Introduction
Neuroblastoma is the most common type of
extracranial solid cancer in children. It is a neuroendocrine
tumor, that may arise from any neural crest element of the
sympathetic nervous system (1). It
comprises 6–10% of all childhood cancers, and 15% of cancer-related
mortalities in children. The annual mortality rates are 10 per
million children aged 0–4 years and 4 per million children aged 4–9
years (2). Although there has been
an improvement in the treatment methods, the mortality rate of
neuroblastoma remains extremely high. The most frequently mutated
gene among all genes known to be involved in human cancer,
including human neuroblastoma, is the tumor suppressor p53
(3). p21WAF, a
cyclin-dependent kinase inhibitor, is known to be the principle
mediator of p53 in the apoptotic pathway and has been shown inhibit
the cyclin-dependent kinase p53R2 (4–6) and
several mitochondrial proteins, including Bax, Noxa and mouse
double minute 2 homolog (7–9). The
products of these genes have diverse functions, including
cell-cycle arrest, apoptosis, DNA repair, angiogenesis and
transcription. Apoptosis is hypothesized to be vital in preventing
cells from undergoing malignant transformation by eliminating
damaged cells (10). A number of
studies have indicated that the mitochondrial and the
death-receptor apoptotic pathways are significant in this process
(7,8,10).
However, identification of a number of p53-regulated genes clearly
indicated that there are numerous p53-regulated apoptotic genes
that are not involved in these two pathways, implying that the
mechanism for p53-dependent apoptosis remains unclear.
Unc5B is one of four related receptors for netrin-1
(Unc5A, Unc5B, Unc5C and Unc5D). Notably, Unc5B was directly
regulated by p53 (11), providing
a novel perspective on the role of the p53-regulatory system in
apoptosis. In the present study, Unc5D was demonstrated to be a
direct target of the p53 response to DNA damage and possibly form a
type of feedback to induce p53-dependent apoptosis by
phosphorylation of serin-15.
Materials and methods
Cell culture and transfection
SK-N-BE (p53−/−) and SH-SY5Y
(p53+/+) human neuroblastoma cells, and H1299
(p53−/−) human lung carcinoma cells were maintained in
RPMI-1640 medium containing 10% heat-inactivated fetal bovine serum
(Invitrogen Life Technologies, Carlsbad, CA, USA) and
penicillin/streptomycin. Cultures were maintained at 37°C in a
water-saturated atmosphere of 5% CO2 in air for
transfection. The pCDNA3.1-p53 plasmid was transfected into H1299
cells in a dose- and time-dependent manner with the indicated
combination expressing plasmid using Lipofectamine™ 2000
transfection reagent according to the manufacturer’s instructions
(Invitrogen Life Technologies).
3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide
solution (MTT) assay
The SH-SY5Y and SK-N-BE cells were seeded in 96-well
culture plates as 5×103/well and were allowed to attach
overnight. The cells were treated with 1 μM of adriamycin (ADR).
Following treatment of 0, 6, 12, 24 and 48 h, 10 μl of a modified
MTT (Dojindo Laboratories, Kumamoto, Japan) was added to the
culture medium and incubated at 37°C for 1 h. The absorbance
readings for each action were carried out at 570 nm using the
microplate reader (Model 450, Bio-Rad Laboratories, Hercules, CA,
USA).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
The total RNA was prepared from cells using an
RNeasy Mini kit (Qiagen, Valencia, CA, USA). The total RNA (2 μg)
was reverse transcribed using random primers and SuperScript II
reverse transcriptase (Invitrogen Life Technologies). The resultant
cDNA was subjected to PCR-based amplification. The oligonucleotide
primers used were as follows: Forward: 5′-GGGACACTGCCTCATTTCAT-3′
and reverse: 5′-CATGGAAGTCCTCCACCTGT-3′ for Unc5D; forward:
5′-ATTTGATGCTGTCCCCGGACGATATTGAAC-3′ and reverse:
5′-ACCCTTTTTGGACTTCAGGTGGCTGGAGTG-3′ for p53; forward:
5′-ATGAAATTCACCCCCTTTCC-3′ and reverse: 5′-CCCTAGGCTGTGCTCACTTC-3′
for P21WAF1; forward: 5′-AGGTGGACCTGTTTCGTGAC-3′ and reverse:
5′-ACCCTGTGATCCACCAGAAG-3′ for Bax; forward:
5′-ACCTGACCTGCCGTCTAGAA-3′ and reverse: 5′-TCCACCACCCTGTTGCTGTA-3′
for GAPDH. The PCR products were separated on 2% gel
electrophoresis and visualized by ethidium bromide staining.
