Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide, and it occurs more
frequently in males compared with females. Despite recent advances
in clinical and experimental oncology, the prognosis of lung cancer
remains unfavorable, with a 5-year overall survival rate of ~11%,
threatening human health (1,2).
Thus, a detailed understanding of the mechanisms underlying lung
cancer development and progression are essential for improving the
diagnosis, prevention and treatment of this disease.
Recently, a five-center collaborative study
(3) reported that genetic variants
at 13q31.3 alter GPC5 expression and are associated with
susceptibility to lung cancer in non-smokers. GPC5 gene expression
levels in normal lung tissues were found to be significantly lower
in individuals who carry high-risk alleles, and that the GPC5
expression level in adenocarcinoma tissue was significantly lower
compared with matched normal lung tissue. Reduction of the
expression of GPC5 may lead to the development of lung cancer,
suggesting that this gene normally functions as a tumor
suppressor.
MicroRNAs (miRNAs) are a family of endogenous, small
non-coding, ~22 nucleotide RNA molecules, which bind to the target
gene mRNA through complete or incomplete complementarity with its
3′-untranslated region (3′-UTR), leading to target mRNA degradation
or translational repression (4).
To date, ~60% of the protein-coding genes are regulated by miRNAs
and a large number of studies show that miRNAs are involved in
specific biological processes, including cell growth, apoptosis and
differentiation (5–8). In the current study, epigenetic
silencing by hypermethylation of the CpG-rich region of GPC5 was
observed to lead to loss of GPC5 function, and the roles of miRNAs
in the reduction of GPC5 in lung adenocarcinoma were explored.
TargetScan, miRDB, and www.microrna.org
were used to predict biological targets of miRNAs, and a series of
miRNAs were found to target the 3′-UTR of GPC5. The luciferase
assay verified that miR-620 directly targets the 3′-UTR of GPC and
regulates its expression in A549 and H1299 lung adenocarcinoma
cells. Further in vitro assays indicate that knockdown of
the expression of GPC5 with GPC5 small interfering (si)RNA rescues
the changed phonotype of lung adenocarcinoma cells caused by
miR-620. Finally, the GPC5 expression level was observed to be
negatively associated with miR-620 expression in lung cancer
tissue. These results suggest that miRNA regulating GPC5 expression
may be important in the development of lung adenocarcinoma and,
which may in turn lead to carcinogenesis.
Materials and methods
Clinical samples and cell lines
A total of nine matched lung adenocarcinoma and
adjacent normal human lung tissues used in the current study were
obtained from the Pingjin Hospital, (Tianjin, China). Specimens
were snap-frozen in liquid nitrogen. The collection and use of the
patient samples were reviewed and approved by Pingjin Hospital
Institutional Ethics Committees, and written informed consent from
all patients was appropriately obtained. The characteristics of the
patients with lung cancer is presented in Table I. A549 and H1299 cells were
purchased from the American Type Culture Collection (Manassas, VA,
USA). The A549 and H1299 cells were cultured at 37°C in 5%
CO2 in RPMI-1640 media (Gibco-BRL, Grand Island, NY,
USA) supplemented with 10% fetal bovine serum (FBS). A549 and H1299
cells were transfected with Lipofectamine 2000 reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA). Enriched small RNAs were
extracted using the mirVana™ miRNA isolation kit (Ambion, Austin,
TX, USA). Total RNA was extracted using TRIzol reagent (Invitrogen
Life Technologies).
 | Table ICharacteristics of lung cancer
patients in this study. |
Table I
Characteristics of lung cancer
patients in this study.
| Patient
characteristics | No. of patients |
|---|
| Gender |
| Male | 6 |
| Female | 3 |
| Age (years) |
| ≥60 | 5 |
| <60 | 4 |
| Histological
type |
| Adenocarcinoma | 9 |
| Squamous cell
carcinoma | 0 |
| Differentiation |
| Well | 1 |
| Moderately | 5 |
| Poorly | 3 |
| Stage |
| I | 0 |
| II | 1 |
| III | 5 |
| IV | 3 |
| Smoking status |
| Smoker | 0 |
| Non-smoker | 9 |
Selection of candidate miRNAs
The candidate miRNAs selected to bind to GPC5 were
identified using three web-based bioinformatic algorithms.
TargetScan, miRDB and microRNA predict miRNA-binding sites based on
complementary nucleotide sequences in the 3′-UTR of GPC5 mRNA.
Luciferase reporter assay
Plasmids containing GPC5-3′-UTR and GPC5-3′-UTR
mutation were constructed using technical support from Dajin
Company (Guangdong, China). The 3′-UTR sequence of GPC5, predicted
to interact with miR-620 and a mutated sequence of the 3′-UTR
sequence by three miRNA target prediction algorithms, was inserted
into pGL3 vectors (Promega Corporation, Madison, WI, USA).
Following transfection of miR-620 or miR-620 (antisense
oligonucleotide) ASO for 24 h, A549 cells were transfected with
pGL3/GPC5-3′-UTR and pGL3/GPC5-3′-UTR mutant plasmids. Following 48
h, the luciferase activity of A549 cells was measured 96 h after
transfection using the Dual-Luciferase reporter assay system
(Promega Corporation).
