Introduction
The incidence of renal cell carcinoma (RCC) has
increased worldwide over the past three decades. Global estimates
in 2008 indicated that ~209,000 new cases were being diagnosed each
year (1). RCC is heterogeneous and
comprises several histological types, which have different genetic
and clinicopathological features that determine clinical course and
outcome. Clear cell RCC (ccRCC), also called conventional RCC, is
the most common histological type of RCC, representing ~80% of
cases (2). Despite the development
of imaging techniques and surgical innovations, ccRCC carries an
extremely high risk of invasiveness and metastasis, with 50% of
patients eventually developing metastatic disease (3). Furthermore, ccRCC exhibits resistance
to chemotherapy and radiation, and only ~10% of patients suffering
from metastatic disease survive for five years following diagnosis
(4). As a result, an enhanced
understanding of the molecular pathogenesis of ccRCC is required
for the development of novel diagnostic methods and therapeutic
strategies.
Wnts are a family of cysteine-rich, secreted
glycoproteins with functions in numerous cellular processes,
including embryogenesis and oncogenesis, the induction of cell
polarity, the maintenance of tissue homeostasis and cell growth
control. The Wnt signaling pathway is composed of canonical Wnt
signaling via Wnt/β-catenin and non-canonical Wnt signaling via the
Wnt/Ca2+ pathway and Wnt/c-Jun N-terminal kinase
(5). Deregulation of canonical Wnt
signaling has been indicated for a variety of types of human cancer
and other diseases (6). Activation
of this signal cascade is a complex process; Wnt ligands bind to
their cognate Frizzled (Fz)/Low-density lipoprotein
receptor-related protein (Lrp) receptor complex and relay a signal
via cytoplasmic transduction intermediates to β-catenin. A number
of extracellular Wnt antagonists modulate the activity of this
signaling cascade. Members of the secreted Fz-related protein
(Sfrp) family and Wnt-inhibitory factor-1 (Wif-1) directly
sequester Wnt ligands, whereas members of the Dickkopf (Dkk) family
of proteins interfere with the co-receptors Lrp and Kremen (Krm),
leading to the internalization of Lrp, and thus preventing Wnt from
binding to a functional receptor complex (7).
Of the four known mammalian Dkk members, Dkk1 is the
most studied, and has been demonstrated to be an antagonist of the
canonical Wnt signaling pathway. By contrast, Dkk3 is the most
divergent member of the human Dkk family and does not modulate Wnt
signaling (8). Furthermore, it has
been indicated that Dkk3 has an important role in tumor
suppression, and therefore may represent a notable therapeutic
target against aberrant Wnt signaling in the treatment of human
cancer. Dkk1 and Dkk3 expression has been investigated in numerous
tumors, including hepatocellular carcinomas (9), Wilms’ tumors (10), pancreatic adenocarcinomas (11), ovarian (12) and colorectal (13) cancer, melanoma cells (14) and lung cancer (15). Dkk expression in RCC has also been
investigated by Ueno et al (16) and Urakami et al (17). Ueno et al (16) studied the potential epigenetic
mechanisms regulating Dkk3 expression in RCC cells and demonstrated
that the mRNA expression of Dkk3 was regulated by histone
modifications. The study also found that Dkk3 inhibited renal
cancer growth through modulation of the cell cycle and apoptotic
pathways. Urakami et al (17) demonstrated that the methylation
levels of all Wnt antagonists, including sFRP-1, sFRP-2, sFRP-4,
sFRP-5, Wif-1 and Dkk3, were significantly higher in RCC tissues
compared with normal renal tissues. However, the expression levels
of Dkk1 and Dkk3 in ccRCC, the correlation between the two
proteins, and whether Dkk1 and Dkk3 contribute to the diagnosis and
treatment of ccRCC have not yet been investigated. The present
study investigated the serum levels of Dkk1 and Dkk3 in ccRCC
patients, the correlation between Dkk1 and Dkk3, the expression of
Dkk1 and Dkk3 in ccRCC tissue samples, and the correlation with the
clinicopathological characteristics by statistical analysis.
Materials and methods
Patients, serum samples and tissue
specimens
The selection criteria for the patients with ccRCC
were as follows: i) Pathologically confirmed ccRCC; ii) a
nephrectomy performed in Shanghai Tenth People’s Hospital
(Shanghai, China) without pre-operative adjuvant therapy, including
radiotherapy or chemotherapy, and with complete clinical data; and
iii) no previous history of other types of cancer. The selection
criteria for the controls were as follows: i) Diagnosis of ureteral
stones or urethral caruncles; ii) no disease of the vital organs,
including the heart, liver and lung; and iii) no family history of
cancer.
A fasting blood sample was taken from all
participants, and serum was collected and stored at −80°C. All
samples were collected prior to surgery. Pairs of cancerous and
adjacent normal tissues were obtained from 20 patients with ccRCC
following radical nephrectomy and once written, informed consent
had been obtained from each patient. The demographic and
pathological data, including age, gender and tumor stage, were
obtained by a review of the patients’ medical records (the data
were used with the consent of the patients and with the approval of
the Ethics Committee of Tongji University, Shanghai, China). Tumor
stage was determined according to the 2009 tumor-node-metastasis
(TNM) staging classification system and American Joint Committee on
Cancer (AJCC), and tumor grade was determined according to the
Fuhrman classification system (well-differentiated, grades I and
II; moderately-differentiated, grade III; and
poorly-differentiated, grade IV) (18,19).
For statistical evaluations, grades I and II tumors were considered
as low-grade and grades III and IV as high-grade. Similarly, stage
III and IV tumors were considered in the advanced-stage category.
From the 20 tissue samples collected, 6, 8 and 10 samples were
analyzed using immunohistochemistry, western blot analysis and
quantitative polymerase chain reaction (qPCR) analysis,
respectively.
