Introduction
Esophageal carcinoma is one of the most common types
of malignancy, with highest mortality rate worldwide (1). Previous studies, which have led to
advances in the diagnosis, staging and treatment of esophageal
carcinoma have improved patient survival (2,3).
However, effective approaches to treat esophageal cancer remain
elusive due to the incomplete understanding of the molecular
mechanisms of esophageal cancer. Therefore, it is crucial to
investigate the mechanisms underlying esophageal cancer incidence
and progression.
The serine/threonine kinase liver kinase B1 (LKB1)
is a known tumor suppressor responsible for the inherited human
cancer disorder Peutz-Jeghers syndrome (PJS) (4). Previous studies demonstrated that
LKB1 mutations or abnormal expression of LKB1 are also associated
with lung, colorectal, testis, ovarian and pancreatic cancer
(5–8). LKB1 inhibits tumor cell cycle
progression by inducing p21 and p53 gene expression dependent on
its kinase activity (9,10). LKB1 deficiency leads to the
induction of matrix metaloproteinase-2 (MMP2), MMP9 and vascular
endothelial growth factor (VEGF) (11,12).
Notably, these genes are also regulated by signal transducer and
activator of transcription 3 (Stat3) activity in cell growth and
tumorigenesis (13–16). Stat3 has been identified as an
oncogene that is frequently activated in various cancer cells,
including esophageal cancer (12,17–19).
However, the biological functions of LKB1 in esophageal carcinoma
have not been elucidated. Although it has been reported that LKB1
inhibits activation of Stat3 in papillary thyroid carcinoma
(12), the association between
LKB1 expression and Stat3 activity in esophageal cancer remains
unclear.
In the current study, esophageal carcinoma and
adjacent non-cancer tissue were collected from 60 patients for
quantitative polymerase chain reaction (qPCR) analysis. LKB1
expression was observed to be significantly lower in esophageal
carcinoma tissue compared with the adjacent normal epithelium,
correlating with tumor node metastasis (TNM) stages. The in
vitro study using the esophageal carcinoma cell line, TE1,
revealed that LKB1 overexpression inhibits TE1 cell proliferation
and impairs the characteristics of cancer cells. Furthermore, LKB1
functions as a tumor suppressor to downregulate cyclin D1
expression through inhibition of Stat3 activity. These findings
indicate that downregulation of LKB1 expression may derepress Stat3
activity to promote esophageal carcinoma genesis, which provides an
important theoretical basis for diagnosis and treatment of
esophageal cancer.
Materials and methods
Samples and cell lines
A total of 60 surgically resected esophageal
carcinoma specimens and adjacent normal epithelium were collected
at the General Hospital of the People’s Liberation Army (Beijing,
China) from January 2009 to December 2011. Tumors were classified
histologically using the Guide Lines for the Clinical and
Pathologic Studies on Carcinoma of the Esophagus (20). There were five stage I; 24 stage
II; 19 stage III; and 12 stage IV esophageal cancer patients and
their mean age was 57.5 years (41–72 years). The conditions of
patients are summarized in Table
I. All samples were individually fresh-frozen in a −80°C
refrigerator. Equal weight of cancer or normal samples were pooled
together, respectively, according to different phases for western
blot analysis, and each sample was individually subjected to RNA
extraction and qPCR evaluation. Informed consent was obtained from
each patient prior to surgery. The study was approved by the
General Hospital of the People’s Liberation Army Clinical Research
Ethics Committee. Survival was calculated from the date of surgery
to the date of the latest follow-up visit or mortality due to
recurrent esophageal cancer. TE1 cells were originally obtained
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
and cultured according to ATCC guidelines.
 | Table IStatistical analysis of 2-YS rate in
esophageal carcinoma. |
Table I
Statistical analysis of 2-YS rate in
esophageal carcinoma.
