Introduction
Coronary heart disease (CHD) is a significant health
problem and one of the leading causes of morbidity and mortality in
China. The most common risk factors include tobacco smoking,
alcohol consumption, family history, hypertension, obesity,
diabetes, lack of exercise, stress and hyperlipidemia. These risk
factors interact with host genetic factors to impact the
development of CHD. To date, >30 common genetic susceptibility
loci have been identified by genome-wide association studies (GWAS)
and are independently associated with CHD risk (1–4),
however, the majority are based on European populations. For the
Chinese Han population, polymorphisms in chromosome 9p21 (5,6) are
the only loci demonstrated to have a CHD risk, with the carriers
having a 30% increased risk of CHD compared with non-carriers
(7). Thus, further studies on gene
polymorphisms are required in order to identify those that result
in susceptibility to CHD by interacting with environmental risk
factors and therefore, lead to the development of preventive and
therapeutic strategies for CHD.
Towards this end, the present study focused on
ATP-binding cassette sub-family G member 5 (ABCG5) and ABCG8, loci
that are localized on human chromosome 2p21. ABCG5 and ABCG8 are
hemi-transporters that regulate the absorption of sterols from the
intestine and promote the canalicular conversion and secretion of
cholesterol and plant sterols into bile (8). Their proteins are exclusively
expressed in the intestine and liver cells (9) and, together with a number of
transporter proteins, are important in the maintenance of lipid
homeostasis in the human body. Mutations in either gene is
associated with sitosterolemia, accumulation of dietary
cholesterol, accelerating atherosclerosis and premature CHD
(10,11). Several studies have demonstrated
that common variants in ABCG5/8 correlate with serum lipid levels
with concordant changes in the risk of CHD. The populations in
these studies were predominantly Caucasian (12–16),
Chilean (17), Czech (18) or Taiwanese consuming the
traditional Chinese Southern diet (19) and the results from diverse ethnic
groups were not consistently reproducible. For example, several
previous studies in China indicated that polymorphisms of
ABCG8 are associated with the upregulated absorption and
esterification of cholesterol in the small intestine and increase
the incidence of gallstone disease in a gender-specific manner
(20,21). However, the responsible variants
and the molecular mechanisms underlying the incidence of CHD have
not yet been identified.
Due to the colder climate, the Han Chinese
population in Northern China consume a high-fat, high-energy and
low-fiber diet compared with the Southern Han Chinese. Since the
launch of China’s reform and opening-up policies in 1978, the Han
Chinese have adopted a Westernized diet and other habits, including
smoking, alcohol consumption, accelerated aging, increased working
hours and complex interpersonal relations, increasing the incidence
and risk of CHD. Indeed, a number of studies have indicated that
dietary constituents perturb cholesterol homeostasis and
consequently affect expression of hepatic transporters. Yamazaki
et al (22) demonstrated
that a high calorie diet induces lipid loading in the liver and
causes significant increases in the expression of ABCG5/8 in bile
canaliculi compared with individuals on an ordinary diet. Another
study demonstrated that the increased expression of ABCG5/8
attenuates diet-induced hypercholesterolemia in Ldlr−/− mice,
resulting in a significant reduction in plasma levels of
cholesterol and aortic atherosclerotic lesions (23). Thus, the present study hypothesized
that ABCG5/8 loci polymorphisms are crucial in the maintenance of
lipid homeostasis and CHD susceptibility in the northern Han
Chinese population. The present study aimed to confirm the novel
susceptibility variants for CHD that were previously identified in
a European population using a case-control study. The association
of four candidate variants in ABCG5/8 genes, including i7892T>C
in ABCG5 and Tyr54CysA>G, Thr400LysC>A and 5U145A>C in
ABCG8 were evaluated using quantitative PCR high-resolution melting
(qPCR-HRM) analysis, a novel genotyping method. The plasma
concentrations and risk of CHD were then analyzed in the northern
Han Chinese population.
Materials and methods
Study population
A total of 684 patients (417 with CHD and 267
healthy controls) were recruited from the Department of Cardiology,
The Fourth Hospital of Jilin University (Changchun, Jilin, China).
Informed consent was obtained from each patient included in the
present study and ethical approval was provided by the Ethics
Committee of The Fourth Hospital of Jilin University. The mean age
of the study subjects was 62.67±11.63 years and the CHD patients
were angiographically diagnosed as having CHD with 50% stenosis in
one or more arteries and stable or unstable angina. The healthy
controls were free of any clinical symptoms (including ischemia or
asymptomatic myocardial infarction as determined by exercise
electrocardiography or 24 h ambulatory electrocardiography
monitoring). The present study was approved by the institutional
review board of The Fourth Hospital of Jilin University and all
participants signed a consent document prior to participation.
Data information
All participants were personally interviewed by our
participating physicians and the initial clinical data, including
age, sex, ethnicity, tobacco smoking status and alcohol consumption
were recorded. The subjects were categorized into smokers (current
smokers and former smokers), non-smokers and drinkers (current
drinkers and former drinkers) and non-drinkers. Diabetes mellitus
was diagnosed as a fasting plasma glucose of >7.0 mmol/l or a
120 min plasma glucose of >11.1 mmol/l, or by current use of
insulin or hypoglycemic drugs. Hypertension was defined as having a
systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure
≥90 mmHg, and/or by current use of antihypertensive
medications.
DNA extraction and real-time PCR
All study subjects donated 1 ml of venous blood that
was collected in an anticoagulant tube through vein-puncture
following an overnight fast of 12 h. These blood samples were
centrifuged at 2,000 × g for 20 min at 4°C and then aliquoted and
stored immediately at −86°C until use. For genotyping of these
single nucleotide polymorphisms (SNPs), genomic DNA was extracted
using a DNeasy blood and tissue kit (Qiagen, Valencia, CA, USA).