ELISA
Dkk1 and Dkk3 were measured using an ELISA kit
(Miltenyi Biotec, Bergisch Gladbach, Germany) in accordance with
the manufacturer’s instructions. The optical density (OD) at 450 nm
was determined. A standard curve was established using
OD450 as the y axis and the concentration of a standard
substance as the x axis, and from this standard curve the level of
protein was determined. Results are presented as the concentration
of Dkk1 or Dkk3 (ng/ml) in samples.
Immunohistochemistry
Immunostaining was performed on paraffin-embedded
4-μm sections of formalin-fixed tumor tissues, placed on chrome
alum gelatin-coated glass slides and dried for 30 min at 70°C.
Following rehydration, the tissue sections were incubated in 3%
hydrogen peroxide (Bio Basic Inc, Amherst, NY, USA) to inhibit
endogenous peroxidase activity. Following citrate buffer antigen
retrieval, the sections were blocked by incubation in 5% bovine
serum albumin (BSA) in phosphate-buffered saline (PBS) (Bio Basic
Inc). Expression of Dkk1 and Dkk3 was assessed using goat
anti-human Dkk1 monoclonal antibody and rabbit anti-human Dkk3
monoclonal antibody (Abcam, Cambridge, UK) at a 1:50 dilution.
Expression was detected using an Envision™ Detection kit,
(peroxidase/DAB, rabbit/mouse; Gene Tech, Shanghai, China) in
accordance with the manufacturer’s instructions. The slides were
then stained with DAB, washed, counterstained with hematoxylin,
dehydrated, treated with xylene and mounted.
qPCR analysis
Total RNA was extracted from the tissues using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) in accordance with
the manufacturer’s instructions, and was then used for the
synthesis of first-strand complementary DNA (cDNA) using the
PrimeScript® 1st Strand cDNA Synthesis kit (Takara Bio,
Inc., Shiga, Japan). A total of 1 μl reverse transcription (RT)
product was then used as the template to amplify specific Dkk1 and
Dkk3 fragments. Primers for human Dkk1, Dkk3 and β-actin genes were
designed using Primer Express 2.0 software (Applied Biosystems,
Inc., Foster City, CA, USA) and synthesized by Sangon (Shanghai,
China). The primer sequences used were as follows: Dkk1 forward,
5′-ATTCCAACGCTATCAAGAACC-3′ and reverse,
5′-CCAAGGTGCTATGATCATTACC-3′; Dkk3 forward,
5′-AGGACACGCAGCACAAATTG-3′ and reverse, 5′-CCAGTCTGGTTGTTGGTTATCTT;
β-actin forward, 5′-GGAGTCCTGTGGCATCCACG-3′ and reverse, 5′-CTA
GAAGCATTTGCGGTGGA-3′. qPCR was performed in triplicate for each
sample in a 20 μl reaction mixture, containing 2 μl template DNA, 1
μl primers, 10 μl SYBR premix and 7 μl ddH2O, using an
ExScript Real-time PCR kit (Takara Bio, Inc.). PCR was performed in
a 7900HT Fast Real-Time PCR machine (Applied Biosystems, Inc.)
under the following conditions: 95°C for 30 sec, then 40 cycles of
95°C for 5 sec and 60°C for 30 sec. Gene expression was presented
using a modification of the 2−ΔΔCt method, first
described by K. Livak in PE Biosystems Sequence Detector User
Bulletin 2 (20).
Western blot analysis
Total protein was extracted using lysis buffer. The
protein concentration was determined using the bicinchoninic acid
protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Equal quantities of protein were loaded on each lane and
electrophoresed on SDS-polyacrylamide gels with tris-glycine
running buffer, prior to being transferred to nitrocellulose
membranes. Blots were saturated with 5% skimmed milk and 0.1% Tween
in Tris-buffered saline, and incubated with antibodies against Dkk1
(1:200), Dkk3 (1:200) and β-actin (1:1,000) (Abcam). β-actin was
used as a control. The membranes were washed and then incubated
with biotinylated secondary antibody labeled with horseradish
peroxidase (1:1,000; Amersham Pharmacia Biotech, Amersham, UK) for
1 h at 37°C, washed again and developed using an enhanced
chemiluminescence (ECL) western blotting system. Membranes were
then apposed to autoradiographic films (Amersham Hyperfilm ECL,
Buckinghamshire, UK).
Statistical analysis
Statistical analyses were performed using SPSS 13.0
statistical software (SPSS, Inc., Chicago, IL, USA) and GraphPad
Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Data are
presented as the mean ± standard deviation and analyzed using
independent t-tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinicopathological characteristics of
the selected patients
A total of 64 patients with ccRCC and 30 controls,
who were recruited from the Department of Urology, Shanghai Tenth
People’s Hospital between July 2010 and June 2012, were included in
this study. The mean age was 62.1±13.2 years for the ccRCC group
and 59.8±12.2 years for the control group (P=0.281). The clinical
characteristic of the 64 patients with ccRCC and the 30 controls
are summarized in Table I.
 | Table IClinical characteristics of the
patients with ccRCC and the controls. |
Table I
Clinical characteristics of the
patients with ccRCC and the controls.