Cell growth assay
Cells were seeded in 96-well plates at 8,000
cells/well and transfected the following day. The MTT assay was
used to determine relative cell growth 24, 48 and 72 h following
transfection. MTT solution (20 μl) was added to 100 μl culture
media and cells were incubated for a subsequent 4 h at 37°C. Next,
the optical density was measured at 570 nm (A570) by Bio-Rad 680
Microplate reader (Bio-Rad, Hercules, CA, USA).
Colony formation assay
The cells were seeded into a 12-well plate at a
density of 200 cells/well following transfection. The medium was
changed every three days. Approximately 10 days later, the majority
of the cell clones contained >50 cells. The clones were washed
with 1X phosphate-buffered saline and stained with crystal violet
for ~5 min. Finally images were captured of the clones by a Canon
camera (Cannon, Taiwan, China) and clones were counted. The colony
formation rate was calculated to = (number of clones)/(number of
seeded cells) × 100%.
Transwell invasion and migration
assay
The cell invasion and migration ability was
performed using the Transwell chamber with or without Matrigel
(Millipore, Billerica, MA, USA). The transfected cells were plated
into the upper chamber with 250 μl serum-free medium, while the
lower chamber was filled with 750 μl cell medium with 10% FBS. The
cells were allowed to invade for ~20 h, and were then washed, fixed
and stained with 5% crystal violet. The cells, which did not invade
into the membrane were scraped with cotton tips. Finally, images
were captured of the invaded or migrated cells and cell numbers
were counted by microscopy (Olympus, Tokyo, Japan).
Quantitative polymerase chain reaction
(qPCR) analysis and western blotting
The total RNA, including miRNA was isolated using
the TRIzol reagent according to the manufacturer’s instructions.
The RNA concentration was determined using a NanoDrop-1000
spectrophotometer (Thermo Scientific, Waltham, MA, USA). For the
reverse transcription reaction of the miRNA, specific miRNA RT
primers were used
(miR-620:5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGAGCTTTA-3′). RNU6B
(U6 small nuclear B non-coding RNA) was used as an internal
control. The qPCR was performed using the SYBR-Green PCR Master mix
(Applied Biosystems, Foster City, CA, USA) according to the
following conditions: 95°C for 5 min followed by 40 cycles of
amplification at 95°C for 30 sec, 57°C for 30 sec and 72°C for 30
sec. The cells were plated into a 6-well plate at a density of
3×104 cells/well and transfected on the second day when
the cell confluency reached ~80%. At 48 h after transfection, the
cells were lysed by RIPA buffer (50 mM Tris-HCl, pH 8.8, 150 mM
NaCl, 1% NP-40, 1% sodium deoxycholate and 0.1% SDS) for 30 min at
4°C. The protein concentration was measured using the bicinchoninic
acid (Haoran Bio Technologies Co., Ltd., Shanghai, China) assay
method. The primary antibody was rabbit monoclonal anti-GPC5
antibody (1:200 dilutions) and anti-GAPDH antibody (1:1,000
dilutions; both Abcam, Cambridge, MA, USA). The secondary antibody
was goat anti-rabbit IgG conjugated with horseradish peroxidase at
a dilution of 1:1,000. The bound antibodies were detected with the
use of Enhanced Chemiluminescence Plus Western Blotting Detection
system (GE Healthcare, Buckinghamshire, United Kingdom) and the
chemiluminescent signals were detected with the use of
high-performance chemiluminescence film (GE Healthcare). GAPDH was
used as an internal control to normalize GPC5 levels.
DNA methylation analysis
A total of 1 μg genomic DNA was treated with sodium
bisulphite using the EZ DNA methylation™ kit (Zymo Research,
Irvine, CA, USA), and subsequently analyzed for GPC5 methylation by
the MethyLight technique (9).
Statistical analysis
Data are expressed as the mean ± standard deviation,
and a Students-Newman-Keuls post hoc test was performed. P<0.05
was considered to indicate a statistically significant difference.
All data were analyzed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA)
to confirm the statistical significance.
