facilitate the in vitro expansion of chondrocytes from
meniscal fibrocartilage (10,11).
Certain studies have used gene transfer technology to improve the
in vitro growth of chondrocytes from meniscal fibrocartilage
(12), while others have combined
scaffold materials with growth factors to facilitate the healing of
meniscal fibrocartilage (13).
Further studies have explored stem cell technologies in combination
with scaffold materials (13,15).
These studies have indicated that it is crucial to select
appropriate cell types and an effective route of treatment in order
to achieve the most favorable outcome for meniscal fibrocartilage
injury repair.
CDMP-2 is able to facilitate cartilage repair
without local tissue ossification and also to promote the ectopic
generation of new tendons and ligaments (containing mainly type I
collagen similar to fibrocartilage), and improve the quality of
fibrocartilage tissue repair (16).
A previous study reported that myoblasts acted as
adult stem cells for skeletal muscle, and that they maintained
similar functions to multipotent mesenchymal cells that exhibit
myogenic, osteogenic and adipogenic differentiation (17). Myoblast gene therapy has been
successful in the treatment of muscle diseases and bone and joint
problems.
The present study showed that canine myoblasts are
able to produce hCDMP-2 RNA and protein in vitro following
lentiviral infection with the hCDMP-2 gene. The infected canine
myoblasts were transplanted into the meniscal injury site via a
PLA/PGA scaffold in order to observe their effect on meniscal
injury repair. H&E histology studies and Safranin-O staining,
which assess cartilage GAGs in cells or tissue, showed that the
treatments in groups A, B and C did not lead to the regeneration of
new tissue in the canine meniscal injury region. By contrast, group
D showed newly repaired tissue in different regions (red-red and
white-white zones) with the characteristics of fibrocartilage
tissue, demonstrating that the treatment of group D was able to
promote the meniscal repair by fibrocartilage-like tissue
regeneration. Furthermore, quantitative assessment of the
fibrocartilage tissue-specific components, including collagen I,
collagen II and GAGs, indicated that the red-red zone was
regenerated more rapidly than the white-white zone during the
fibrocartilage-like tissue repair of meniscal injury. This is
consistent with the previous hypothesis, and may be due to the
differences in the blood supply of these two regions.
In all four groups, the PLA/PGA scaffold completely
dissolved at 8 weeks post-transplantation, indicating that the
PLA/PGA scaffold used in this study did not affect meniscal injury
repair. The scaffold material can be degraded in vivo, and
the time for the complete degradation is approximately eight
weeks.
Meniscal fibrocartilage has the characteristics of
cartilage tissue as well as fibrous tissue. The white-white zone is
more similar to the cartilage tissue (18), while the red-red zone resembles the
fibrous tissue (19). Collagen
fibers in the red-red zone are generally distributed longitudinally
in a horizontal plain and aligned in a ‘C’ shape, while collagen
fibers in the white-white zone exhibit a radial distribution
(20). The IHC results of the
present study revealed that the regenerated collagen fibers in the
red-red zone exhibited a ‘C’-shaped distribution. Collagen I was
aligned along the fibers, while collagen II was scattered
irregularly among the mesenchyma of the tissue irrelevant to the
fiber alignment. These observations are consistent with the
previous description of the histology and structure of the meniscal
fibrocartilage, indicating that the treatment adopted in the
present study resulted in the meniscal regeneration by tissue that
was similar to normal meniscal fibrocartilage tissue. Furthermore,
a previous study indicated that the structure and components of the
red-red and white-white regions are different from each other, as
the former contains collagen I, which forms large beam fibers,
while the latter contains high levels of collagen II and
proteoglycans (21). These
components have distinct functions to confer meniscal biomechanical
properties. The content proportion of collagen I and II and the
GAGs in the normal meniscal tissue obtained in the present study
was similar to that of previous studies. However, the amounts and
distribution of each component of the repaired tissue from group D
showed differences from the normal tissue. For example, the red-red
zone showed higher levels of the assessed proteins as compared to
the white-white zone at all the examined time-points. Further
evaluation is, however, required to assess whether these
differences are capable of affecting the function of meniscal
fibrocartilage.
The present study indicated that myoblasts infected
with the hCDMP-2 gene are able to facilitate the regeneration of
meniscal fibrocartilage-like tissue in the canine model of local
meniscal injury, and that the repair of the red-red zone is more
rapid than that of the white-white zone. Further studies are
required to address the mechanism of the hCDMP-2-mediated effect on
meniscal fibrocartilage injury repair. In addition, due to the
limitation in the clinical use of lentiviral infections, further
studies are required to explore the best way to combine the hCDMP-2
growth factor with myoblasts to be used in the clinic.