DNA yield and purity were determined by the A260/A280 ratio with a
Nanodrop 1000 spectrophotometer (Nanodrop Technologies, Wilmington,
DE, USA).
For optimal real-time qPCR-HRM genotyping analysis,
primers of the target loci (Table
II) were designed using the freely available software
Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast) and
these primers were synthesized by Invitrogen Life Technologies
(Shanghai, China).
 | Table IIPrimer sequences for genotyping of
ABCG5/8 SNPs using qPCR-HRM analysis. |
Table II
Primer sequences for genotyping of
ABCG5/8 SNPs using qPCR-HRM analysis.
| SNP | Primer
sequence | Allele |
|---|
| ABCG5
rs4131229 |
5′-GCTTGCTTGGAGGCATCTTG-3′ | C |
|
5′-CGGTGGGTTTACCTGTGGC-3′ | T |
| ABCG8
rs4148211 |
5′-TCTCCTCTGAAAGTGACAACAGC-3′ | A |
|
5′-CCTTGAACCCAGGCGTG-3′ | G |
| ABCG8
rs4148217 |
5′-CCGAGTCCTACGAAGATGCC-3′ | A |
|
5′-GGGGGGCGGGTTCAGT-3′ | C |
| ABCG8
rs3806471 |
5′-CATGGGGCCCACAGGTCT-3′ | C |
|
5′-CTGCTCCAGGAAACAGAGTGAA-3′ | A |
For real-time PCR, mixtures of 1 μl of 10X PCR
buffer, 1 μl of 25 mM MgCl2, 0.25 μl of 2.5 mM dNTPs,
0.5 μl of 20X Eva-green saturation dye, 0.25 μl of each forward and
reverse primer (10 μmol/l), 1 μl of template DNA (10 ng/μl), 0.1 μl
of 5U Taq enzyme and 5.65 μl of nuclease-free water, were added in
triplicate to a 96-well PCR plate and amplified in a CFX96
real-time PCR system (Bio-Rad, Hercules, CA, USA). The cycling
parameters of PCR were as follows: an initial cycle at 95°C for 5
min, followed by 50 cycles of 95°C for 10 sec, 60°C for 15 sec and
72°C for 25 sec. Following qPCR, DNA genotyping was performed using
a high-resolution melting curve i.e. 95°C for 1 min and 40°C for 1
min. Fluorescence data were then collected ~40 times for every
degree change during warm-up from 60–90°C. The samples were then
cooled at 40°C for 10 sec. As an example, the amplification curve
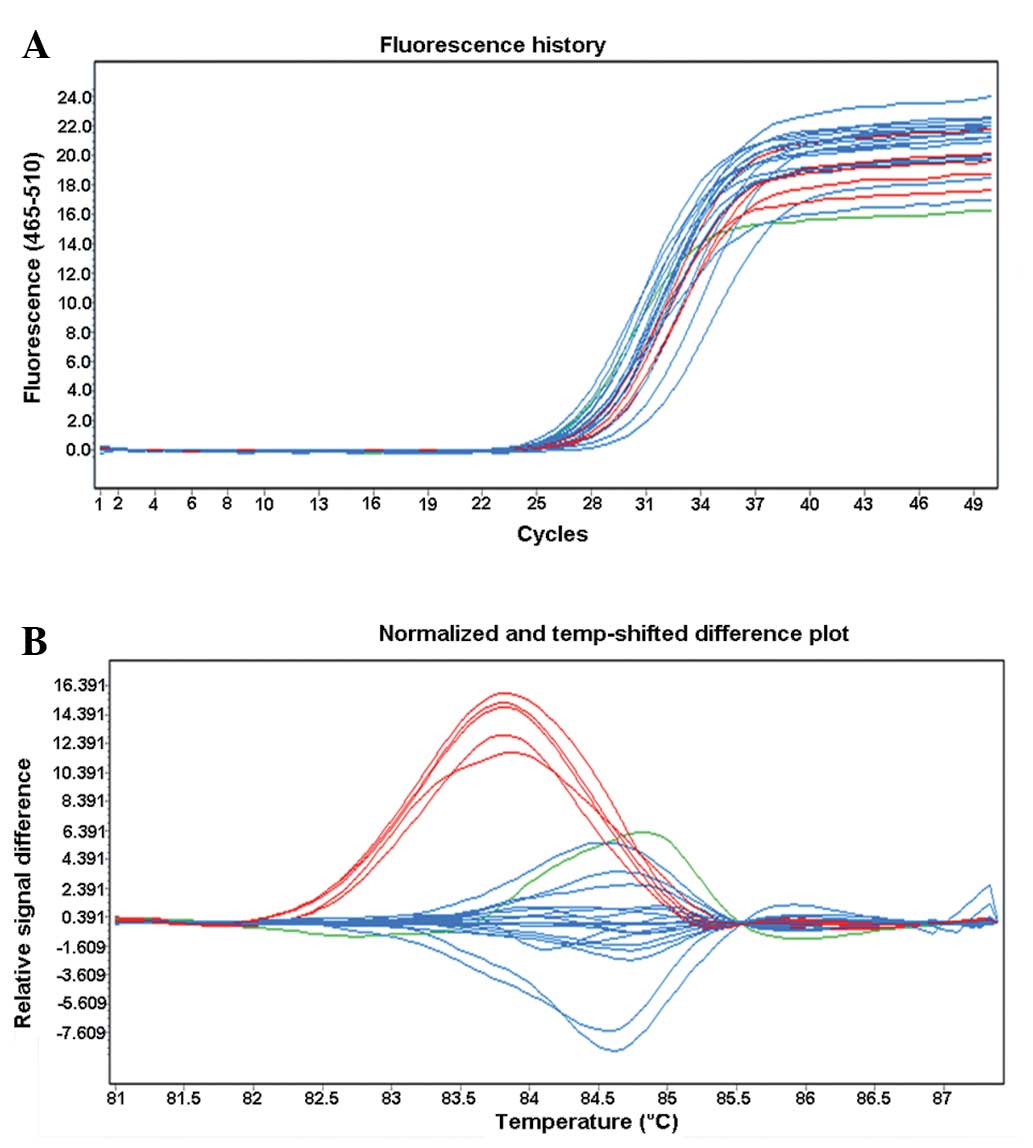
and typing results for s4148217 (A/C) are shown in Fig. 1. DNA samples detected by the
qPCR-HRM were also confirmed by direct DNA sequencing (each
genotype in five samples). DNA sequences were then analyzed using
an ABI PRISM 3100 Genetic Analyzer sequencer (PE Biosystems, Foster
City, CA, USA).