| Phase | N (%) | Male/female | 2-YS rate (%) |
|---|
| I | 5 (8.3) | 2/3 | 100 |
| II | 24 (40.0) | 14/10 | 70.8 |
| III | 19 (31.7) | 10/9 | 42.1 |
| IV | 12 (20.0) | 7/5 | 0 |
RNA extraction and qPCR
Total RNA was isolated from tissue samples with
TRIzol Reagent (Life Technologies, Carlsbad, CA, USA), following
the manufacturer’s recommendations. Total RNA (2.5 mg) was reverse
transcribed using Superscript III Reverse Transcriptase (Invitrogen
Life Technologies, Carlsbad, CA, USA) and amplified with SYBR-Green
Real-time PCR Master mix (Toyobo, Osaka, Japan). The primers
(Sangon Biotech, Shanghai, China) used for amplification of
encoding genes were as follows: Forward:
5′-CGAAGTCAACGGATTTGGTCGTAT-3′ and reverse:
5′-AGCCTTCTCGGTGGTGAAGAC-3′ for glyceraldehyde 3-phosphate
dehydrogenase (GAPDH); forward: 5′-GAGCTGATGTCGGTGGGTATG-3′ and
reverse: 5′-CACCTTGCCGTAAGAGCCT-3 for LKB1; forward:
5′-GCTGCGAAGTGGAAACCATC-3′ and reverse:
5′-CCTCCTTCTGCACACATTTGAA-3′ for cyclin D1; forward:
5′-TACCCTCTCAACGACAGCAG-3′ and reverse: 5′-TCTTGACATTCTCCTCGGTG-3′
for Myc; forward: 5′-TGTCCGTCAGAACCCATG-3′ and reverse:
5′-TGGGAAGGTAGAGCTTGG-3′ for p21; and forward:
5′-TAGTATTGTATTAGGTAGGGGCGC-3′ and reverse:
5′-TATCGATAACCCGAAAAACGTT-3′ for p16.
Western blot analysis
The tissues or cells were harvested in lysis buffer
containing 150 mM NaCl, 50 mM Tris-HCl (pH 8.0), 0.1% SDS, 0.5%
sodium deoxycholate, 5 mM EDTA, 1% Nonidet P-40, 0.25 mM
phenylmethanesulfonyl fluoride and protease inhibitors. The lysates
were separated by SDS-PAGE and then proteins were transferred to
polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA),
followed by blocking in 5% skimmed milk and immunoblotting with
anti-LKB1 mouse monoclonal antibody (1:500), anti-Stat3 rabbit
polyclonal antibody (1:1,000) (both from Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), anti-pY705-Stat3 rabbit monoclonal
antibody (p-Stat3) (1:1,000; Cell Signaling Technology, Danvers,
MA, USA) and anti-GAPDH rabbit polyclonal antibody (1:10,000;
Abcam, Cambridge, MA, USA).
Transient transfection and reagents
TE1 cells were transfected using Lipofectamine 2000
reagents (Life Technologies) following the manufacturer’s
instructions, when the cells reached 60–70% confluency. Stat3C, a
constitutively active form of Stat3, was purchased from Addgene
plasmid repository (Cambridge MA, USA). Stat3 inhibitor Stattic was
purchased from Calbiochem (La Jolla, CA, USA).
Cell proliferation assay
Cell proliferation was detected by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay as previously described (21). Optical density (OD)570 values were
determined from cultured TE1 cells at days 0–4. The mean values of
three wells were calculated as the final OD value. Cell
proliferation was considered to be in linear proportion to the
color measurements.
Statistical analysis
Data are expressed as mean counts ± standard error
of the mean. All results were obtained from three independent
experiments for analysis. The Statistical Package for the Social
Sciences (SPSS; SPSS Inc., Chicago, IL, USA) was used to analyze
data. Student’s t-test was analyzed and P<0.05 was considered to
indicate a statistically significant difference.