| Variables | Patients, n
(%) | Controls, n
(%) | P-value |
|---|
| Total subjects | 64 (100) | 30 (100) | |
| Age in years |
| <45 | 19 (30) | 9 (30) | |
| 45–65 | 24 (37) | 9 (30) | |
| >65 | 21 (33) | 12 (40) | |
| Average | 62.1±13.2 | 59.8±12.2 | 0.281 |
| Gender |
| Male | 38 (59) | 17 (57) | 0.323 |
| Female | 26 (41) | 13 (43) | |
| TNM |
| T1 29 (45) | - | | |
| T2 21 (33) | - | | |
| T3 8 (13) | - | | |
| T4 6 (8) | - | | |
| N049 (77) | - | | |
| N111 (17) | - | | |
| N24 (6) | - | | |
| M0 | 57 (87) | - | |
| M1 | 7 (13) | - | |
| Fuhrman |
| I | 28 (44) | - | |
| II | 20 (31) | - | |
| III | 12 (19) | - | |
| IV | 4 (6) | - | |
| AJCC |
| I | 22 (34) | - | |
| II | 21 (33) | - | |
| III | 15 (23) | - | |
| IV | 6 (10) | - | |
ELISA assay
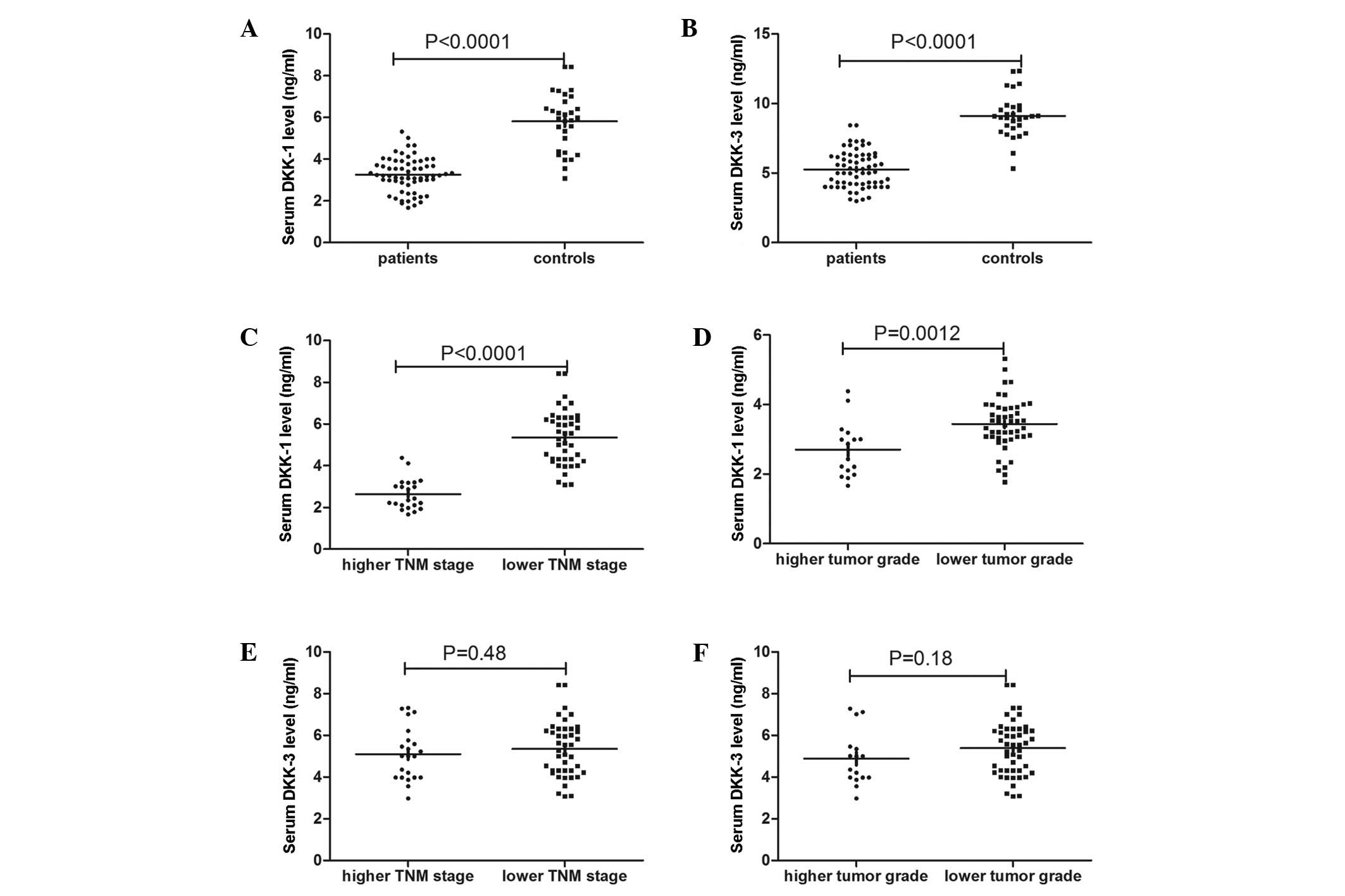
Serum Dkk1 levels were found to be significantly
lower in the patients with ccRCC compared with the controls. The
mean serum Dkk1 level was 3.26±0.81 ng/ml in the ccRCC group
compared with 5.81±1.38 in the control group (P<0.0001)
(Fig. 1A). Furthermore, serum Dkk1
levels were significantly lower at higher TNM stages (2.64±0.74
ng/ml) and higher tumor grades (2.70±0.79 ng/ml) compared with
lower TNM stages (3.58±0.66 ng/ml, P<0.001) and lower tumor
grades (3.44±0.74 ng/ml, P=0.003) (Fig. 1C and D). Similarly, serum Dkk3
levels were significantly lower in the patients with ccRCC compared
with the controls. The mean serum Dkk3 level was 5.26±1.31 ng/ml in
the ccRCC group compared with 9.11±1.55 ng/ml in the control group
(P<0.0001) (Fig. 1B). However,
no significant difference was found in Dkk3 levels between lower
and higher TNM stages (5.35±1.34 and 5.10±1.28 ng/ml respectively;
P=0.482) and lower and higher tumor grades (5.39±1.30 and 4.89±1.30
ng/ml, respectively; P=0.183) (Fig. 1E
and F).