Laboratory analysis of serum lipid
levels
All laboratory data were determined using fasting
sera without any lipid-lowering drug usage for at least 4 weeks.
Lipid levels were measured using an Hitachi 7170 automatic
biochemical analyzer (Hitachi, Tokyo, Japan). Total cholesterol and
triglyceride were measured by an enzyme method with reagents
supplied by The DiaSys Diagnostic System Limited Company (Shanghai,
China). low density lipoprotein-cholesterol (LDL-C) and high
density lipoprotein-cholesterol (HDL-C) levels were detected by a
direct enzyme method using reagents provided by Beijing Jiuqiang
Biological Ltd. (Beijing, China) according to the manufacturer’s
instructions.
Statistical analysis
Epidemiological data were collected and analyzed
against SNP and laboratory data using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). The baseline characteristics of participants
were presented as the mean ± SD or as percentages of the study
population. The differences in normal variables were analyzed using
the Student’s t-test (for categorical variables) and chi-square
test or Fisher’s exact test (for continuous variables). The
Hardy-Weinberg equilibrium was assessed by the chi-square test.
Genotype and allele frequencies for polymorphisms were calculated
through direct counting. The chi-square test was used for genotype
distributions and allele frequencies comparison. The impact of
ABCG5/8 variants was analyzed in a dominant genetic model
(heterozygous and homozygous for minor allele carriers were
combined and compared with carriers of the common variant). To
assess differences in plasma cholesterol concentrations among
genotypes, the Chi-square test was performed. A multiple logistic
regression analysis was used to evaluate the impact of ABCG5/8 gene
polymorphisms on CHD and adjusted for significant risk factors by
using an analysis of covariance. The results are expressed as odds
ratios (OR) and 95% CI. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of the study
population
The characteristics of all the participants are
shown in Table I. Among these
variables, there were significant differences in age (P<0.05),
tobacco smoking (P<0.05), HDL-C (P<0.05) and diabetes
(P<0.01) between CHD patients and the healthy controls. However,
no significant difference was observed for gender, alcohol
consumption, hypertension status or levels of total cholesterol
(TC), triglyceride (TG) and LDL-C between the cases and controls
(P>0.05 for all).
 | Table IGeneral characteristics and lipid
levels in controls and CHD patients. |
Table I
General characteristics and lipid
levels in controls and CHD patients.
| Characteristic | Control
(n=417) | Cases (n=267) |
|---|
| Age (years)b | 60.49±12.03 | 64.85±11.23 |
| Female, n (%) | 237 (56.83) | 129 (48.31) |
| Body mass index
(kg/m2) | 21.6±2.9 | 21.8±2.8 |
| Non-smokers, n
(%)a | 261 (62.59) | 126 (47.19) |
| Current smokers, n
(%)a | 141 (33.81) | 132 (49.44) |
| Former smokers, n
(%) | 15 (3.60) | 9 (3.37) |
| Non-alcohol, n
(%) | 336 (80.58) | 187 (70.04) |
| Current alcohol, n
(%) | 72 (17.27) | 73 (27.34) |
| Former alcohol, n
(%) | 9 (2.16) | 6 (2.25) |
| Diabetes, n
(%)b | 48 (11.51) | 74 (27.72) |
| Hypertension, n
(%) | 258 (61.87) | 184 (68.91) |
| TC (mmol/l) | 5.26±1.06 | 5.38±0.98 |
| TG (mmol/l) | 2.18±0.47 | 2.25±0.58 |
| HDL-C
(mmol/l)a | 1.34±0.27 | 1.25±0.30 |
| LDL-C (mmol/l) | 3.27±0.72 | 3.34±0.71 |
Distribution of genotype and allele
frequencies in CHD patients and controls
Genotypic and allelic frequencies of candidate
ABCG5/8 SNPs are shown in Table
III. In particular, the genotype distributions observed for
these four SNPs were consistent with the Hardy-Weinberg equilibrium
(P>0.05). Among the SNPs examined, only the genotypic and
allelic frequencies of ABCG8_Thr400LysC>A were significantly
different (P<0.05) between the CHD patients (CC-83.91%,
CA-13.79% and AA-2.30%; A=9.20%) and the healthy controls
(CC-71.22%, CA-27.34% and AA-1.44%; A=15.10%). By contrast, no
significant differences in genotype frequencies of the remaining
three SNPs were identified between cases and controls (P>0.05
for all).
 | Table IIIGenotype distribution and allele
frequencies of ABCG5/8 polymorphisms. |
Table III
Genotype distribution and allele
frequencies of ABCG5/8 polymorphisms.