Western blot analysis
Cells were extracted directly with lysis buffer
containing 25 mM Tris-HCl, pH 8.0, 137 mM NaCl, 2.7 mM KCl, 1%
Triton X-100, 1 mM PMSF and protease inhibitor mixture (Sigma, St.
Louis, MO, USA). The total protein concentrations were determined
using the Bradford protein assay according to the manufacturer’s
instructions (Bio-Rad Laboratories). Equal quantities of protein
(30–50 μg) were boiled for 5 min in an SDS sample buffer containing
62.5 mM Tris-HCl, pH 6.8, 2% SDS, 2% β-mercaptoethanol and 0.01%
bromophenol blue. Whole cell lysates were separated on 10% SDS-PAGE
and transferred onto a polyvinylidene difluoride membrane
(Millipore, Billerica, MA, USA). The membrane was blocked with
Tris-buffered saline [50 mM Tris-HCl, (pH 8.0), 100 mM NaCl and
0.1% Tween-20] containing 5% non-fat dried milk, and then probed
with the monoclonal anti-p53 (Clone Pab1801), monoclonal
anti-phosphorylation (Ser-15) p53, goat anti-human Unc5D (R&D
Systems, Minneapolis, MN, USA) or with polyclonal anti-actin
(Sigma) antibody. The immunoreactive bands were visualized using
horseradish peroxidase-conjugated anti-mouse, anti-rabbit or
anti-goat immunoglobulin G antibodies (Jackson ImmunoResearch
Laboratories, West Grove, PA, USA) and enhanced chemiluminescence
(Amersham Biosciences, Piscataway, NJ, USA). In order to detect the
endogenous Unc5D protein, the collected cells were lysed directly
in 5X SDS sample buffer [125 mM Tris-HCl, (pH 6.8), 4% SDS, 20%
glycerol, 10% β-mercaptoethanol and 0.4 mg/ml bromophenol
blue].
Construction of luciferase reporter
plasmids
The indicated luciferase reporter constructs driven
by putative p53-responsive elements of the Unc5D gene were
generated using the following primers: Forward:
5′-GAGCTCATGTTGGCCAGGCTAGTC-3′ and reverse:
5′-GTGCTCACAGGGCAATGACTCACCTC-3′ for RE1; and forward:
5′-GGTACCTCACCTCTGAACGTTAAC-3′ and reverse:
5′-GGTACCTAAAGGGACTAGATCATG-3′ for RE2. The resultant PCR products
were gel-purified and inserted into appropriate restriction sites
of the pGL3-promoter plasmid (Promega Corporation, Madison, WI,
USA), to generate p53-RE1 and p53-RE2, and the constructs were
verified by DNA sequencing.
Luciferase reporter assay
The H1299 cells, which contain deficient p53, were
seeded into 12-well cell culture plates. Following overnight
culture, the cells were transiently co-transfected with 100 ng
pGL3-promoter plasmid (Promega Corporation), p53-RE1 or p53-RE2 and
10 ng plasmids. The total quantity of DNA was kept constant (510
ng) with pcDNA3. At 48 h after transfection, the cells were lysed
and their luciferase activity was measured using the
Dual-Luciferase Assay system (Promega Corporation).
Establishment of Unc5D stable clones in
H1299 cells
The H1299 cells were transfected with empty plasmid
pcDNA3.1 or with the expression plasmid of pcDNA3.1-Unc5D. At 48 h
following transfection, the cells were transfected into the fresh
medium containing G418 (Sigma) at a final concentration of 800
μg/ml and incubated for two weeks. Next, G418-resistant clones were
selected and cultured in the presence of G418 (500 μg/ml). A total
of two mock and two stable clones were selected for future
experiments.
Statistical analysis
The data are expressed as the mean ± standard
deviation. A statistical analysis was performed using Student’s
t-test and P<0.05 was considered to indicate a statistically
significant difference.