Results
Downregulation of GPC5 in lung
adenocarcinoma cells is independent of DNA methylation
A total of nine lung adenocarcinoma tissues were
analyzed by methylation-specific PCR (MSP) to detect the
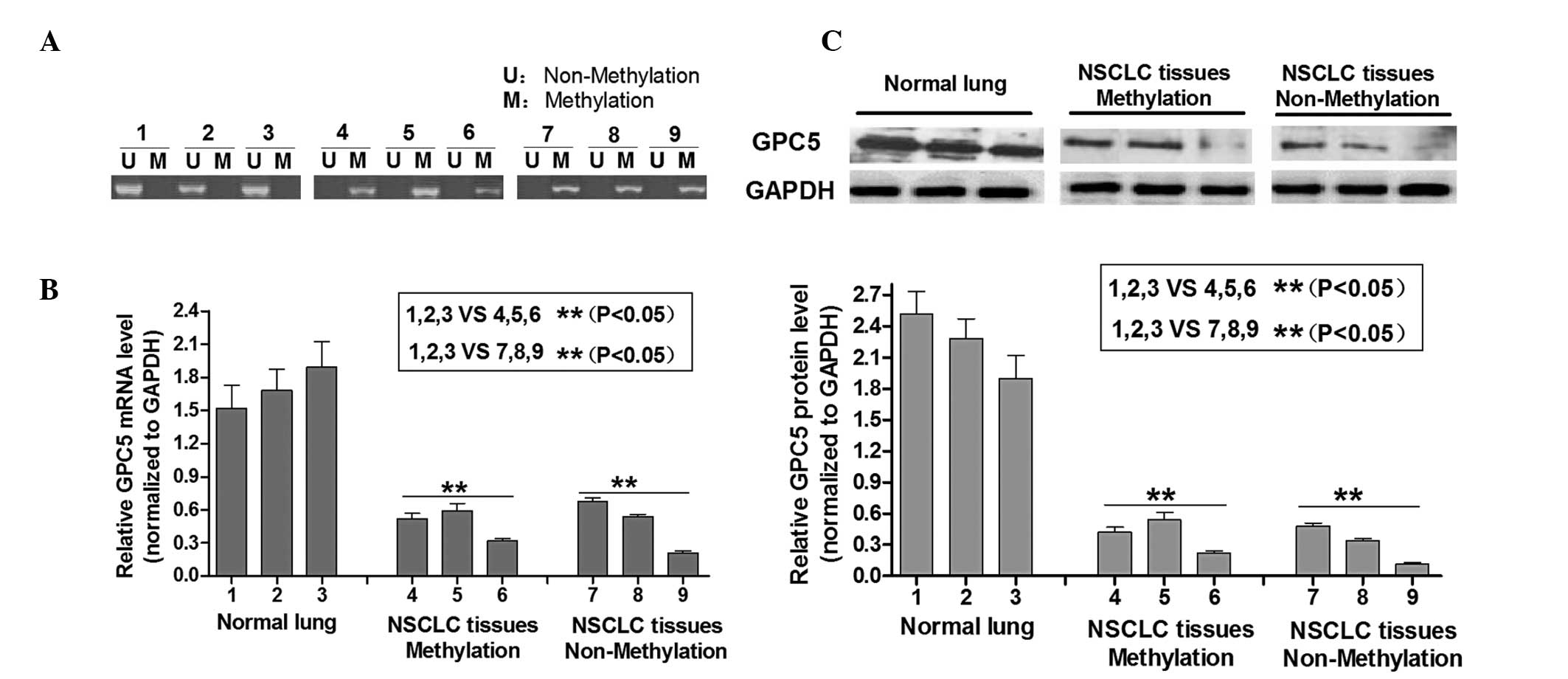
methylation status of the GPC5 CpG islands. As shown in Fig. 1A, tumors 1, 2 and 3 were
unmethylated at the GPC5 CpG islands, whereas 4, 5, 6, 7, 8 and 12
were fully methylated at this locus (Fig. 1A). Next, the expression levels of
GPC5 protein were determined in unmethylated and methylated lung
adenocarcinoma tissues. Fig. 1B
shows that the GPC5 mRNA and protein levels exhibited a loss of
expression in the lung adenocarcinoma tissues compared with the
normal lung tissues irrespective of the methylation status in CpG
islands. Western blotting results showed that regardless of whether
GPC5 CpG islands were methylated or not, the GPC5 protein was
consistently expressed at a lower level in laryngeal carcinoma
tissues compared with normal lung tissue, which was normalized to
GAPDH (Fig. 1C). These results
indicated that GPC5 was downregulated in lung adenomacarcinoma
cells, which is consistent with a previous study (3) that implies that there may be another
potential mechanism attributed to its downregulation.
miR-620 targets GPC5 transcription and
downregulates its expression
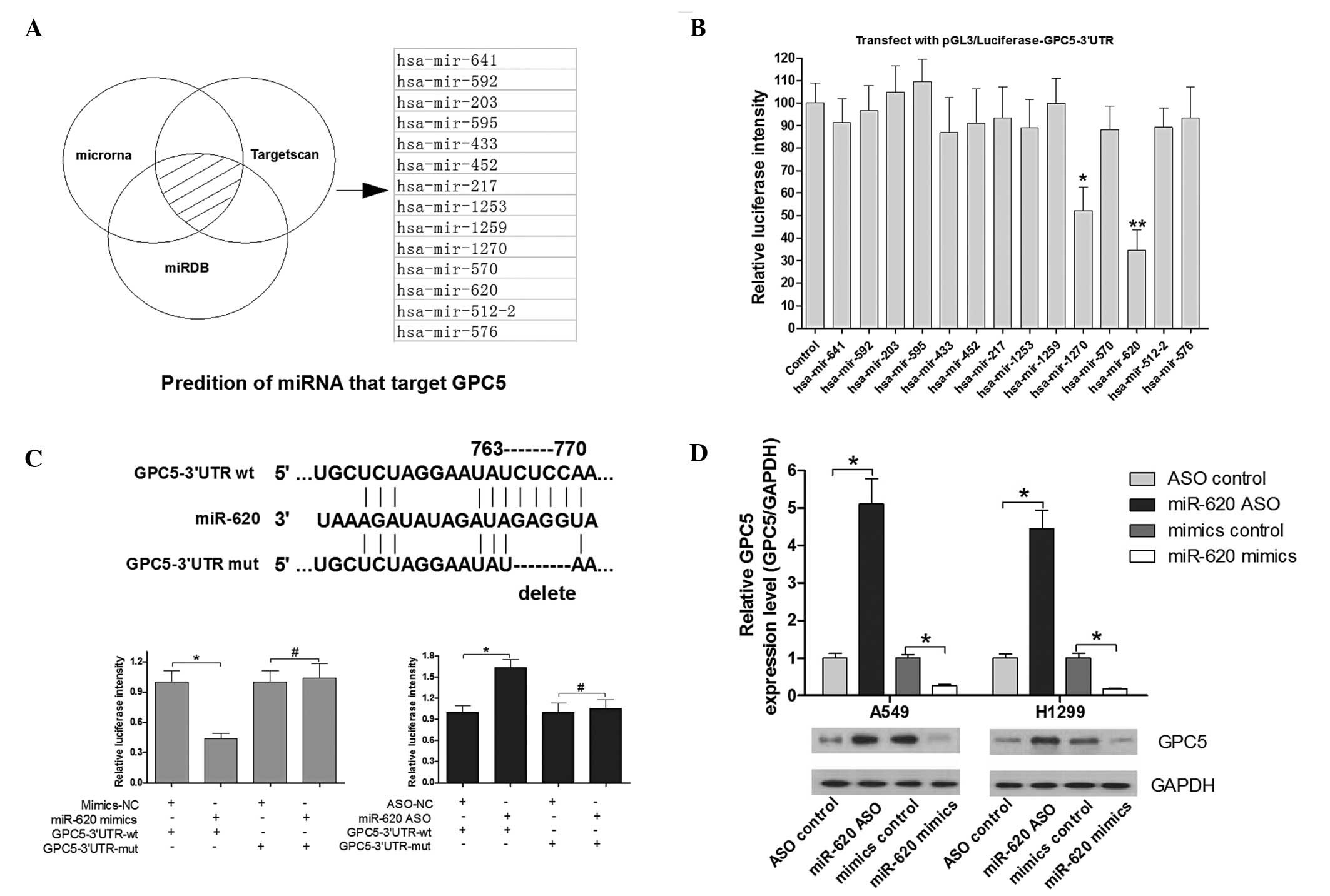
TargetScan, microRNA and miRDB were selected for
miRNA prediction. As shown in Fig.
2A, 14 candidate miRNAs were identified. To determine which
miRNA is the most effective in regulating GPC5, a luciferase
reporter assay was performed. A549 cells were transfected with the
reporter vector along with the 14 miRNA mimics. Notably, miR-620
significantly downregulated luciferase intensity of
pGL3/luciferase-GPC5-3′-UTR, and miR-1270 was also downregulated to
a lesser extent (Fig. 2B). Thus,