| | i7892T>C
(rs4131229) | Tyr54CysA>G
(rs4148211) | Thr400LysC>A
(rs4148217) | 5U145A>C
(rs3806471) |
|---|
| |
|
|
|
|
|---|
| SNP | Control | Case | Control | Case | Control | Case | Control | Case |
|---|
| N | | 417 | 261 | 417 | 261 | 417 | 261 | 417 | 261 |
| Genotype, n
(%) | AA | 294 (70.50) | 186 (71.26) | 288 (69.06) | 207 (79.31) | 297 (71.22) | 219
(83.91)* | 288 (69.06) | 201 (77.01) |
| AB | 114 (27.34) | 72 (27.59) | 120 (28.78) | 48 (18.39) | 114 (27.34) | 36
(13.79)* | 123 (29.50) | 57 (21.84) |
| BB | 9 (2.16) | 3 (1.15) | 9 (2.16) | 6 (2.30) | 6 (1.44) | 6 (2.30) | 6 (1.44) | 3 (1.15) |
| MAF (%) | A | 15.83 | 14.94 | 16.55 | 11.49 | 15.10 | 9.20 | 16.19 | 12.07 |
| N | | 141 | 126 | 141 | 126 | 141 | 126 | 141 | 126 |
| Smokers | AA | 99 (70.21) | 87 (69.05) | 102 (72.34) | 99 (78.57) | 195 (70.65) | 117 (86.67)a | 99 (70.21) | 96 (76.19) |
| AB+BB | 42 (29.79) | 39 (30.95) | 39 (27.66) | 27 (21.43) | 81 (29.35) | 18 (13.33)a | 42 (29.79) | 30 (23.81) |
| | 276 | 135 | 276 | 135 | 276 | 135 | 276 | 135 |
| Non-smokers | AA | 195 (70.65) | 99 (73.33) | 186 (67.39) | 108 (80.00) | 102 (72.34) | 102 (80.95) | 189 (68.48) | 105 (77.78) |
| AB+BB | 81 (29.35) | 36 (26.67) | 90 (32.61) | 27 (20.00) | 39 (27.66) | 24 (19.05) | 87 (31.52) | 30 (22.22) |
Association between ABCG5/8 polymorphism
and susceptibility to CHD following adjusting for established risk
factors
A stepwise multiple regression analysis was used to
identify the genetic and environmental risk factors for CHD
(Table IV). Age (OR=1.033; 95%
[CI]=1.008–1.057; P=0.008), tobacco smoking (OR=1.827; 95%
[CI]=1.056–3.160; P=0.031), and diabetes (OR=2.929; 95%
[CI]=1.452–5.907; P=0.003) were major environmental risk factors
for CHD in the studied population, while the levels of plasma HDL-C
were a potent protective factor (OR=0.265; 95% [CI]=0.084–0.832;
P=0.023) against CHD.
 | Table IVUnivariate analyses of risk factors
for CHD. |
Table IV
Univariate analyses of risk factors
for CHD.
| Age | Gender | Smokers | Drinker | Hypertension | Diabetes | TC (mmol/l) | TG (mmol/l) | HDL-C (mmol/l) | LDL-C (mmol/l) | i7892T>C | Tyr54
CysA>G | Thr400
LysC>A | 5U145 A>C |
|---|
| P-value | 0.008 | 0.270 | 0.031 | 0.090 | 0.232 | 0.003 | 0.397 | 0.594 | 0.023 | 0.453 | 0.903 | 0.093 | 0.032 | 0.196 |
| OR | 1.033 | 1.353 | 1.827 | 1.737 | 1.411 | 2.929 | 0.889 | 0.961 | 0.265 | 0.869 | 1.028 | 1.717 | 2.107 | 1.501 |
| 95% CI | 1.008–1.057 | 0.791–2.316 | 1.056–3.160 | 0.917–3.291 | 0.803–2.482 | 1.452–5.907 | 0.678–1.167 | 0.831–1.112 | 0.084–0.832 | 0.601–1.254 | 0.573–1.852 | 0.913–3.228 | 1.068–4.157 | 0.811–2.777 |
Binary logistic regression analysis of the four
genetic variants studied revealed that CC subjects of the
ABCG8_Thr400LysC>A SNP had a significant risk of developing CHD
following adjusting for established risk factors (adjusted
OR=2.304; 95% [CI]=0.983–4.207; P=0.048; Table V) compared with A allele carriers.
The minor allele carriers of Tyr54CysA>G and 5U145A>C were
also at an increased risk of CHD, however, the association was not
statistically significant (Table
IV).
 | Table VAssociation of ABCG8_ Thr400LysC>A
with CHD risk by subgroup analyses. |
Table V
Association of ABCG8_ Thr400LysC>A
with CHD risk by subgroup analyses.
| Genotype | | All
participants | Tobacco smoke | Non-smokers | Male | Female | Alcohol
drinking | Non-drinkers |
|---|
| Thr400Lys C>A
(allele) | OR (95%CI) | 2.107
(1.068–4.157) | 2.700
(1.024–7.120) | 1.625
(0.597–4.421) | 2.059
(0.774–5.476) | 2.108
(0.816–5.447) | 2.722
(0.612–12.101) | 1.926
(0.895–4.143) |
| P-value | 0.032 | 0.045 | 0.342 | 0.148 | 0.124 | 0.188 | 0.094 |
| Adjusted
log-dominant model | OR (95%CI) | 2.034
(0.983–4.207) | 2.663
(0.973–7.286) | 1.472
(0.467–4.640) | 2.220
(0.746–6.608) | 1.975
(0.717–5.439) | 2.152
(0.359–12.914) | 1.972
(0.873–4.454) |
| P-value | 0.048 | 0.049 | 0.509 | 0.152 | 0.188 | 0.402 | 0.102 |
Interaction between ABCG5/8 polymorphism
and tobacco smoking in the development of CHD
Subgroup analyses of interactions between ABCG5/8
and tobacco smoking were performed and their impacts on the risk of
CHD were assessed. The dominant model analysis demonstrated that
among non-smokers, the genotype frequency of ABCG8_Thr400LysC>A
SNP in CHD patients (CC=86.67%; CA + AA=13.13%) was significantly
different from that in healthy controls (70.65 vs. 29.35; P=0.024;
Table III). However, no
significant differences in gender and alcohol consumption between
cases and controls were identified (data not shown).