Results
Induction of endogenous Unc5D by DNA
damage in a p53-dependent manner
In order to examine the effect of Unc5D for cell
survival, two neuroblastoma cell lines SH-SY5Y (that contains
wild-type p53) and SK-N-BE (which has deficient p53), were tested
for cell viability following ADR treatment. SH-SY5Y cells underwent
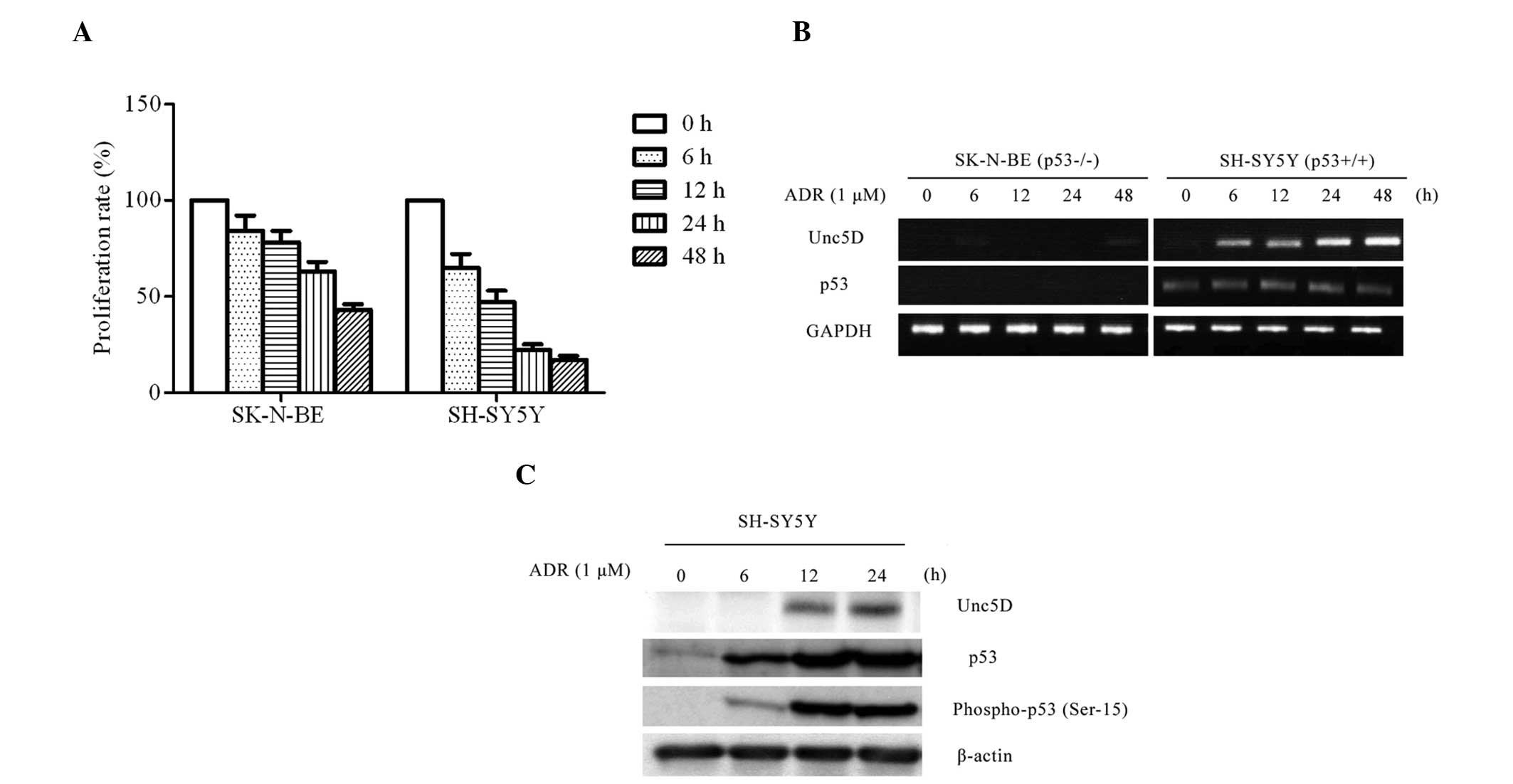
marked cell death compared with SK-N-BE cells (Fig. 1A). DNA damage by ADR treatment
clearly induced transcription of Unc5D in SH-SY5Y but not in
SK-N-BE cells (Fig. 1B and C). The
immunoblot analysis revealed that ADR-mediated apoptosis resulted
in an evident induction of endogenous Unc5D. The results indicate
that Unc5D was induced in ADR-mediated apoptosis in a p53-dependent
manner at the mRNA and protein level, indicating that Unc5D is a
target of p53 and also is involved in the DNA damage response.