Results
Downregulation of LKB1 expression during
esophageal carcinoma progression
Previously, defects of LKB1 were reported to be
associated with lung, ovarian and pancreatic cancer (5,6). To
evaluate the association between LKB1 expression and esophageal
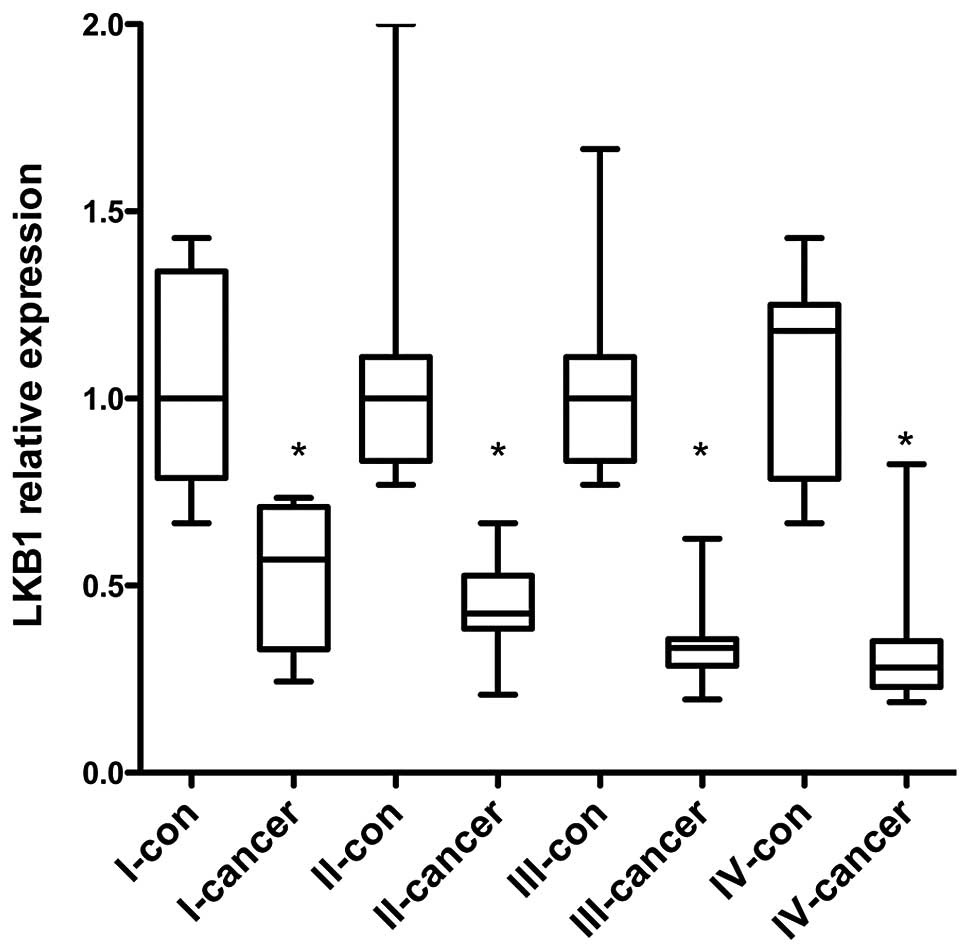
carcinoma, 60 esophageal cancer patients at phases I–IV were
collected for qPCR and 2-year survival rate analysis. The 2-year
survival rates at phase I–IV were 100, 70.8, 42.1 and 0,
respectively (Table I), suggesting
that the 2-year survival rate was highly associated with esophageal
carcinoma progression. Therefore, it is valuable to investigate the
mechanism of esophageal cancer during the early stage. The LKB1
mRNA levels in 60 specimens were examined by qPCR and the results
show that LKB1 expression was significant in cancer tissues
compared with the adjacent normal epithelium. Furthermore, LKB1
expression was gradually decreased from phase I to IV (Fig. 1). This demonstrates that
progressive LKB1 downregulation may be associated with esophageal
cancer development.