Furthermore, a stepwise multiple regression analysis
revealed that CC subjects of the ABCG8_Thr400LysC>A SNP had a
significantly increased risk of CHD in non-smokers (OR=2.663; 95%
CI=0.973–7.286; adjusted P=0.049) compared with the A allele
carriers. The ORs were adjusted by age, gender, history of diabetes
mellitus and HDL-C level. However, no significant interaction
between gender and alcohol consumption leading to a risk of CHD was
identified (Table V).
Association of ABCG5/8 polymorphism with
plasma lipid level in different genotypes
TG levels in carriers of the minor A allele of the
ABCG8_Thr400LysC>A SNP were significantly lower than that of the
homozygous CC (P=0.017; Table
VI). CHD patients carrying the minor A allele of the
ABCG8_Thr400LysC>A SNP had significantly lower baseline TG
levels than CC homozygotes (P<0.05). However, no significant
difference was observed in TC, LDL-C and HDL-C levels among the
remaining genotypes in the two cases and controls (P>0.05).
 | Table VIAssociation of ABCG5/8 polymorphism
with plasma lipid levels in different genotypes. |
Table VI
Association of ABCG5/8 polymorphism
with plasma lipid levels in different genotypes.
| | TC | TG | HDL-C | LDL-C |
|---|
| |
|
|
|
|
|---|
| SNP | Genotype | Control | Case | Control | Case | Control | Case | Control | Case |
|---|
| i7892T>C | TT | 5.224±1.019 | 5.404±0.925 | 2.022±0.457 | 2.372±0.459 | 1.335±0.269 | 1.263±0.330 | 3.281±0.824 | 3.370±0.748 |
| TC+CC | 5.306±1.102 | 5.352±1.029 | 2.195±0.486 | 2.130±0.697 | 1.349±0.267 | 1.239±0.271 | 3.260±0.615 | 3.311±0.665 |
| Tyr54CysA>G | AA | 5.271±1.050 | 5.388±0.922 | 2.129±0.462 | 2.302±0.576 | 1.322±0.279 | 1.241±0.328 | 3.268±0.772 | 3.351±0.747 |
| AG+GG | 5.259±1.071 | 5.368±1.032 | 2.088±0.481 | 2.200±0.580 | 1.362±0.257 | 1.261±0.273 | 3.273±0.667 | 3.330±0.666 |
|
Thr400LysC>A | CC | 5.280±1.048 | 5.432±1.032 | 2.139±0.464 |
2.534±0.460* | 1.336±0.278 | 1.263±0.320 | 3.287±0.734 | 3.361±0.735 |
| CA+AA | 5.250±1.073 | 5.324±0.922 | 2.078±0.479 | 1.968±0.696 | 1.348±0.258 | 1.239±0.281 | 3.254±0.705 | 3.320±0.678 |
| 5U145A>C | AA | 5.210±0.998 | 5.390±0.927 | 1.992±0.439 | 2.330±0.512 | 1.349±0.276 | 1.248±0.321 | 3.291±0.797 | 3.335±0.738 |
| AC+CC | 5.320±1.123 | 5.366±1.027 | 2.225±0.504 | 2.172±0.644 | 1.335±0.260 | 1.254±0.280 | 3.250±0.642 | 3.346±0.675 |
| Smokers |
|
Thr400LysC>A | CC | 5.293±1.173 | 5.382±1.028 | 2.300±0.515 | 2.712±0.586a | 1.307±0.182 | 1.231±0.394 | 3.279±0.864 | 3.447±0.634 |
| CA+AA | 5.214±0.992 | 5.246±1.025 | 1.934±0.486 | 1.998±0.575 | 1.324±0.262 | 1.225±0.276 | 3.357±0.519 | 3.411±0.665 |
| Non-smokers |
|
Thr400LysC>A | CC | 5.281±1.081 | 5.361±1.023 | 2.208±0.452 | 2.256±0.663 | 1.378±0.383 | 1.288±0.280 | 3.241±0.793 | 3.274±0.666 |
| CA+AA | 5.132±0.996 | 5.491±0.832 | 1.992±0.433 | 2.038±0.488 | 1.359±0.245 | 1.260±0.252 | 3.206±0.702 | 3.230±0.861 |
In order to determine the effect of the interaction
between ABCG5/8 and tobacco smoking on lipid levels, further
subgroup analyses were performed. The data demonstrated that in
tobacco smokers, TG levels in carriers of the minor A allele of the
ABCG8_Thr400LysC>A SNP were significantly lower than in CC
homozygotes (P<0.05). However, no significant differences in
lipid concentrations between CHD subjects and controls in
non-smokers were identified.
Discussion
The present study assessed four ABCG5 and
ABCG8 SNPs and serum lipid levels for an association with
the risk of developing CHD or dyslipidemia. The present
case-control study included 417 CHD patients and 267 controls. Our
data demonstrated clear associations between variants of ABCG5/8
and serum lipid levels and the risk of CHD in the northern Han
Chinese population. However, in these four candidate SNPs, only the
genotype and allele frequencies of ABCG8_T400K SNP were
significantly different between the CHD and healthy control groups.