miR-620 was observed in regard to the regulation of GPC5. To
further determine whether miR-620 directly regulates GPC5, another
luciferase-reporter assay was used to validate the target site in
the GPC5-3′-UTR (Fig. 2C). A549
cells were transfected with the reporter vector along with miR-620
mimics, control mimics, miR-620 ASO or control ASO. Notably, the
intensity of luciferase in cells transfected with miR-620 mimics
was significantly decreased compared with that in control cells,
whereas the intensity of luciferase in cells transfected with
miR-620 ASO was significantly increased (Fig. 2C). By contrast, the luciferase
intensity from the reporter vector containing the mutant
GPC5-3′-UTR was not affected by miR-620 mimics or miR-620 ASO
(Fig. 2C). These results indicate
that GPC5 is a direct target of miR-620. Furthermore, the effect of
miR-620 on the expression of endogenous GPC5 was examined by
western blot analysis. The GPC5 protein level was increased by
~5-fold in A549 and 4-fold in H1299 cells transfected with miR-620
ASP when compared with the control group (Fig. 2D). The GPC5 protein level was
decreased by ~80% in A549 and 85% in H1299 cells transfected with
miR-620 mimics when compared with the control group (Fig. 2D).
GPC5 expression level is inversely
associated with miR-620 levels in human lung adenocarcinoma
tissues
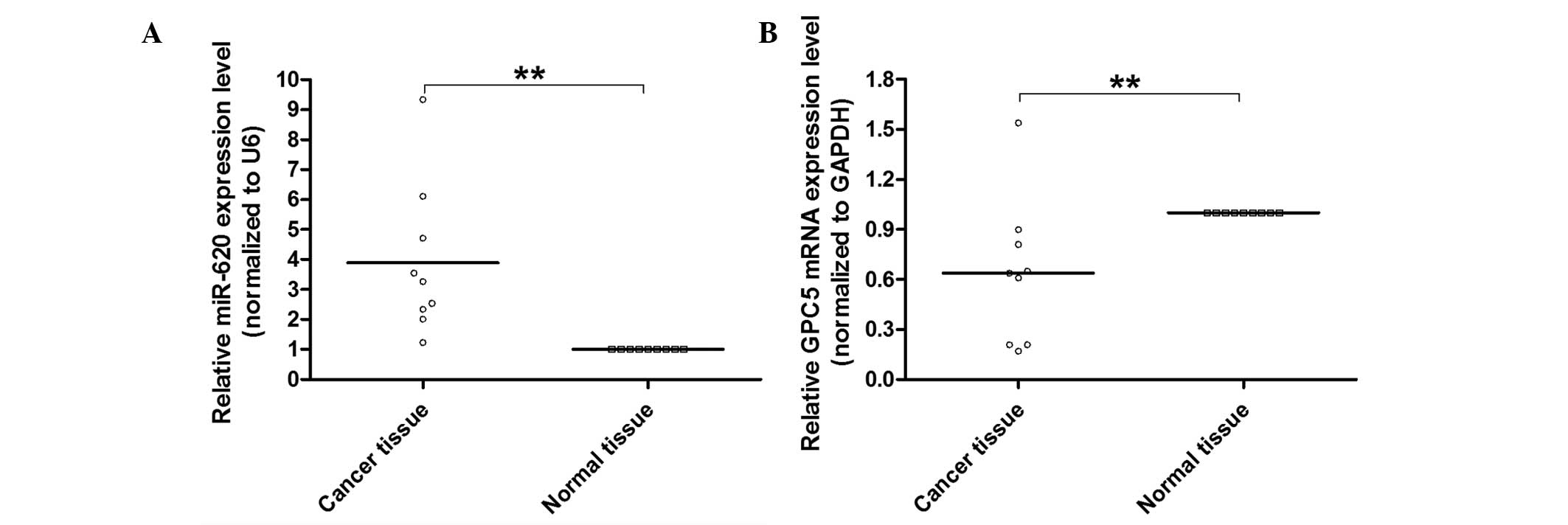
To further explore whether miR-620 regulates GPC5
expression in lung adenocarcinoma, the levels of miR-620 and GPC5
were examined in nine human lung adenocarcinoma tissues. qPCR was
performed to detect miR-620 and GPC5 mRNA. As shown in Fig. 3A and B, the expression of miR-620
was inversely associated with GPC5 in the examined lung
adenocarcinoma tissue, further indicating that miR-620 may have an
important role in the reduction of GPC5 in lung adenocarcinoma
tumorigenesis.