Revisable association between LKB1
expression and Stat3 activity in esophageal carcinoma
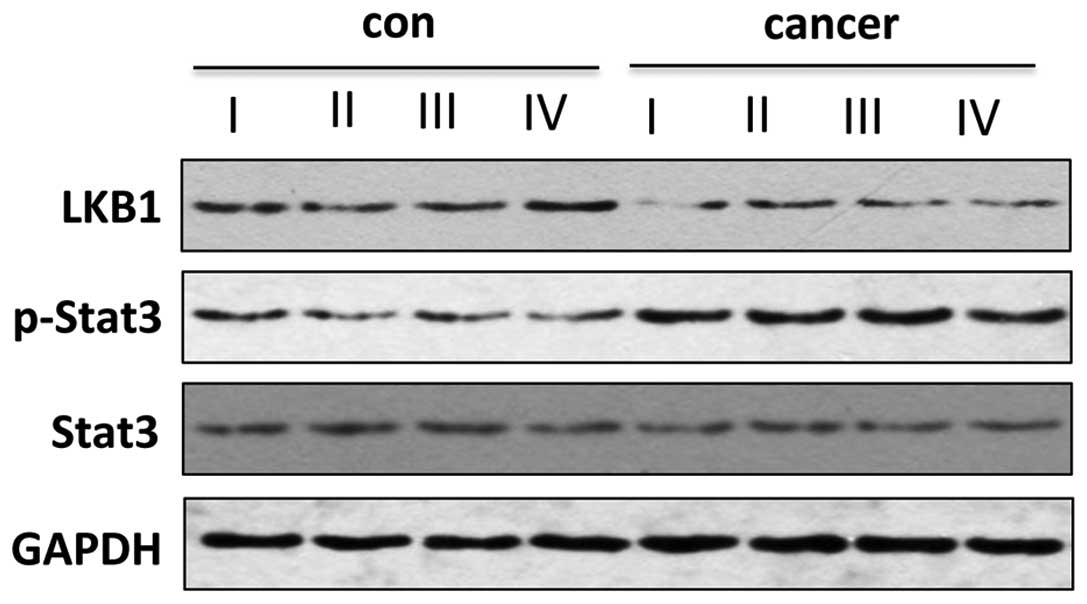
To verify the protein level change of LKB1 in
esophageal carcinoma, the tissues from the same phases were pooled
together for western blot analysis. LKB1 protein level was observed
to be markedly lower in cancer tissues (phase I–IV) compared with
the adjacent normal epithelium (Fig.
2), which is similar with the mRNA level change (Fig. 1). It was hypothesized that Stat3
was activated by interleukin (IL)-6 in esophageal cancer (19) and that the same genes are regulated
by Stat3 activation and LKB1 deficiency (11,12,16).
To investigate the association between LKB1 expression and Stat3
activity, the phosphorylated Stat3 level was also examined in the
present study. Notably, Stat3 phosphorylation was detected in
cancer tissues from phase I–IV, while it was maintained at an
extremely low level in the adjacent normal tissue, and Stat3
expression was not altered in the cancer and normal specimens
(Fig. 2). Thus, LKB1
downregulation was associated with Stat3 phosphorylation in
esophageal cancer.
LKB1 overexpression inhibits the
proliferation of esophageal carcinoma cells and represses Stat3
activity
The tumor suppressor LKB1 led to cell growth in PJS
(9,10). To investigate whether LKB1
functions as a tumor suppressor in esophageal carcinoma, the TE1
esophageal cancer cell line was applied for functional
investigation. Since LKB1 expression was extremely low in TE1
cells, LKB1 was overexpressed to examine the cell proliferation
rate by MTT assays in TE1 cells. LKB1 overexpression inhibited cell
growth (Fig. 3A). Notably, the
characteristics of cancer cells were impaired by LKB1. The
expression of tumor suppressor genes p16 and p21 was upregulated
and the mRNA level of cyclin D1 and Myc was downregulated (Fig. 3B), suggesting that LKB1 may also
result in cell growth arrest in esophageal carcinoma to inhibit
tumor growth.