TG levels in the CC homozygotes were significantly greater in CHD
patients than in healthy controls. Binary logistic regression
analysis demonstrated that the homozygous C allele at
Thr400LysC>A resulted in a more than two-fold greater risk of
developing CHD compared with those who carried the A allele in a
dominant model. Multivariate analysis supported that such an effect
was independent of several other risk factors, including age,
gender, history of diabetes mellitus and HDL-C level. Furthermore,
tobacco smoking CC homozygotes at Thr400LysC>A had a 2.7-fold
higher risk for CHD than either the CA or AA genotype. The current
study did not provide evidence of an interaction between genotype
and alcohol consumption in terms of CHD risk, and also did not find
an association between genotype and gender of the subjects as
previous studies have reported in other ethnicities (16,24).
However, a future study with a larger sample size is required to
verify our current findings prior to using the information for CHD
prevention in high-risk populations in a clinical setting.
At the cell biology level, ABCG5 and ABCG8 proteins
are hemi-transporters that limit intestinal absorption, however,
promote biliary excretion of sterols (8), which are localized on human
chromosome 2p21, and are tandemly arrayed in a head-to-head
orientation separated by 374 bp. The two proteins have 13 exons and
12 introns and span ~28 kb DNA sequences (10). To date, several SNPs have been
identified in ABCG5 and ABCG8 (10,25).
In the current study, rs4131229 for ABCG5 and rs4148211, rs4148217
and rs3806471 for ABCG8 were selected. However, rs11887534 and
rs6544718 were not included in the present study as they were rare
variants in the Chinese population (i.e. minor allele frequency
<1%) (26). Several previous
studies demonstrated that the ABCG8 Thr400LysC>A SNP may have an
ethnic specificity (15,27–30).
In the present study, the genotype distribution of these four
variants in Northern Han Chinese participants deviated slightly
from expectations of the Hardy-Weinberg equilibrium (P>0.05).
For example, frequencies of CC, CA and AA at ABCG8_Thr400LysC>A
SNP were 83.91, 13.79 and 2.30%, respectively in the CHD group,
while they were 71.22, 27.34 and 1.44%, respectively in the healthy
controls, which were similar to a previous study on Chinese
patients with gallstones (CC, CA and AA genotypes were 83.1, 16.4
and 0.5%, respectively) (31). The
frequencies observed in the present study were different from those
in the Boston Puerto Rican Health cohort (27) (frequencies of CC and CA/AA
genotypes were 60.0 and 40.0%, respectively), a German study
(15) on gallstones (frequencies
of CC and CA/AA genotypes were 58.3% and 41.7%, respectively) and a
Czech study (18) (frequencies of
CC, CA and AA genotypes were 65.4, 31.3 and 3.3%, respectively).
These genetic diversities among various ethnicities may result from
the consumption of different food, dietary history and other
environmental factors, indicating gene-environment interactions
that alter human gene activities and the risk of disease
development. Thus, genetic diversity may also produce potentially
contradictory polymorphisms between genes and disease risk in
different ethnic groups.
In the current study in a northern Han Chinese
population, the homozygous CC ABCG8 Thr400LysC>A SNP carriers
exhibited significantly higher TG levels than the A allele
carriers. This conclusion corroborates the findings of a previous
study, which demonstrated that females with the A allele had lower
plasma TG than C homozygotes (31). Several other studies have also
demonstrated a significant association between baseline TG levels
and the p.T400K polymorphism (16,32,33).
Notably, several association studies have also demonstrated that
there is no significant association between the T400K polymorphism
and plasma levels of TG (28,29,34,35).
A study of siblings with gallstones (36) demonstrated that male ABCG8 CC
homozygotes had greater decreases in TG and LDL-C levels than CA/AA
carriers following dietary alteration. Furthermore, neither ABCG8
T400K nor D19H polymorphisms are independently associated with the
risk of CHD in a cohort containing 2,012 heterozygous FH patients
(37). Another study suggests that
ABCG5/G8 polymorphisms are not associated with the risk of CHD
(38). An additional study
suggests that this locus affects risk of CHD either directly
through its effect on plasma phytosterol levels or through
primary/secondary changes in LDL-cholesterol (39). Notably, ABCG8 alleles at rs41360247
are associated with reduced phytosterol levels, thus indicating
further association with reduced risk of CHD (40). However, to date, to the best of our
knowledge, there has been no study that linked these SNPs to CHD in
the Han Chinese population. In the current study, ABCG8 CC
homozygotes had >two-fold risk of CHD compared with A allele
carriers, which may imply that ABCG8 T400K variants are a novel
gene marker for CHD risk in the northern Han Chinese population.
Indeed, the T400K variant is a missense mutation that changes the
amino acid residue at position 400 from arginine to histidine in
ABCG8. Nationally representative surveys in China indicate that the
long-term nutritional trend of Chinese has shifted towards a
Westernized lifestyle, i.e. a high-fat, high-energy and low-fiber
diet. Several studies have indicated that dietary constituents
perturb cholesterol homeostasis and consequently affect expression
of hepatic transporters. Diets rich in cholesterol and cholic acid
reportedly enhance the expression of ABCG5/8 in the liver (41,42).
Hubacek et al (18)
suggested that the Thr400 allele may be a ‘diet-responsive’ allele
and a long-term change in dietary composition may affect the
expression of ABCG5/8 genes. Thus, it may be expected that the
expression of the ABCG5/8 gene, in C allele carriers at Thr400Lys,
is able to increase markedly while consuming a high fat diet to
limit cholesterol absorption but promote hepatic secretion.