miR-620/GPC5 signal regulates the growth
of human lung adenocarcinoma cells
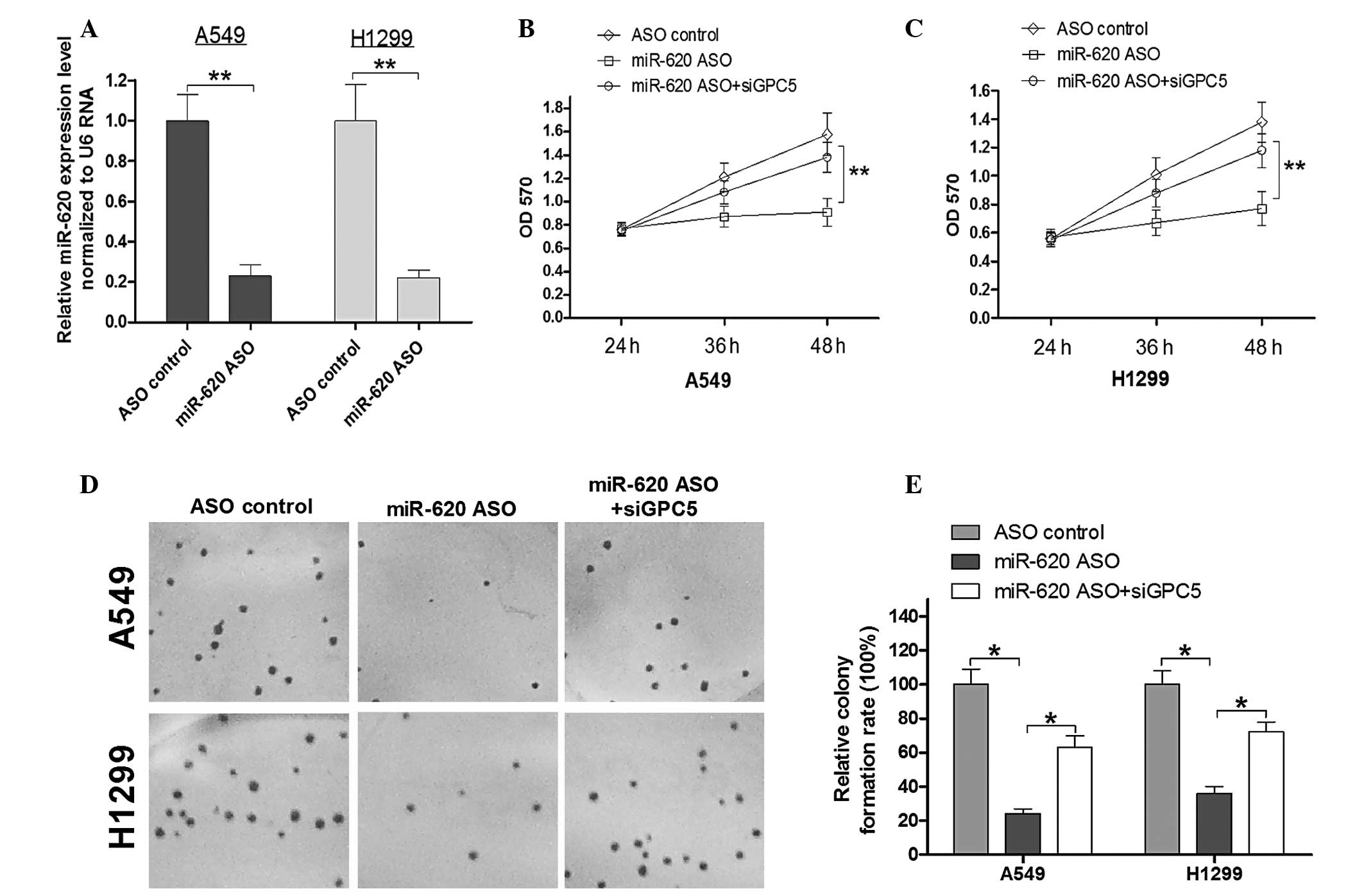
To determine the role of miR-620 and GPC5 in the
proliferation of lung adenocarcinoma cells, miR-620 ASO was used to
block endogenous miR-620 expression in the A549 and H1299 lung
adenocarcinoma cell lines. The efficiency of ASO-mediated blocking
of miR-620 was confirmed by qPCR (Fig.