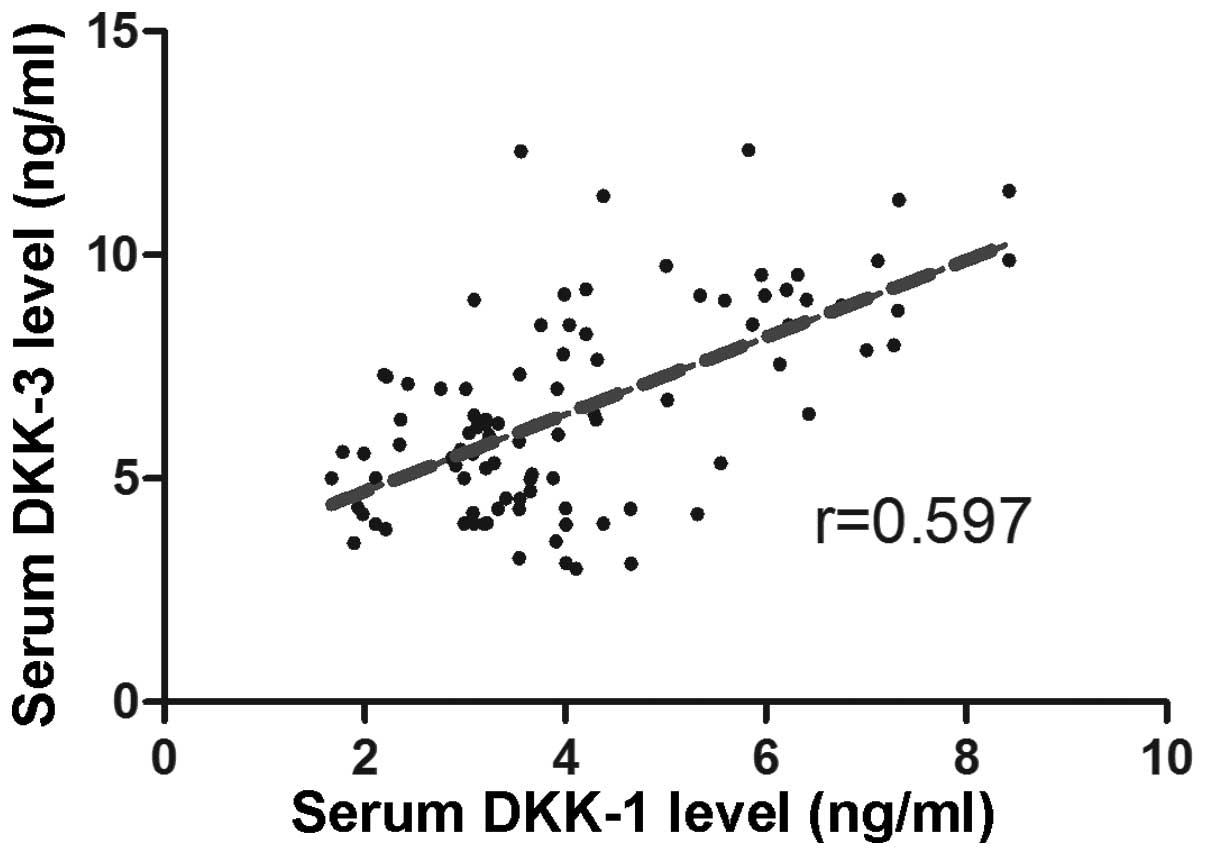
The correlation between Dkk1 and Dkk3 serum levels
in patients was measured using a correlation analysis, and the
results revealed a positive correlation (r=0.597) (Fig. 2).
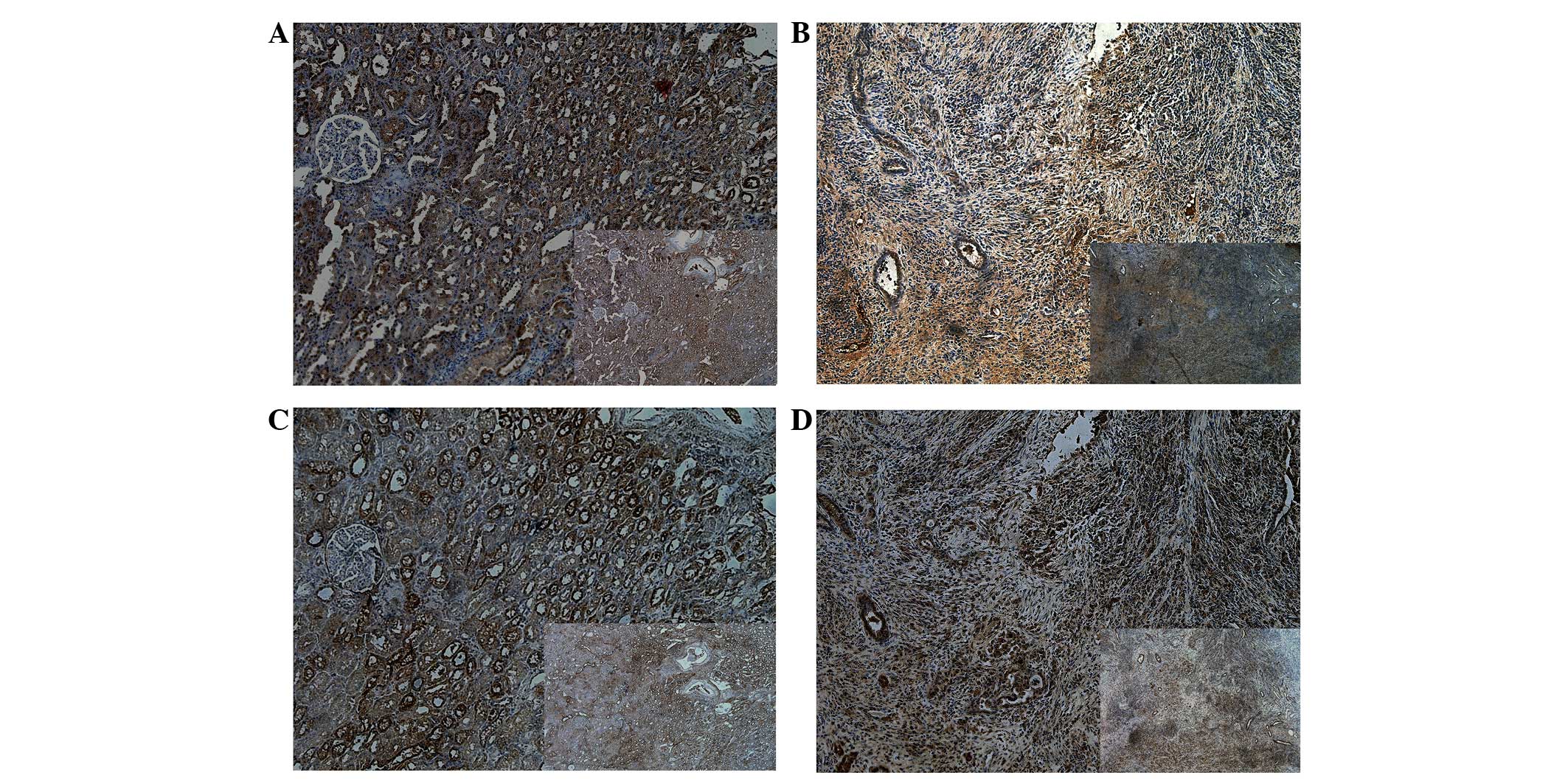
Immunohistochemistry analysis
Immunochemistry was performed to analyze the protein
expression of Dkk1 and Dkk3 in tumor and adjacent healthy tissues
obtained from six patients with ccRCC (Table II). It was observed that there was
a significant decrease in the expression of Dkk1 in the tumor
tissue compared with the adjacent normal tissue. The same result
was observed for DKK3 expression, with a significant decrease in
the expression in the tumor tissue compared with adjacent normal
tissue (Fig. 3).
 | Table IIResults from the immunohistochemistry
analysis. |
Table II
Results from the immunohistochemistry
analysis.
| Patient | Tissue type | Gender | AJCC | Fuhrman | DKK-1 | DKK-3 |
|---|
| 1 | Tumor tissue | Male | III | III | + | ++ |
| Adjacent normal
tissues | | | | +++ | +++ |
| 2 | Tumor tissue | Male | IV | III | + | −/+ |