Cigarette smoke is an established risk factor for
developing CHD and is considered as one of the major risk factors
for the development of atherosclerosis disease (43). A previous study suggested that
smokers who are carriers of the ABCG8 Thr400 allele exhibit lower
HDL-C concentrations and thereby have a potential increased risk
for atherosclerosis (37), while
smokers who carry the minor alleles at ABCG5 (i7892A>G,
i18429C>T or i11836G>A) SNPs exhibit significantly lower
HDL-C, higher TC and higher TG, respectively. In the current study,
the dominant model analysis demonstrated that tobacco smokers,
CC-homozygous at the ABCG8_Thr400LysC>A SNP, had increased TG
levels and risk of CHD compared with CA/AA tobacco smokers. The
present study hypothesized that tobacco smoke may lower the
transcription of ABCG5/8 and lead to intracellular cholesterol
accumulation, development of CHD and premature atherosclerosis.
However, the current study had a relatively small sample size and
did not allow us to conduct haplotype analyses. Further functional
analyses of the variants examined in the present study have not yet
been conducted. Additionally, further study is required in order to
elucidate the mechanisms of ABCG5/8 variants on CHD development in
the Han Chinese population.
Acknowledgements
This study was funded by the Science and Technology
Department of Jilin Province (no. 20130102087JC).
References
|
1
|
Deloukas P, Kanoni S, Willenborg C, et al:
Large-scale association analysis identifies new risk loci for
coronary artery disease. Nat Genet. 45:25–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schunkert H, König IR, Kathiresan S, et
al: Large-scale association analysis identifies 13 new
susceptibility loci for coronary artery disease. Nat Genet.
43:333–338. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
The IBC 50K CAD Consortium. Large-scale
gene-centric analysis identifies novel variants for coronary artery
disease. PLoS Genet. 7:e10022602011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sampietro ML, Trompet S, Verschuren JJ, et
al: A genome-wide association study identifies a region at
chromosome 12 as a potential susceptibility locus for restenosis
after percutaneous coronary intervention. Hum Mol Genet.
20:4748–4757. 2011. View Article : Google Scholar
|
|
5
|
Cheng X, Shi L, Nie S, et al: The same
chromosome 9p21.3 locus is associated with type 2 diabetes and
coronary artery disease in a Chinese Han population. Diabetes.
60:680–684. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou L, Zhang X, He M, Cheng L, Chen Y, Hu
FB and Wu T: Associations between single nucleotide polymorphisms
on chromosome 9p21 and risk of coronary heart disease in Chinese
Han population. Arterioscler Thromb Vasc Biol. 28:2085–2089. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang W, Peng WH, Lu L, et al: Polymorphism
on chromosome 9p21.3 contributes to early-onset and severity of
coronary artery disease in non-diabetic and type 2 diabetic
patients. Chin Med J (Engl). 124:66–71. 2011.PubMed/NCBI
|
|
8
|
Yu L, Li-Hawkins J, Hammer RE, Berge KE,
Horton JD, Cohen JC and Hobbs HH: Overexpression of ABCG5 and ABCG8
promotes biliary cholesterol secretion and reduces fractional
absorption of dietary cholesterol. J Clin Invest. 110:671–680.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Repa JJ, Berge KE, Pomajzl C, Richardson
JA, Hobbs H and Mangelsdorf DJ: Regulation of ATP-binding cassette
sterol transporters ABCG5 and ABCG8 by the liver X receptors α and
β. J Biol Chem. 277:18793–18880. 2002.
|
|
10
|
Berge KE, Tian H, Graf GA, et al:
Accumulation of dietary cholesterol in sitosterolemia caused by
mutations in adjacent ABC transporters. Science. 290:1771–1775.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hubacek JA, Berge KE, Cohen JC and Hobbs
HH: Mutations in ATP-cassette binding proteins G5 (ABCG5) and G8
(ABCG8) causing sitosterolemia. Hum Mutat. 18:359–360. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pandit B, Ahn GS, Hazard SE, Gordon D and
Patel SB: A detailed Hapmap of the Sitosterolemia locus spanning 69
kb; differences between Caucasians and African-Americans. BMC Med
Genet. 7:132006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berge KE, von Bergmann K, Lutjohann D, et
al: Heritability of plasma noncholesterol sterols and relationship
to DNA sequence polymorphism in ABCG5 and ABCG8. J Lipid Res.
43:486–494. 2002.PubMed/NCBI
|
|
14
|
Koeijvoets KC, van der Net JB,
Dallinga-Thie GM, et al: ABCG8 gene polymorphisms, plasma
cholesterol concentrations, and risk of cardiovascular disease in
familial hypercholesterolemia. Atherosclerosis. 204:453–458. 2009.
View Article : Google Scholar
|
|
15
|
Acalovschi M, Ciocan A, Mostean O, et al:
Are plasma lipid levels related to ABCG5/ABCG8 polymorphisms? A
preliminary study in siblings with gallstones. Eur J Intern Med.
17:490–494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gylling H, Hallikainen M, Pihlajamäki J,
et al: Polymorphisms in the ABCG5 and ABCG8 genes associate with
cholesterol absorption and insulin sensitivity. J Lipid Res.
45:1660–1665. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caamaño JM, Pacheco A, Lanas F and Salazar
LA: Single nucleotide polymorphisms in ABCG5 and ABCG8 genes in
Chilean subjects with polygenic hypercholesterolemia and controls.
Clin Chem Lab Med. 46:1581–1585. 2008.PubMed/NCBI
|
|
18
|
Hubácek JA, Berge KE, Stefková J, Pitha J,
Skodová Z, Lanska V and Poledne R: Polymorphisms in ABCG5 and ABCG8
transporters and plasma cholesterol levels. Physiol Res.