Unc5D is a transcriptional target of
p53
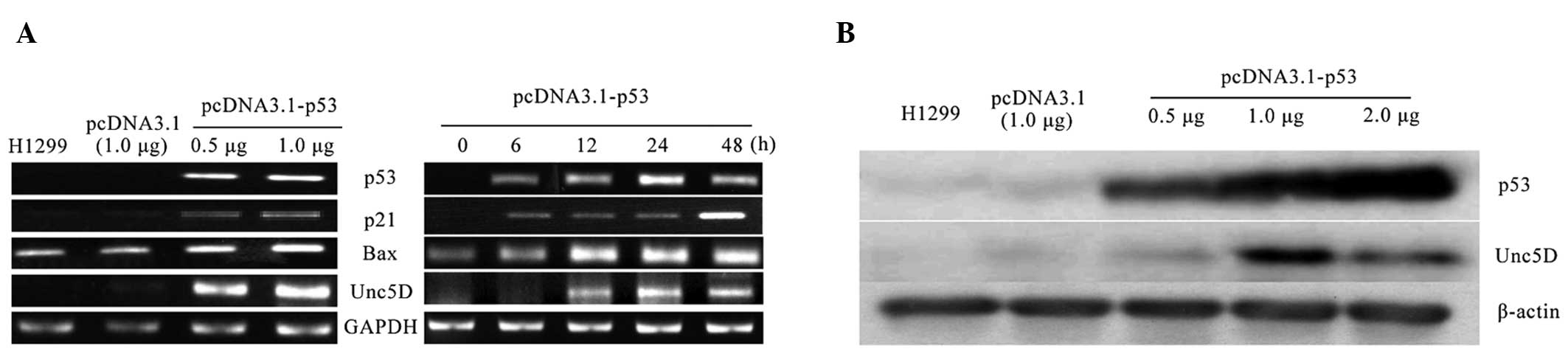
In order to examine whether Unc5D is a
transcriptional target of p53, H1299 cells with deficient p53 were
transfected with an expression plasmid encoding p53. Further
experiments demonstrated that p53 induced the expression of Unc5D,
p21WAF1 and Bax in a time- and dose-dependent manner
(Fig. 2A). Unc5D was induced 12 h
after p53 transfection, and 6 h after p53 expression at the mRNA
level (Fig. 2A). Unc5D protein
expression was induced in a dose-dependent manner following p53
expression (Fig. 2B). Transfection
with the empty plasmid alone did not exhibit a detectable effect on
Unc5D, indicating that Unc5D is a direct transcriptional target of
p53.