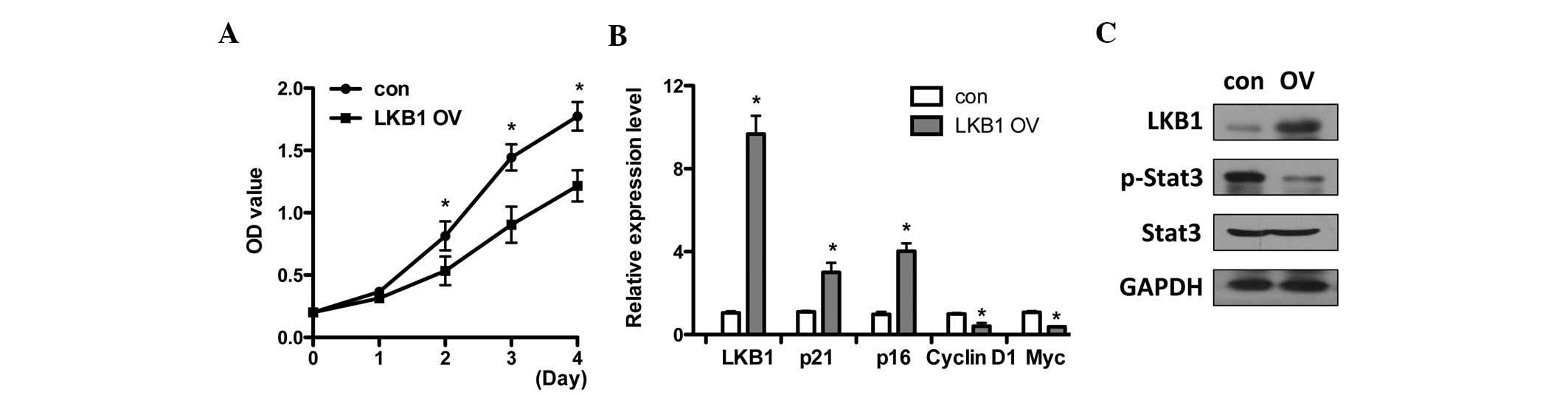 | Figure 3LKB1 OV inhibits the proliferation of
esophageal carcinoma cells and represses Stat3 activity. (A) LKB1
was overexpressed in TE1 cells by transfection of pcDNA3-LKB1 or
pcDNA3 vector as a con. MTT assays were performed on the indicated
days with OD570 evaluation. (B) Control and LKB1 overexpressing TE1
cells were collected at day four for mRNA expression analysis and
the expression of LKB1, p16, p21, cyclin D1 and Myc were examined
by qPCR. (C) Western blot analysis of LKB1, phosphorylated
(p)-Stat3, Stat3 and GAPDH levels in control and
LKB1-overexpressing TE1 cells. *P<0.05, compared with
the control. LKB1, liver kinase B1; OV, overexpression; OD, optical
density; Stat3, signal transducer and activator of transcription 3;
con, control; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; qPCR,
quantitative PCR; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
Based on LKB1 expression level and Stat3 activation
change (Fig. 2), it was
investigated whether Stat3 activity was modulated by LKB1. The
phosphorylated Stat3 level was determined following LKB1
overexpression and the results show that Stat3 phosphorylation was
suppressed by LKB1 (Fig. 3C). This
is consistent with the expression patterns in normal epithelial
cells with high LKB1 and low Stat3 phosphorylation levels (Fig. 2).
Constitutively active Stat3 activity
blocks the LKB1-elicited suppression of proliferation in esophageal
carcinoma cells
As LKB1 suppresses cell proliferation of TE1 cells
and inhibits Stat3 phosphorylation, it was investigated whether
LKB1 functions through downregulating Stat3 activity. A chemical
Stat3 inhibitor, Stattic, was used to block Stat3 transactivity and
it was observed that Stattic significantly inhibited cell
proliferation, similar to that of the LKB1 overexpression effects
(Fig. 4A). By contrast,
overexpression of a constitutively active Stat3 form, Stat3C,
promoted TE1 cell growth. When LKB1 and Stat3C were co-transfected
into TE1 cells, LKB1-elicited suppression of cell proliferation was
fully inhibited by forced activation of Stat3 (Fig. 4A). The expression of cyclin D1
under the same conditions was also investigated. Cyclin D1
expression was observed to be downregulated by LKB1 overexpression,
and Stattic and Stat3C markedly upregulated the cyclin D1
transcriptional level. Furthermore, a decrease in LKB1-induced
cyclin D1 was completely rescued by constitutively active Stat3C.
Thus, LKB1 suppresses TE1 cell proliferation by inhibiting Stat3
activity and further downregulating cyclin D1 gene expression.