4A). An MTT assay and colony forming assay were used to
determine the effects of miR-620 in lung adenocarcinoma cells. The
optical density (OD)570 means in each group (Fig. 4B) indicated that transfection of
A549 cells with miR-620 ASO resulted in the suppression of A549
cell proliferation 48 h post-infection when compared with the
controls. To confirm whether the A549 growth alters with miR-620,
ASO was mediated by GPC5, and a specific siRNA (siGPC5) was used to
knockdown GPC5 expression. As expected, the cell viability was
rescued by siGPC5 compared with the viability in the miR-620 ASO
group. Thus, miR-620 regulates A549 cell proliferation by
regulating GPC5 expression. A similar trend was observed in the
H1299 cells (Fig. 4C). Next, the
colony forming ability in the transfected A549 and H1299 cells was
determined. The colony formation ability of A549 and H1299 cells
were reduced to ~78 and 60%, respectively, in the miR-620 ASO
group. A549 and H1299 cells simultaneously transfected with miR-620
ASO and siGPC5, exhibited rescued colony forming ability compared
with the miR-620 ASO group with a statistical significance
(Fig. 4D and E). This implied that
the miR-620/GPC5 signal regulates cell growth in human lung
adenocarcinoma.
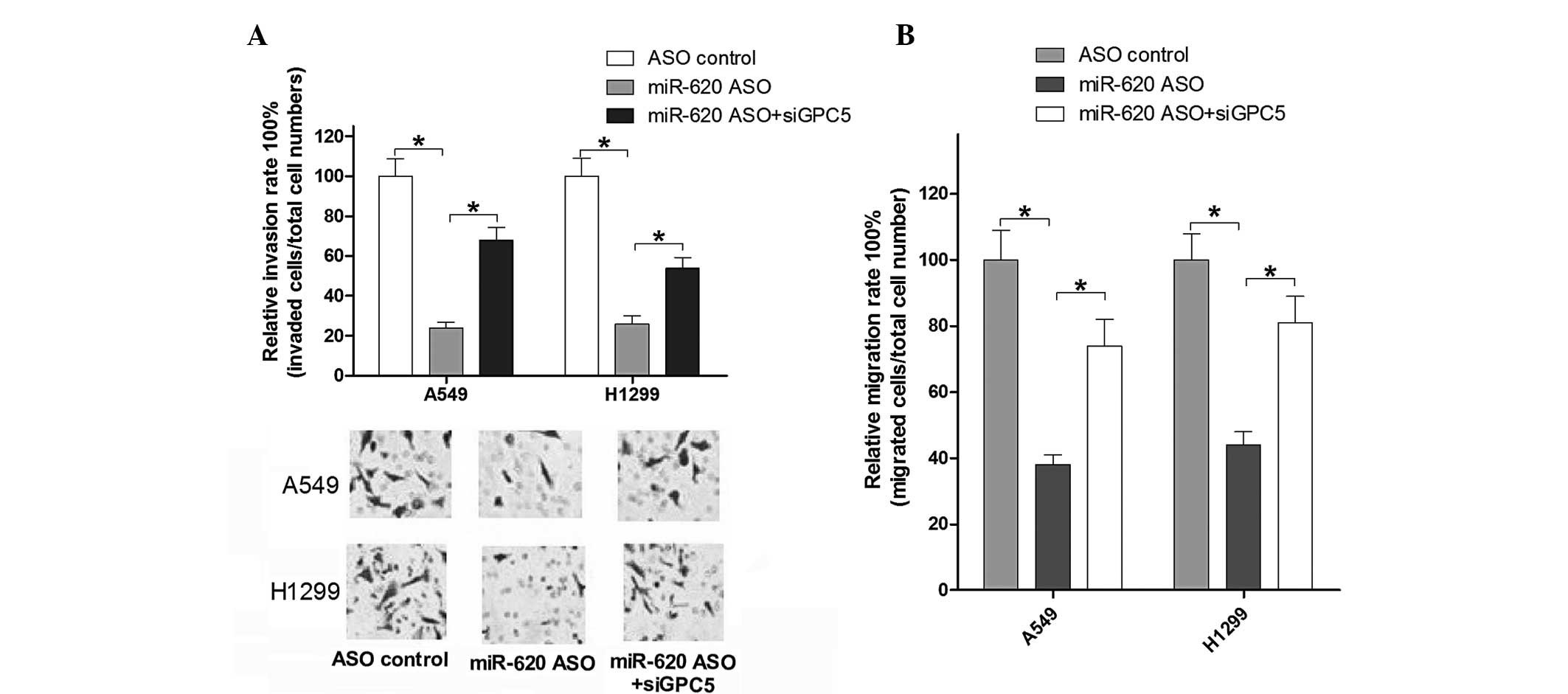
miR-620/GPC5 signal regulates the
invasion and migration of human lung adenocarcinoma cells
To further investigate the effect of miR-620/GPC5
signal on cell motility, Transwell invasion and migration assays
were measured post-infection for 24 h in A549 and H1299 cells. As
shown in Fig. 5A and B, the
results suggested that blocking miR-620 suppressed the cell
migration and invasion abilities in A549 cells, and similar results
were obtained in H1299 cells. siGPC5 rescued the changed migration
and invasion ability of A549 and H1299 cells compared with that in
the miR-620 ASO group. These data investigated whether miR-620/GPC5
signal regulates the invasion and migration of human lung
adenocarcinoma cells.