| Adjacent normal
tissues | | | | ++ | ++ |
| 3 | Tumor tissue | Male | III | II | ++ | ++ |
| Adjacent normal
tissues | | | | +++ | ++++ |
| 4 | Tumor tissue | Female | III | II | + | + |
| Adjacent normal
tissues | | | | +++ | +++ |
| 5 | Tumor tissue | Male | II | II | + | ++ |
| Adjacent normal
tissues | | | | ++ | +++ |
| 6 | Tumor tissue | Female | II | I | −/+ | −/+ |
| Adjacent normal
tissues | | | | ++ | ++ |
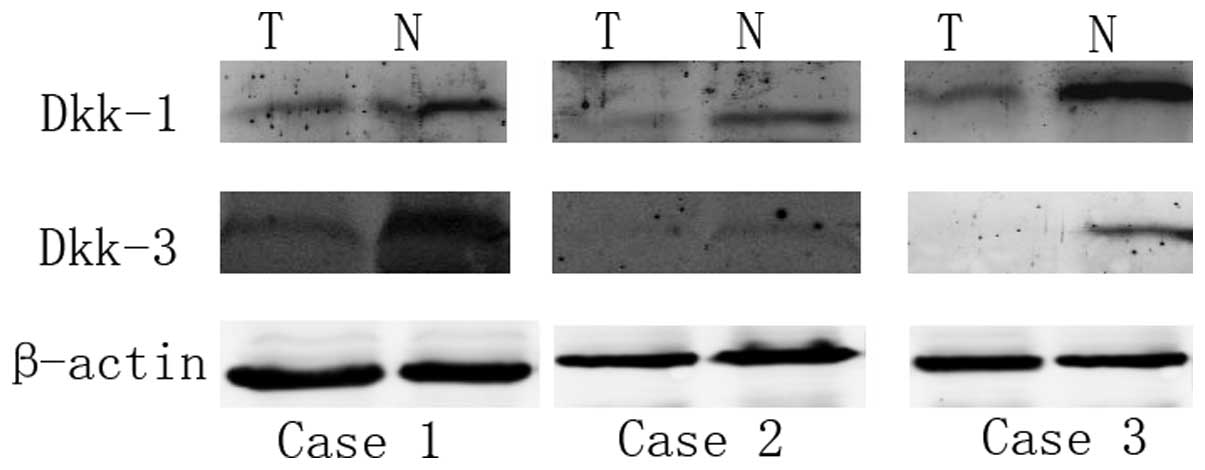
Western blot analysis
Western blot analysis was performed to analyze the
protein expression of Dkk1 and Dkk3 in samples of tumor and
adjacent normal tissue obtained from eight patients with ccRCC.
Western blot analysis revealed that the protein expression of Dkk1
was higher in the adjacent normal tissue compared with the tumor
tissue. Low protein expression levels of Dkk1 were found in 75%
(6/8) of the tumor tissues. The expression of Dkk3 was also higher
in the adjacent normal tissue compared with the tumor tissue. Lower
expression levels of Dkk3 were found in 62.5% (5/8) of the tumor
tissues (Fig. 4).