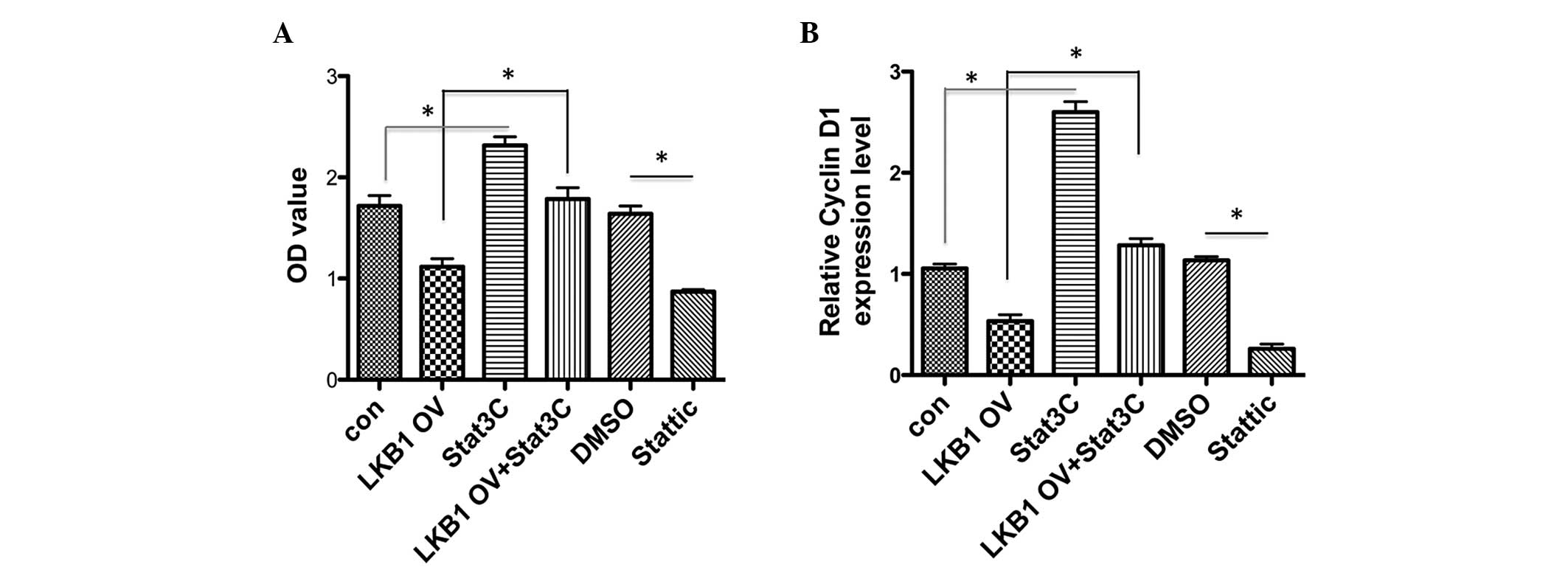 | Figure 4Constitutive Stat3 activity blocks
LKB1-elicited suppression of proliferation in esophageal carcinoma
cells. (A) Constitutively active Stat3 form Stat3C was transfected
alone or co-transfected with LKB1 into TE1 cells. Empty vectors
served as con. TE1 cells were supplemented with Stattic (10 μM) or
DMSO. MTT assays were performed to evaluate cell proliferation
under indicated conditions following four days of culture. (B) RNA
samples were collected under the same conditions in (A) and the
cells were subjected to qPCR analysis of cyclin D1 expression.
*P<0.05. Stat3, signal transducer and activator of
transcription 3; LKB1, liver kinase B1; OV, overexpression; OD,
optical density; DMSO, dimethylsulfoxide; con, control; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; qPCR,
quantitative PCR; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
Discussion
The tumor suppressor LKB1 has intrinsic
serine/threonine kinase activity and its defects cause PJS and
various cancers (4–6,22,23).
It is unknown whether LKB1 is associated with the incidence of
esophageal carcinoma. In the current study, qPCR analysis of 60
esophageal cancer specimens revealed that LKB1 mRNA and protein
level is progressively downregulated during the progression of
esophageal cancer compared with the adjacent normal epithelium.
This observation indicated that downregulation of LKB1 in the
esophageal epithelium may be applied as an early diagnostic marker
in the clinical process.
As a tumor suppressor, LKB1 downregulation may cause
normal cells to lose their ability to suppress tumorigenesis, which
has been observed in other types of tumors (9,10).
Therefore, LKB1 was hypothesized to be capable of suppressing the
esophageal carcinomagenesis. To verify this hypothesis, LKB1 was
overexpressed in the TE1 esophageal carcinoma cell line cells in
vitro. LKB1 overexpression also resulted in cell growth
inhibition in TE1 cells, which is consistent with previous studies
(9,10). The gene expression signature of
LKB1-overexpressing TE1 cells includes p16 and p21 elevation, as
well as cyclin D1 and Myc downregulation. It demonstrates that LKB1
may suppress cell growth dependent on modulating cell cycle arrest,
as in other types of tumor cells (10,12).