53:395–401. 2004.PubMed/NCBI
|
|
19
|
Chen ZC, Shin SJ, Kuo KK, Lin KD, Yu ML
and Hsiao PJ: Significant association of ABCG8:D19H gene
polymorphism with hypercholesterolemia and insulin resistance. J
Hum Genet. 53:757–763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Jiang ZY, Fei J, et al: ATP
binding cassette G8 T400K polymorphism may affect the risk of
gallstone disease among Chinese males. Clin Chim Acta. 384:80–85.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rudkowska I and Jones PJ: Polymorphisms in
ABCG5/G8 transporters linked to hypercholesterolemia and gallstone
disease. Nutr Rev. 66:343–348. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamazaki Y, Hashizume T, Morioka H,
Sadamitsu S, Ikari A, Miwa M and Sugatani J: Diet-induced lipid
accumulation in liver enhances ATP-binding cassette transporter
g5/g8 expression in bile canaliculi. Drug Metab Pharmacokinet.
26:442–450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wilund KR, Yu L, Xu F, Hobbs HH and Cohen
JC: High-level expression of ABCG5 and ABCG8 attenuates
diet-induced hypercholesterolemia and atherosclerosis in Ldlr−/−
mice. J Lipid Res. 45:1429–1436. 2004.PubMed/NCBI
|
|
24
|
Santosa S, Demonty I, Lichtenstein AH, et
al: Single nucleotide polymorphisms in ABCG5 and ABCG8 are
associated with changes in cholesterol metabolism during weight
loss. J Lipid Res. 48:2607–2613. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee MH, Lu K, Hazard S, et al:
Identification of a gene, ABCG5, important in the regulation of
dietary cholesterol absorption. Nat Genet. 27:79–83. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei KK, Zhang LR, Zhang Y and Hu XJ:
Interactions between CYP7A1 A-204C and ABCG8 C1199A polymorphisms
on lipid lowering with atorvastatin. J Clin Pharm Ther. 36:725–733.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Junyent M, Tucker KL, Smith CE, et al: The
effects of ABCG5/G8 polymorphisms on plasma HDL cholesterol
concentrations depend on smoking habit in the Boston Puerto Rican
Health Study. J Lipid Res. 50:565–573. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Szilvási A, Andrikovics H, Pongrácz E,
Kalina A, Komlósi Z, Klein I and Tordai A: Frequencies of four
ATP-binding cassette transporter G8 polymorphisms in patients with
ischemic vascular diseases. Genet Test Mol Biomarkers. 14:667–672.
2010.PubMed/NCBI
|
|
29
|
Kajinami K, Brousseau ME, Ordovas JM and
Schaefer EJ: Interactions between common genetic polymorphisms in
ABCG5/G8 and CYP7A1 on LDL cholesterol-lowering response to
atorvastatin. Atherosclerosis. 175:287–293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Plat J, Bragt MC and Mensink RP: Common
sequence variations in ABCG8 are related to plant sterol metabolism
in healthy volunteers. J Lipid Res. 46:68–75. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Q, Wei XL and Yin RX: Association of
ATP binding cassette transporter G8 rs4148217 SNP and serum lipid
levels in Mulao and Han nationalities. Lipids Health Dis.
11:462012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Jiang ZY, Fei J, et al: ATP
binding cassette G8 T400K polymorphism may affect the risk of
gallstone disease among Chinese males. Clin Chim Acta. 384:80–85.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chan DC, Watts GF, Barrett PH, Whitfield
AJ and van Bockxmeer FM: ATP-binding cassette transporter G8 gene
as a determinant of apolipoprotein B-100 kinetics in overweight
men. Arterioscler Thromb Vasc Biol. 24:2188–2191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jakulj L, Vissers MN, Tanck MW, Hutten BA,
Stellaard F, Kastelein J and Dallinga-Thie GM: ABCG5/G8
polymorphisms and markers of cholesterol metabolism: systematic
review and meta-analysis. J Lipid Res. 51:3016–3023. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kajinami K, Brousseau ME, Nartsupha C,
Ordovas JM and Schaefer EJ: ATP binding cassette transporter G5 and
G8 genotypes and plasma lipoprotein levels before and after
treatment with atorvastatin. J Lipid Res. 45:653–656. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Acalovschi M, Ciocan A, Mostean O, et al:
Are plasma lipid levels related to ABCG5/ABCG8 polymorphisms? A
preliminary study in siblings with gallstones. Eur J Intern Med.
17:490–494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Garcia-Rios A, Perez-Martinez P, Fuentes
F, et al: Genetic variations at ABCG5/G8 genes modulate plasma
lipids concentrations in patients with familial
hypercholesterolemia. Atherosclerosis. 210:486–492. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gylling H, Hallikainen M, Rajaratnam RA,
Simonen P, Pihlajamäki J, Laakso M and Miettinen TA: The metabolism
of plant sterols is disturbed in postmenopausal women with coronary
artery disease. Metabolism. 58:401–407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ordovas JM and Tai ES: The babel of the
ABCs: novel transporters involved in the regulation of sterol
absorption and excretion. Nutr Rev. 60:30–33. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Teupser D, Baber R, Ceglarek U, et al:
Genetic regulation of serum phytosterol levels and risk of coronary
artery disease. Circ Cardiovasc Genet. 3:331–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chan J, Donalson LM, Kushwaha RS,
Ferdinandusse S, VandeBerg JF and VandeBerg JL: Differential
expression of hepatic genes involved in cholesterol homeostasis in
high- and low-responding strains of laboratory opossums.
Metabolism. 57:718–724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Orolin J, Vecera R, Markova I, Zacharova A
and Anzenbacher P: Differences in hepatic expression of genes
involved in lipid homeostasis between hereditary
hypertriglyceridemic rats and healthy Wistar rats and in their
response to dietary cholesterol. Food Chem Toxicol. 47:2624–2630.
2009. View Article : Google Scholar
|
|
43
|
Ma W, Xu J, Wang Q, et al: Interaction of
functional NPC1 gene polymorphism with smoking on coronary heart
disease. BMC Med Genet. 11:1492010. View Article : Google Scholar : PubMed/NCBI
|