p53 enhances the promoter activity of the
Unc5D gene
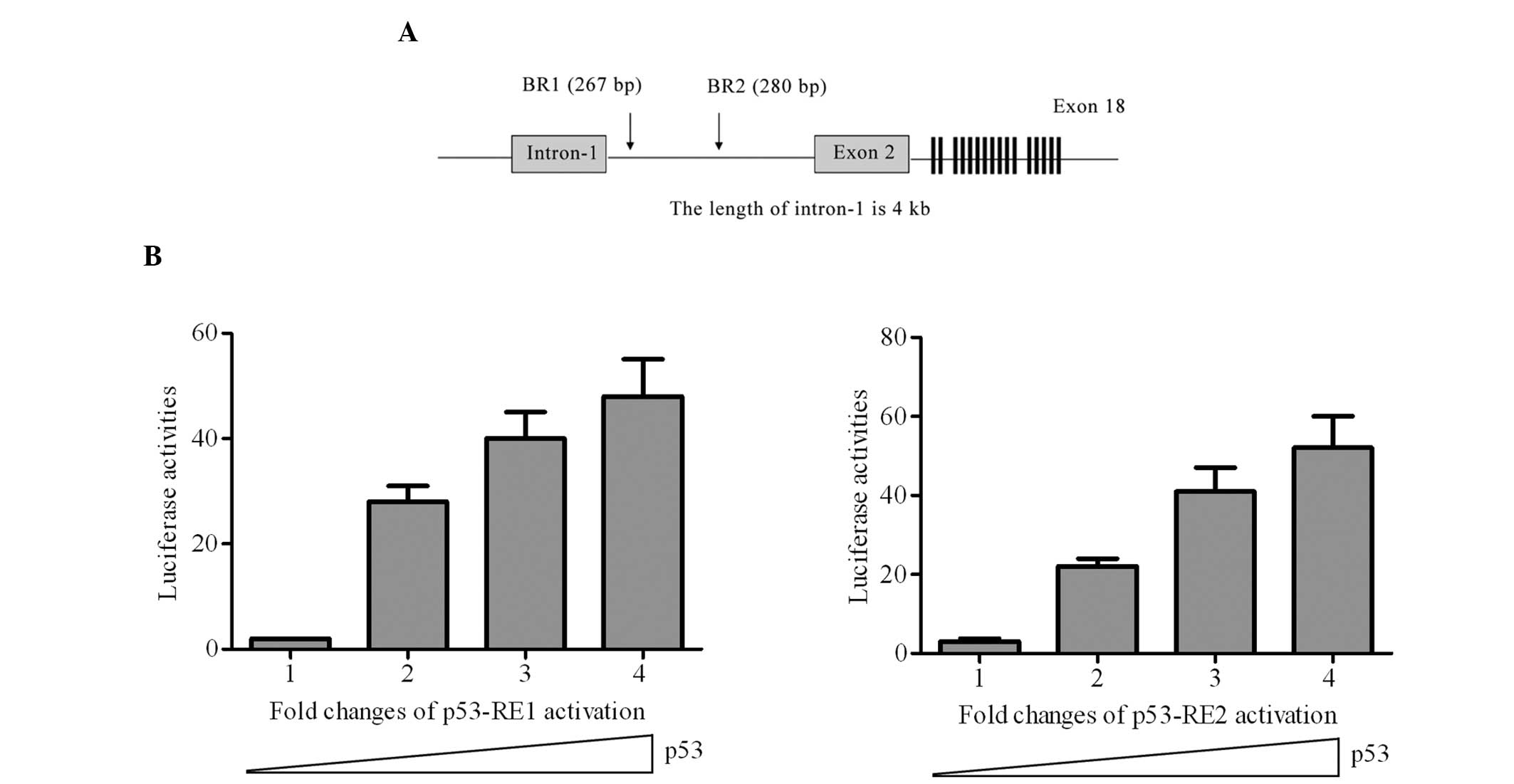
As Unc5D was induced in the DNA damage response in a
p53-dependent manner, it was important to further evaluate the
transcription-enhancing activity of the possible p53-responsive
elements on the Unc5D gene. The genomic structure of Unc5D and
exon-intron organization was presented (Fig. 3A) and two candidate p53-responsive
elements were identified as p53-RE1 and p53-RE2. Cotransfection of
p53-RE1 or p53-RE2 with the wide-type p53 expression plasmid
significantly increased the luciferase activity compared with the
control (Fig. 3B). Therefore, the
p53 responsive elements identified in the present study led to the
conclusion that Unc5D is a direct target of p53.