Stat3 is a well-known oncogene that is activated in
multiple types of cancers, including esophageal carcinoma (17–19).
A previous study reported that Stat3 transactivity was suppressed
by LKB1 (12), while the
association between Stat3 activity and LKB1 in esophageal cancer
remains unclear. Generally, Stat3 phosphorylation levels represent
Stat3 activity (24), thus,
p-Stat3 level was also determined in phase I–IV patients. Stat3
phosphorylation was observed to be downregulated in cancer tissue
with low p-Stat3 levels, which is negatively correlated with high
LKB1 expression. LKB1 overexpression repressed Stat3
phosphorylation in TE1 cells when LKB1 inhibited cell
proliferation. Another tumor suppressor, p53, was also reported to
regulate Stat3 phosphorylation and DNA binding activity in prostate
cancer cells (25). This suggests
that it is possible for tumor suppressors to inhibit Stat3
transcriptional activity.
Stat3 activity inhibition and LKB1 overexpression
showed inhibitory effects on TE1 cell growth, while constitutively
active Stat3 promoted cell proliferation. Notably, LKB1-elicited
suppression of cell growth was fully inhibited by forced activation
of Stat3, demonstrating that LKB1 functions in cell proliferation
through modulation of Stat3 phosphorylation. This is observed in
the functional correlation between LKB1 and Stat3 activity in
esophageal carcinoma cells. A previous study proposed that LKB1
reduces the binding of Stat3 to cyclin D1 and the VEGF promoter to
inhibit the target gene expression (12). This is consistent with the
observations of the present study that LKB1 downregulated cyclin D1
expression through suppression of Stat3 activity. The results of
these studies demonstrate that LKB1-mediated suppression of Stat3
target genes is dependent on the phosphorylation status of
Stat3.
In conclusion, the current study revealed that the
downregulation of LKB1 expression is associated with esophageal
carcinoma progression in 60 cases. The esophageal cancer
development was also accompanied with an increase in the p-Stat3
level. Functional investigation in TE1 cells showed that
suppression of esophageal cancer cell growth and cyclin D1
expression by LKB is dependent on inhibition of Stat3 activity.
Thus, LKB1 is hypothesized to act as a tumor suppressor to inhibit
cell growth through counteracting oncogenic Stat3 activity and that
downregulation of LKB1 may derepress its inhibition on Stat3
activation to promote esophageal carcinomagenesis. The present
study may provide a potential mechanism underlying the initiation
of esophageal carcinomagenesis and it provides information for the
exploration of the latent therapy target for esophageal cancer.
Acknowledgements
The study was supported by Wu Jieping medical fund:
The animal experimental study on 11C-gefitinib therapy
of esophageal carcinoma (number: 320.6799.1141).
References
|
1
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knisely JP, Burtness BA and Salem RR:
Surgical treatment of esophageal cancer. N Engl J Med.
348:1177–1179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kocher HM and Tekkis PP: Surgical
treatment of esophageal cancer. N Engl J Med. 348:1177–1179. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hemminki A, Markie D, Tomlinson I, et al:
A serine/threonine kinase gene defective in Peutz-Jeghers syndrome.
Nature. 391:184–187. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Avizienyte E, Loukola A, Roth S, et al:
LKB1 somatic mutations in sporadic tumors. Am J Pathol.
154:677–681. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Avizienyte E, Roth S, Loukola A, et al:
Somatic mutations in LKB1 are rare in sporadic colorectal and
testicular tumors. Cancer Res. 58:2087–2090. 1998.PubMed/NCBI
|
|
7
|
Sato N, Rosty C, Jansen M, et al:
STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal
papillary-mucinous neoplasms of the pancreas. Am J Pathol.