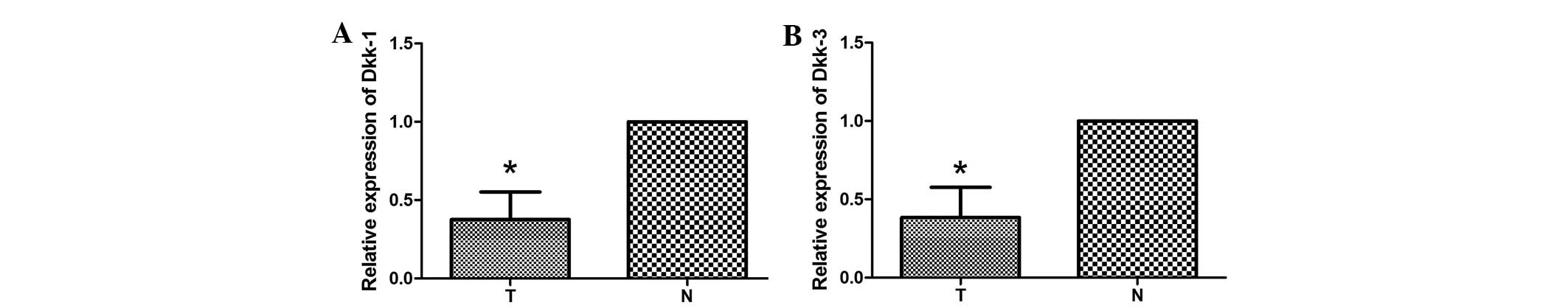
qPCR analysis
qPCR was performed to assess Dkk1 and Dkk3 mRNA
expression in 10 tumor samples and the corresponding adjacent
normal tissues. A decrease in the expression levels of Dkk1 mRNA in
the tumor tissue compared with the adjacent normal tissue was
observed in 70% of the samples (Fig.
5A). Consistently, 80% of samples showed a decrease in the
expression levels of Dkk3 mRNA in the tumor tissue compared with
the adjacent normal tissue (Fig.
5B). These results demonstrated that the average expression
levels of Dkk1 and Dkk3 mRNA in tumor tissues are significantly
lower than the expression levels in corresponding adjacent normal
tissues.
Discussion
Aberrant activation of the Wnt signaling pathway is
a major characteristic of numerous types of cancer in humans,
including colorectal cancer melanoma, non-small cell lung cancer,
leukemia and bladder cancer (21).
Recently, the Wnt signaling pathway has been investigated at
several molecular levels, as its involvement has been indicated in
tumorigenesis and disease progression. ccRCC is the most common
histological type of RCC, and the Wnt signaling pathway has been
indicated to have a significant role in the pathogenesis and
progression of ccRCC. Previous trials have investigated the Wnt
signaling pathway in order to develop novel cancer therapeutics for
ccRCC (22,23). Studies have shown that the secreted
Wnt antagonist Dkk and its regulators may contribute to the
diagnosis of human cancer, in addition to providing novel
therapeutic targets (7,24–26).
It is well known that Dkk1 affects Wnt signaling
either physically or functionally by interacting with LRP5 and
LRP6. Dkk proteins also bind to Krm1 and Krm2, other single-pass
transmembrane receptors. When Dkk binds to Krm, a Dkk/LRP/Krm
protein complex forms, which is endocytosed rapidly. The result of
this action is the removal of LRP5/6 receptors from the cell
membrane. The cell membrane is thus depleted of LRP receptors, and
Wnt signaling is inhibited to a greater extent and for longer
(27). Previous studies have shown
that the expression of Dkk1 is downregulated in human colon cancer,
gastric cancer and melanoma tissues, indicating that Dkk1 may act
as a tumor suppressor in these types of cancer and not as an
antagonist of Wnt signaling (13,14,28,29).
However, Dkk1 has been found to be overexpressed in hepatoblastoma
and hepatocellular carcinoma, indicating that Dkk1 may be regulated
by a feedback loop activated by the Wnt signaling pathway. These
previous studies demonstrated that the function of Dkk1 may differ
depending on the tissue of origin. In the present study, serum Dkk1
levels were found to be lower in the patients with ccRCC compared
with the controls. Serum Dkk1 levels were also associated with AJCC
stage and Fuhrman grade, indicating that Dkk1 may be used as a
novel marker for the diagnosis, staging and prognosis of ccRCC.
However, Urakami et al (17) found that the limited sensitivity
and specificity of the Wnt antagonist genes meant that none of them
could be used as a single reliable biomarker of RCC. One important
reason for this was that only the levels of methylated DNA were
assessed in the study and not the protein levels. Furthermore, in
the present study, it was demonstrated using immunohistochemistry,
western blot analysis and qPCR that Dkk1 levels in the tumor tissue
were lower compared with those in the adjacent normal tissue. This
indicates that Wnt signaling pathway activation may partially occur
due to the functional loss of Wnt antagonists, and that
carcinogenesis may result due to the dysregulation of cell
proliferation and differentiation. It also indicates that Dkk1 may
be used as a therapeutic target in the management of ccRCC.
Hypermethylation of the Wnt antagonist genes has also been
investigated in previous studies (25,30,31),
and promoter methylation of certain Wnt antagonists has been
recognized as a strong prognostic marker for a poor outcome in
patients with primary kidney cancer (17). In the present study, the
methylation of Dkk1 promoter was not investigated; however, the
expression of Dkk1 in the tumor tissue was found to be lower
compared with that in the adjacent normal tissue. This may be the
result of the methylation of the Dkk1 promoter.
Dkk3 is the most divergent member of the human Dkk
family. A previous study showed that Dkk3 regulates canonical Wnt
signaling in lung cancer (32). It
has also been demonstrated that Dkk3 has no effect on Wnt/β-catenin
signaling in prostate cancer cells, but instead induces apoptosis
through the non-canonical c-Jun N-terminal kinase pathway (33,34).