Acknowledgements
This study was supported by grants from the Shanghai
Natural Science Foundation (no. 09ZR1425500) and the National
Natural Science Foundation of China (no. 81101354 ).
References
|
1
|
McDermott ID and Amis AA: The consequences
of meniscectomy. J Bone Joint Surg Br. 88:1549–1556. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moran CJ, Pascual-Garrido C, Chubinskaya
S, et al: Restoration of articular cartilage. J Bone Joint Surg Am.
96:336–344. 2014. View Article : Google Scholar
|
|
3
|
Zhu WH and Wang YB: Advances in molecular
and cellular biology of the healing after meniscal injury.
Zhong-guo Wei-chuang Wai-ke Za-zhi. 8:752–754. 2008.(In
Chinese).
|
|
4
|
Yoon JR, Muzaffar N, Kang JW, et al: A
novel technique for arthroscopic reduction and repair of a
bucket-handle meniscal tear. Knee Surg Sports Traumatol Arthrosc.
17:1332–1335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang KH, Hwang DH, Cho JH, et al:
Arthroscopic direct repair for a complete radial tear of the
posterior root of the medial meniscus. Clin Orthop Surg. 3:332–335.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gu Y, Wang Y, Dai H, et al: Chondrogenic
differentiation of canine myoblasts induced by cartilage-derived
morphogenetic protein-2 and transforming growth factor-β1 in
vitro. Mol Med Rep. 5:767–772. 2012.PubMed/NCBI
|
|
7
|
Zhu W, Wang Y, Qiu G and Chen B:
Characterization of the purification and primary culture of adult
canine myoblasts in vitro. Mol Med Rep. 3:463–468.
2010.PubMed/NCBI
|
|
8
|
Delos D and Rodeo SA: Enhancing meniscal
repair through biology: platelet-rich plasma as an alternative
strategy. Instr Course Lect. 60:453–460. 2011.PubMed/NCBI
|
|
9
|
Swenson TM: The use of exogenous fibrin
clot to supplement meniscal surgery techniques. Orthopedics.
30:718–723. 2007.PubMed/NCBI
|
|
10
|
Fox DB, Warnock JJ, Stoker AM, et al:
Effects of growth factors on equine synovial fibroblasts seeded on
synthetic scaffolds for avascular meniscal tissue engineering. Res
Vet Sci. 88:326–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Riera KM, Rothfusz NE, Wilusz RE, et al:
Interleukin-1, tumor necrosis factor-alpha, and transforming growth
factor-beta 1 and integrative meniscal repair: influences on
meniscal cell proliferation and migration. Arthritis Res Ther.
13:R1872011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goto H, Shuler FD, Lamsam C, et al:
Transfer of lacZ marker gene to the meniscus. J Bone Joint Surg Am.
81:918–925. 1999.PubMed/NCBI
|
|
13
|
Adams SB Jr, Randolph MA and Gill TJ:
Tissue engineering for meniscus repair. J Knee Surg. 18:25–30.
2005.PubMed/NCBI
|
|
14
|
Yamasaki T, Deie M, Shinomiya R, et al:
Meniscal regeneration using tissue engineering with a scaffold
derived from a rat meniscus and mesenchymal stromal cells derived
from rat bone marrow. J Biomed Mater Res A. 75:23–30. 2005.
View Article : Google Scholar
|
|
15
|
Steinert AF, Palmer GD, Capito R, et al:
Genetically enhanced engineering of meniscus tissue using ex vivo
delivery of transforming growth factor-beta 1 complementary
deoxyribonucleic acid. Tissue Eng. 13:2227–2237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu WH and Wang YB: Role of
cartilage-derived morphogenetic protein-2 in repair of chondral
tissues. Chinese J of Trauma. 24:945–948. 2008.
|
|
17
|
Matsushita T, Matsui N, Fujioka H, et al:
Expression of transcription factor Sox9 in rat L6 myoblastic cells.
Connect Tissue Res. 45:164–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Killian ML, Lepinski NM, Haut RC and Haut
Donahue TL: Regional and zonal histo-morphological characteristics
of the lapine menisci. Anat Rec (Hoboken). 293:1991–2000. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petersen W and Tillmann B: Collagenous
fibril texture of the human knee joint menisci. Anat Embryol
(Berl). 197:317–324. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abraham AC, Edwards CR, Odegard GM and
Donahue TL: Regional and fiber orientation dependent shear
properties and anisotropy of bovine meniscus. J Mech Behav Biomed
Mater. 4:2024–2030. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Villegas DF and Donahue TL: Collagen
morphology in human meniscal attachments: a SEM study. Connect
Tissue Res. 51:327–336. 2010. View Article : Google Scholar : PubMed/NCBI
|