159:2017–2022. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang ZJ, Churchman M, Campbell IG, et al:
Allele loss and mutation screen at the Peutz-Jeghers (LKB1) locus
(19p13.3) in sporadic ovarian tumours. Br J Cancer. 80:70–72. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tiainen M, Vaahtomeri K, Ylikorkala A and
Mäkelä TP: Growth arrest by the LKB1 tumor suppressor: induction of
p21(WAF1/CIP1). Hum Mol Genet. 11:1497–1504. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tiainen M, Ylikorkala A and Mäkelä TP:
Growth suppression by Lkb1 is mediated by a G(1) cell cycle arrest.
Proc Natl Acad Sci USA. 96:9248–9251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ylikorkala A, Rossi DJ, Korsisaari N,
Luukko K, Alitalo K, Henkemeyer M and Mäkelä TP: Vascular
abnormalities and deregulation of VEGF in Lkb1-deficient mice.
Science. 293:1323–1326. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim DW, Chung HK, Park KC, et al: Tumor
suppressor LKB1 inhibits activation of signal transducer and
activator of transcription 3 (STAT3) by thyroid oncogenic tyrosine
kinase rearranged in transformation (RET)/papillary thyroid
carcinoma (PTC). Mol Endocrinol. 21:3039–3049. 2007. View Article : Google Scholar
|
|
13
|
Dechow TN, Pedranzini L, Leitch A, Leslie
K, Gerald WL, Linkov I and Bromberg JF: Requirement of matrix
metalloproteinase-9 for the transformation of human mammary
epithelial cells by Stat3-C. Proc Natl Acad Sci USA.
101:10602–10607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Funamoto M, Fujio Y, Kunisada K, et al:
Signal transducer and activator of transcription 3 is required for
glycoprotein 130-mediated induction of vascular endothelial growth
factor in cardiac myocytes. J Biol Chem. 275:10561–10566. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hutt JA, O’Rourke JP and DeWille J: Signal
transducer and activator of transcription 3 activates CCAAT
enhancer-binding protein delta gene transcription in G0
growth-arrested mouse mammary epithelial cells and in involuting
mouse mammary gland. J Biol Chem. 275:29123–29131. 2000. View Article : Google Scholar
|
|
16
|
Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F,
Sawaya R and Huang S: Stat3 activation regulates the expression of
matrix metalloproteinase-2 and tumor invasion and metastasis.
Oncogene. 23:3550–3560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garcia R, Yu CL, Hudnall A, et al:
Constitutive activation of Stat3 in fibroblasts transformed by
diverse oncoproteins and in breast carcinoma cells. Cell Growth
Differ. 8:1267–1276. 1997.PubMed/NCBI
|
|
18
|
Gouilleux-Gruart V, Gouilleux F, Desaint
C, et al: STAT-related transcription factors are constitutively
activated in peripheral blood cells from acute leukemia patients.
Blood. 87:1692–1697. 1996.PubMed/NCBI
|
|
19
|
Leu CM, Wong FH, Chang C, Huang SF and Hu
CP: Interleukin-6 acts as an antiapoptotic factor in human
esophageal carcinoma cells through the activation of both STAT3 and
mitogen-activated protein kinase pathways. Oncogene. 22:7809–7818.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Japanese Society for Esophageal Diseases.
Guide lines for the clinical and pathologic studies on carcinoma of
the esophagus. Jpn J Surg. 6:69–78. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmidt AI, Reismann M, Kübler JF, et al:
Exposure to carbon dioxide and helium reduces in vitro
proliferation of pediatric tumor cells. Pediatr Surg Int. 22:72–77.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hemminki A, Tomlinson I, Markie D, et al:
Localization of a susceptibility locus for Peutz-Jeghers syndrome
to 19p using comparative genomic hybridization and targeted linkage
analysis. Nat Genet. 15:87–90. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Resta N, Stella A, Susca FC, et al: Two
novel mutations and a new STK11/LKB1 gene isoform in Peutz-Jeghers
patients. Hum Mutat. 20:78–79. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wen Z, Zhong Z and Darnell JE Jr: Maximal
activation of transcription by Stat1 and Stat3 requires both
tyrosine and serine phosphorylation. Cell. 82:241–250. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin J, Tang H, Jin X, Jia G and Hsieh JT:
p53 regulates Stat3 phosphorylation and DNA binding activity in
human prostate cancer cells expressing constitutively active Stat3.
Oncogene. 21:3082–3088. 2002. View Article : Google Scholar : PubMed/NCBI
|