Ueno et al (16) reported
that Dkk3 expression was regulated by histone modifications in the
Dkk3 promoter region in RCC cells. Furthermore, the study showed
that Dkk3 expression induced G0/G1 cell cycle
arrest and decreased cell growth in RCC cells through p21
expression. This indicates that Dkk3 acts as a tumor suppressor in
kidney cells and that the downregulation of Dkk3 may be involved in
RCC progression (16). The
expression of Dkk3 has been analyzed in several types of
malignancies, and downregulation of Dkk3 has been observed in
glioma, breast cancer, melanoma, prostate cancer and
gastrointestinal cancer (14,35–38).
Further studies have revealed that the downregulation of Dkk3 in
cancer was a result of epigenetic silencing by DNA methylation
(17). It also has been reported
that transfection of Dkk3 in certain tumor cells affected their
invasive capacity and led to cell apoptosis, indicating that Dkk3
may act as a tumor suppressor (14,32,34,39,40).
However, Dkk3 mutant mice showed no enhanced tumorigenesis
(41). By contrast, Dkk3 has been
found to be overexpressed in hepatoblastoma and hepatocellular and
esophageal squamous cell carcinoma. One possible reason may be due
to an inactive form of Dkk3 in these tumors or an alteration of
Dkk3 activity depending on cell type (40). Kurose et al (23) demonstrated that the decrease in the
mRNA and protein levels of reduced expression in immortalized
cells/Dkk-3 was observed irrespective of tumor grade and stage. In
the present study, Dkk3 levels were analyzed using
immunohistochemistry, western blotting and qPCR, and were found to
be lower in the tumor tissue compared with the adjacent normal
tissue. This was consistent with the results found by Kurose et
al (23). Furthermore, in the
present study, serum Dkk3 levels were found to be decreased in the
patients with ccRCC compared with those in the controls. However,
serum Dkk3 levels were not associated with AJCC stage and Fuhrman
grade. Furthermore, the results demonstrated that Dkk3 expression
was correlated with Dkk1 expression, indicating that Dkk3 and Dkk1
may have an associated mechanism in the development of ccRCC.
To the best of our knowledge, this is the first
study to investigate the expression levels of Dkk1 and Dkk3 in the
serum and tumor specimens of patients with ccRCC, as well as their
correlation with clinicopathological features. However, there were
certain limitations to the study. Firstly, the number of patients
analyzed was small (n=64). Secondly, the detailed mechanism of the
action of the Dkk family in ccRCC was not investigated.
Furthermore, the correlation between Dkk serum level and the
prognosis of ccRCC was not investigated.
In conclusion, a decrease in Dkk1 and Dkk3 mRNA and
protein levels was observed in the ccRCC tissues. The serum Dkk1
and Dkk3 levels in the patients with ccRCC were found to be
significantly lower than those in the controls, and the Dkk1 levels
were associated with the clinicopathological features of the ccRCC
patients. Consequently, Dkk1 and Dkk3 may present a novel molecular
target for the diagnosis and treatment of ccRCC. However, the
detailed mechanisms behind the actions of the Dkk family in ccRCC
require further investigation.
References
|
1
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deng FM, Melamed J and Zhou M: Pathology
of renal cell carcinoma. Renal Cancer. Libertino JA: Springer; New
York, NY: pp. 51–69. 2013, View Article : Google Scholar
|
|
3
|
Vacas E, Bajo AM, Schally AV, et al:
Vasoactive intestinal peptide induces oxidative stress and
suppresses metastatic potential in human clear cell renal cell
carcinoma. Mol Cell Endocrinol. 365:212–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Escudier B, Szczylik C, Porta C and Gore
M: Treatment selection in metastatic renal cell carcinoma: expert
consensus. Nat Rev Clin Oncol. 9:327–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cohen ED, Tian Y and Morrisey EE: Wnt
signaling: an essential regulator of cardiovascular
differentiation, morphogenesis and progenitor self-renewal.
Development. 135:789–798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Veeck J and Dahl E: Targeting the Wnt
pathway in cancer: the emerging role of Dickkopf-3. Biochim Biophys
Acta. 1825:18–28. 2012.PubMed/NCBI
|
|
8
|
Mao B, Wu W, Li Y, et al:
LDL-receptor-related protein 6 is a receptor for Dickkopf proteins.
Nature. 411:321–325. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patil MA, Chua MS, Pan KH, et al: An
integrated data analysis approach to characterize genes highly
expressed in hepatocellular carcinoma. Oncogene. 24:3737–3747.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wirths O, Waha A, Weggen S, et al:
Overexpression of human Dickkopf-1, an antagonist of wingless/WNT
signaling, in human hepatoblastomas and Wilms’ tumors. Lab Invest.
83:429–434. 2003.PubMed/NCBI
|
|
11
|
Takahashi N, Fukushima T, Yorita K, Tanaka
H, Chijiiwa K and Kataoka H: Dickkopf-1 is overexpressed in human
pancreatic ductal adenocarcinoma cells and is involved in invasive
growth. Int J Cancer. 126:1611–1620. 2010.PubMed/NCBI
|
|
12
|
Shizhuo W, Tao J, Shulan Z and Bing Z: The
expression and significance of Dickkopf-1 in epithelial ovarian
carcinoma. Int J Biol Markers. 24:165–170. 2009.PubMed/NCBI
|
|
13
|
González-Sancho JM, Aguilera O, García JM,
et al: The Wnt antagonist DICKKOPF-1 gene is a downstream target of
beta-catenin/TCF and is downregulated in human colon cancer.