Discussion
GPC5 is a member of the glypican family, a group of
membrane-bound heparan sulfate proteoglycans (HSPGs) that are
linked to the exocytoplasmic surface of the plasma membrane via a
glycosylphosphatidylinositol anchor (GPI). To date, six glypicans
have been identified in mammals (GPC1 to GPC6) (10–14).
These proteins have been suggested to be dysregulated in various
types of cancer, including GPC1, which is overexpressed in human
pancreatic and breast cancers (15,16),
GPC3 is overexpressed in neuroblastoma, Wilm’s tumors, and melanoma
(17,18). Alterations at the GPC5 locus are
also a common event in various human tumors, including lymphomas,
breast cancers, and neurological tumors (19–21).
In lung cancer, GPC5 has been reported to be downregulated in
adenocarcinoma tissue compared with matched normal lung tissue
(3). Although epigenetic silencing
by hypermethylation of the CpG-rich region of GPC5 leads to a loss
of GPC5 function and carcinogenesis, the current study indicated
that there remains a proportion of lung adenocarcinoma tissue with
a lower expression level of GPC5 without DNA methylation. Thus, the
current study aimed to further explore the other factors involved
in the regulation of GPC5. Notably, following screening, 14
candidate miRNAs were selected that may target GPC5 and are
involved in the reduction of GPC5 in lung adenocarcinoma. The
luciferase reporter assay confirmed that miR-620 may have the most
potential.
Hundreds of miRNAs have been reported to repress
tumor suppressor genes, and this may be a common mechanism involved
in tumorigenesis of lung cancer. Du et al (22) reported regulated expression of the
tumor suppressor gene FUS1 in non-small cell lung cancer. Liu et
al (23) demonstrated that
miR-21 promotes growth, metastasis and chemoresistance or
radioresistance in non-small cell lung cancer cells by targeting
the tumor suppressor gene PTEN. miR-221 and miR-222 induces TRAIL
resistance and enhances cellular migration through the activation
of the AKT pathway and metallopeptidases by targeting the tumor
suppressors PTEN and TIMP3 in lung cancer (24). Another important miRNA cluster
miR-17–92, has been observed to be overexpressed in lung cancer and
to target the tumor suppressors PTEN and Rb2 (25,26).
These results suggest that a number of miRNAs are important in lung
cancer progression and development, acting as oncogenes by
regulating tumor suppressor gene expression. In the current study,
miR-620 was observed to be upregulated in human adenocarcinoma
compared with the adjacent normal tissues, which acts as an
oncogene by regulating the tumor suppressor GPC5 in lung
adenocarcinoma. Transfection of A549 and H1299 cells with miR-620
ASO was further shown to significantly induce growth, invasion and
migration inhibition by MTT, colony formation assays, Transwell
invasion and migration assays. Knockdown of GPC5 expression using
GPC5-siRNA partly restores phenotype changes in A549 and H1299
cells. These results confirmed that miR-620 is involved in the
tumorigenesis of lung adenocarcioma by repression of the tumor
suppressor gene, GPC5.
In conclusion, the current study is summarized by
several major findings: i) A downregulation of GPC5 in lung
adenocarcinoma is observed irrespective of its methylation in the
CpG region; ii) to the best of our knowledge, we have shown for the
first time that miR-620 directly targets GPC5 and downregulates the
expression of GPC5 in lung adenocarcinoma cells; and iii) the
suppression of miR-620 using miR-620 ASO causes a marked inhibition
of proliferation, invasion and migration in A549 and H1299 lung
adenocarcinoma cell lines, and this phenotype is rescued by GPC5
knockdown. These results provide evidence that, in addition to
hypermethylation, GPC5 may also be inactivated in lung
adenocarcinoma cells by miR-620 overexpression. Further studies
should be performed as follows: i) Since the expression patterns of
GPC5 in different types of cancer are varied, including negative
expression in acute lymphoblastic leukemia, histiocytic lymphoma
and myeloma (27), and positive
expression in lymphomas (28),
further studies are required to evaluate the function of GPC5 in
other tumors. ii) Based on the evidence that one miRNA regulates
numerous target genes, and one tumor suppressor gene may be
regulated by a number of miRNAs, studies are required to detect
more miRNAs, including miR-1270, which regulates GPC5 expression to
establish the miRNA/GPC5 regulation network for characterization of
the progression of lung adenocarcinoma and iii) although the
current study identifies the expression patterns of miR-620 in lung
adenocarcinoma and clarifies the miR-620/GPC5 signal in tumorigenes
of lung adenocarcinoma, further studies should aim to determine
whether the mechanism of miR-620/GPC5 signaling contributes to the
tumorigenesis of other cancers, and determine the expression
patterns of miR-620 in other cancers.
References
|
1
|
Subramaniam S, Thakur RK, Yadav VK, Nanda
R, Chowdhury S and Agrawal A: Lung cancer biomarkers: State of the
art. J Carcinog. 12:32013. View Article : Google Scholar
|
|
2
|
Amorin Kajatt E: Lung cancer: a review of
current knowledge, diagnostic methods and therapeutic perspectives.