Oncogene. 24:1098–1103. 2005.PubMed/NCBI
|
|
14
|
Kuphal S, Lodermeyer S, Bataille F,
Schuierer M, Hoang BH and Bosserhoff AK: Expression of Dickkopf
genes is strongly reduced in malignant melanoma. Oncogene.
25:5027–5036. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livark KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
16
|
Ueno K, Hirata H, Majid S, et al: Wnt
antagonist DICKKOPF-3 (Dkk-3) induces apoptosis in human renal cell
carcinoma. Mol Carcinog. 50:449–457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Urakami S, Shiina H, Enokida H, et al: Wnt
antagonist family genes as biomarkers for diagnosis, staging, and
prognosis of renal cell carcinoma using tumor and serum DNA. Clin
Cancer Res. 12:6989–6997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union Against Cancer (UICC) TNM Classification of
Malignant Tumours. 7th edition. Wiley-Liss; New York, NY: pp.
1–336. 2009
|
|
19
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A; American Joint Committee on Cancer (AJCC).
AJCC Cancer Staging Manual. 7th edition. Springer-Verlag; New York,
NY: pp. 1–649. 2010
|
|
20
|
Winer J, Jung CK, Shackel I and Williams
PM: Development and validation of real-time quantitative reverse
transcriptase-polymerase chain reaction for monitoring gene
expression in cardiac myocytes in vitro. Anal Biochem. 270:41–49.
1999. View Article : Google Scholar
|
|
21
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Forget MA, Turcotte S, Beauseigle D, et
al: The Wnt pathway regulator DKK1 is preferentially expressed in
hormone-resistant breast tumours and in some common cancer types.
Br J Cancer. 96:646–653. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kurose K, Sakaguchi M, Nasu Y, et al:
Decreased expression of REIC/Dkk-3 in human renal clear cell
carcinoma. J Urol. 171:1314–1318. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang T, Huang L and Zhang S: DKK-1 in
serum as a clinical and prognostic factor in patients with cervical
cancer. Int J Biol Markers. 28:221–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Urakami S, Shiina H, Enokida H, et al:
Combination analysis of hypermethylated Wnt-antagonist family genes
as a novel epigenetic biomarker panel for bladder cancer detection.
Clin Cancer Res. 12:2109–2116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin Y, Murata H, Sakaguchi M, et al:
Partial sensitization of human bladder cancer cells to a
gene-therapeutic adenovirus carrying REIC/Dkk-3 by downregulation
of BRPK/PINK1. Oncol Rep. 27:695–699. 2012.PubMed/NCBI
|
|
27
|
Niehrs C: The complex world of WNT
receptor signalling. Nat Rev Mol Cell Biol. 13:767–779. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mikata R, Yokosuka O, Fukai K, et al:
Analysis of genes upregulated by the demethylating agent
5-aza-2′-deoxycytidine in gastric cancer cell lines. Int J Cancer.
119:1616–1622. 2006.
|
|
29
|
Niida A, Hiroko T, Kasai M, et al: DKK1, a
negative regulator of Wnt signaling, is a target of the
beta-catenin/TCF pathway. Oncogene. 23:8520–8526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsieh SY, Hsieh PS, Chiu CT and Chen WY:
Dickkopf-3/REIC functions as a suppressor gene of tumor growth.
Oncogene. 23:9183–9189. 2004.PubMed/NCBI
|
|
31
|
Fukui T, Kondo M, Ito G, et al:
Transcriptional silencing of secreted frizzled related protein 1
(SFRP 1) by promoter hypermethylation in non-small-cell lung
cancer. Oncogene. 24:6323–6327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yue W, Sun Q, Dacic S, et al:
Downregulation of Dkk3 activates beta-catenin/TCF-4 signaling in
lung cancer. Carcinogenesis. 29:84–92. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawano Y, Kitaoka M, Hamada Y, Walker MM,
Waxman J and Kypta RM: Regulation of prostate cell growth and
morphogenesis by Dickkopf-3. Oncogene. 25:6528–6537. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abarzua F, Sakaguchi M, Takaishi M, et al:
Adenovirus-mediated overexpression of REIC/Dkk-3 selectively
induces apoptosis in human prostate cancer cells through activation
of c-Jun-NH2-kinase. Cancer Res. 65:9617–9622. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mizobuchi Y, Matsuzaki K, Kuwayama K, et
al: REIC/Dkk-3 induces cell death in human malignant glioma. Neuro
Oncol. 10:244–253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kloten V, Becker B, Winner K, et al:
Promoter hypermethylation of the tumor-suppressor genes ITIH5,
DKK3, and RASSF1A as novel biomarkers for blood-based breast cancer
screening. Breast Cancer Res. 15:R42013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zenzmaier C, Sampson N, Plas E and Berger
P: Dickkopf-related protein 3 promotes pathogenic stromal
remodeling in benign prostatic hyperplasia and prostate cancer.
Prostate. 73:1441–1452. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Than SS, Kataoka K, Sakaguchi M, et al:
Intraperitoneal administration of an adenovirus vector carrying
REIC/Dkk-3 suppresses peritoneal dissemination of scirrhous gastric
carcinoma. Oncol Rep. 25:989–995. 2011.
|
|
39
|
Koppen A, Ait-Aissa R, Koster J, et al:
Dickkopf-3 expression is a marker for neuroblastic tumor maturation
and is down-regulated by MYCN. Int J Cancer. 122:1455–1464. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hoang BH, Kubo T, Healey JH, et al:
Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma
cells by modulating the Wnt-beta-catenin pathway. Cancer Res.
64:2734–2739. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
del Barrantes IB, Montero-Pedrazuela A,
Guadaño-Ferraz A, et al: Generation and characterization of
dickkopf3 mutant mice. Mol Cell Biol. 26:2317–2326. 2006.PubMed/NCBI
|