Rev Peru Med Exp Salud Publica. 30:85–92. 2013.(In Spanish).
|
|
3
|
Li Y, Sheu CC, Ye Y, et al: Genetic
variants and risk of lung cancer in never smokers: a genome-wide
association study. Lancet Oncol. 11:321–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brennecke J, Hipfner DR, Stark A, Russell
RB and Cohen SM: Bantam encodes a developmentally regulated
microRNA that controls cell proliferation and regulates the
proapoptotic gene hid in Drosophila. Cell. 113:25–36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu P, Vernooy SY, Guo M and Hay BA: The
Drosophila microRNA Mir-14 suppresses cell death and is
required for normal fat metabolism. Curr Biol. 13:790–795.
2003.
|
|
7
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen CZ, Li L, Lodish HF and Bartel DP:
MicroRNAs modulate hematopoietic lineage differentiation. Science.
303:83–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eads CA, Danenberg KD, Kawakami K, et al:
MethyLight: a high-throughput assay to measure DNA methylation.
Nucleic Acids Res. 28:E322000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Filmus J, Capurro M and Rast J: Glypicans.
Genome Biol. 9:2242008. View Article : Google Scholar
|
|
11
|
Filmus J, Church JG and Buick RN:
Isolation of a cDNA corresponding to a developmentally regulated
transcript in rat intestine. Mol Cell Biol. 8:4243–4249.
1988.PubMed/NCBI
|
|
12
|
Filmus J, Shi W, Wong ZM and Wong MJ:
Identification of a new membrane-bound heparan sulphate
proteoglycan. Biochem J. 311:561–565. 1995.
|
|
13
|
Paine-Saunders S, Viviano BL and Saunders
S: GPC6, a novel member of the glypican gene family, encodes a
product structurally related to GPC4 and is colocalized with GPC5
on human chromosome 13. Genomics. 57:455–458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Veugelers M, De Cat B, Ceulemans H, et al:
Glypican-6, a new member of the glypican family of cell surface
heparan sulfate proteoglycans. J Biol Chem. 274:26968–26977. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kleeff J, Ishiwata T, Kumbasar A, et al:
The cell-surface heparan sulfate proteoglycan glypican-1 regulates
growth factor action in pancreatic carcinoma cells and is
overexpressed in human pancreatic cancer. J Clin Invest.
102:1662–1673. 1998. View
Article : Google Scholar
|
|
16
|
Matsuda K, Maruyama H, Guo F, et al:
Glypican-1 is overexpressed in human breast cancer and modulates
the mitogenic effects of multiple heparin-binding growth factors in
breast cancer cells. Cancer Res. 61:5562–5569. 2001.PubMed/NCBI
|
|
17
|
Nakatsura T, Kageshita T, Ito S, et al:
Identification of glypican-3 as a novel tumor marker for melanoma.
Clin Cancer Res. 10:6612–6621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saikali Z and Sinnett D: Expression of
glypican 3 (GPC3) in embryonal tumors. Int J Cancer. 89:418–422.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reardon DA, Jenkins JJ, Sublett JE, Burger
PC and Kun LK: Multiple genomic alterations including N-myc
amplification in a primary large cell medulloblastoma. Pediatr
Neurosurg. 32:187–191. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ojopi EP, Rogatto SR, Caldeira JR,
Barbiéri-Neto J and Squire JA: Comparative genomic hybridization
detects novel amplifications in fibroadenomas of the breast. Genes
Chromosomes Cancer. 30:25–31. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neat MJ, Foot N, Jenner M, et al:
Localisation of a novel region of recurrent amplification in
follicular lymphoma to an approximately 6.8 Mb region of 13q32-33.
Genes Chromosomes Cancer. 32:236–243. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Du L, Schageman JJ, Subauste MC, et al:
miR-93, miR-98, and miR-197 regulate expression of tumor suppressor
gene FUS1. Mol Cancer Res. 7:1234–1243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu ZL, Wang H, Liu J and Wang ZX:
MicroRNA-21 (miR-21) expression promotes growth, metastasis, and
chemo- or radioresistance in non-small cell lung cancer cells by
targeting PTEN. Mol Cell Biochem. 372:35–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garofalo M, Di Leva G, Romano G, et al:
miR-221&222 regulate TRAIL resistance and enhance
tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell.
16:498–509. 2009.
|
|
25
|
Ebi H, Sato T, Sugito N, et al:
Counterbalance between RB inactivation and miR-17–92 overexpression
in reactive oxygen species and DNA damage induction in lung
cancers. Oncogene. 28:3371–3379. 2009.PubMed/NCBI
|
|
26
|
Hayashita Y, Osada H, Tatematsu Y, et al:
A polycistronic microRNA cluster, miR-17–92, is overexpressed in
human lung cancers and enhances cell proliferation. Cancer Res.
65:9628–9632. 2005.
|
|
27
|
Veugelers M, Vermeesch J, Reekmans G,
Steinfeld R, Marynen P and David G: Characterization of glypican-5
and chromosomal localization of human GPC5, a new member of the
glypican gene family. Genomics. 40:24–30. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu W, Inoue J, Imoto I, Matsuo Y, Karpas A
and Inazawa J: GPC5 is a possible target for the 13q31-q32
amplification detected in lymphoma cell lines. J Hum Genet.
48:331–335. 2003.PubMed/NCBI
|