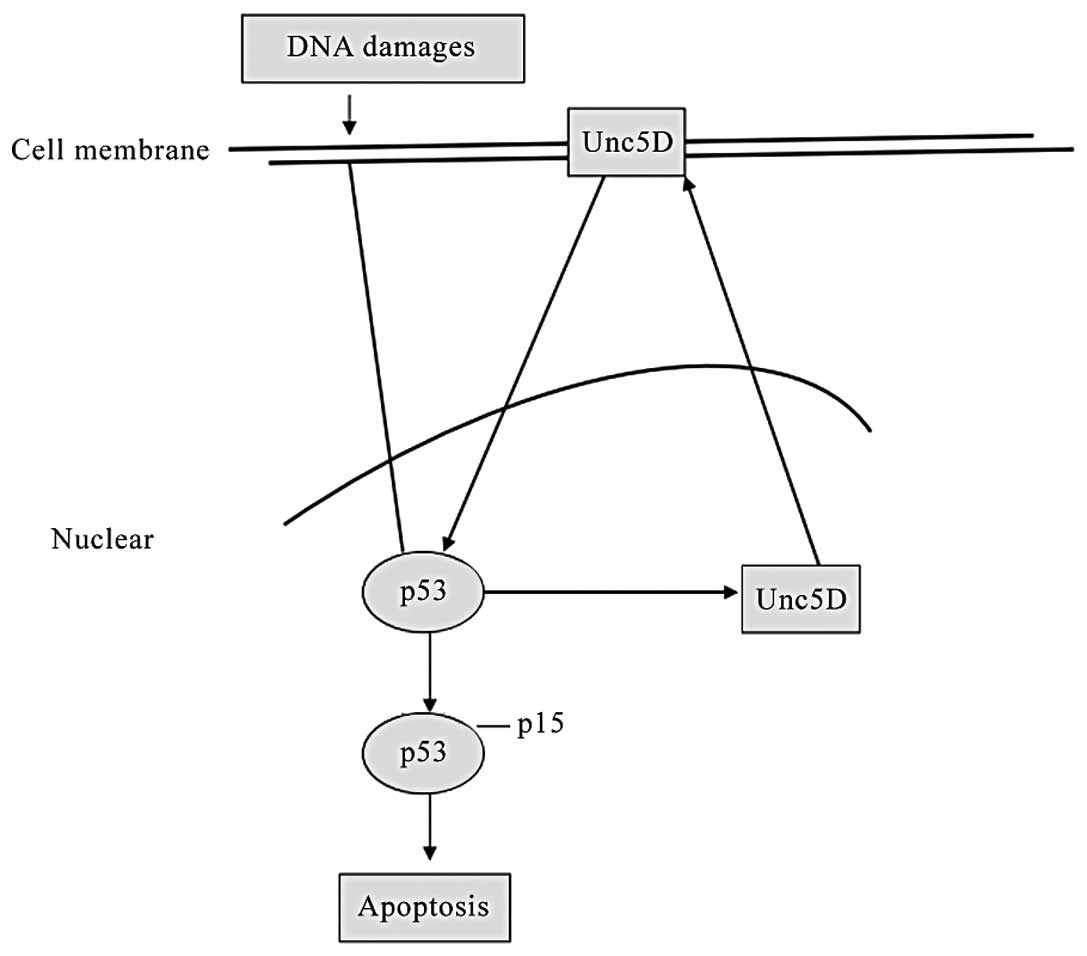
Unc5D is a direct target of p53 and forms
a type of feedback to induce apoptosis with DNA damage signal
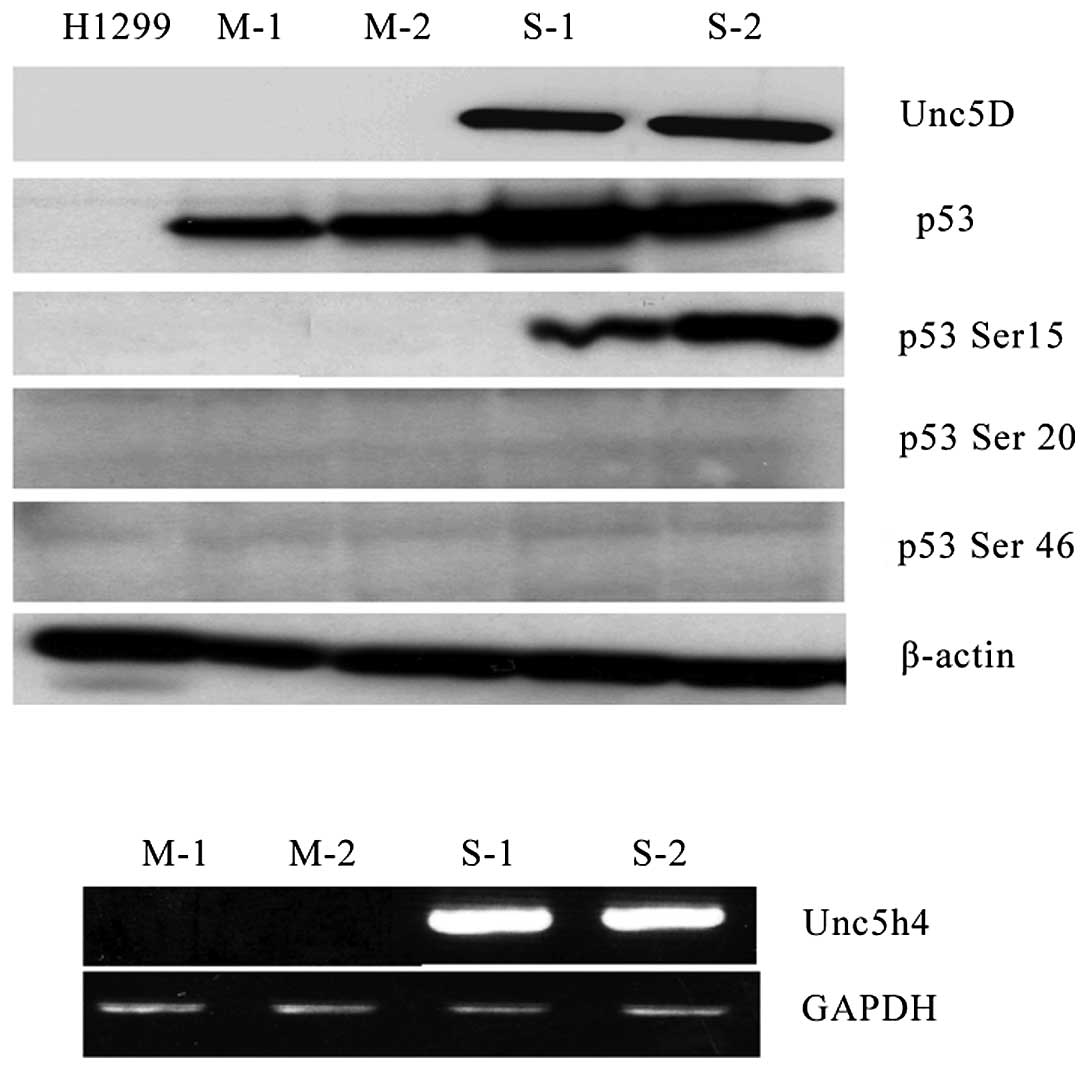
In the present study, Unc5D induction was observed
in SH-SY5Y cells following ADR treatment with an evident
accumulation of p53, phosphorylated at ser-15 (Fig. 1C). Furthermore, Unc5D
stable-expressing H1299 cells were established. A total of two
stable and two mock cell clones were used to detect phosphorylation
at several different sites on p53 following DNA damage by ADR.
Antibodies that recognized p53 phosphorylation at ser-15, ser-20
and ser-46 were used to detect modified p53 subsequent to ADR
treatment and only ser-15 was clearly detectable in Unc5D stable
clones compared with that in the mock stable clones, of which the
other sites were undetectable (Fig.
4). These data reveal that Unc5D is a direct target of p53
following DNA damage, and forms a type of positive feedback to
activate p53 by phosphorylation at serine 15 for p53-dependent
apoptosis (Fig. 5).
Discussion
The present study demonstrated that Unc5D was a
transcriptional target of p53, and induced apoptosis even in
aggressive neuroblastoma dependent on the normal p53 status. Unc5D
is a member of the Unc5s family, which are the netrin-1 receptors
Unc5A, Unc5B, Unc5C and Unc5D. Netrin-1 receptors are a type of
transmembrane receptor possibly mediating the chemorepulsive
activity during the development of neural cells (12,13),
but also promoting cell death induction in the absence of netrin-1,
the latter proapoptotic activity depending on the caspase cleavage
of these receptors and the conserved death domain located at the
C-terminus of their intracellular domains (14,15).
Thus they are also termed dependence receptors, which induce cell
survival or death dependent on the presence or absent of netrin-1.
Unc5B was previously shown to be a direct transcriptional target
for the tumor suppressor p53 and to mediate p53 proapoptotic
activity (15). In the present
study it was shown that Unc5D is significantly induced in DNA
damage. Enforced expression of p53 in p53-deficient H1299 cells
also induced endogenous Unc5D expression. In SH-SY5Y neuroblastoma
cells and in SH-SY5Y p53-proficient cells, Unc5D was induced
following adriamycin treatment, following apoptosis in a
p53-dependent manner. However, this did not occur in SK-N-BE
p53-deficient cells. It remains to be determined how the
proapoptotic signal is transmitted from the cell surface to the
cytoplasm or nucleus. It is well known that Unc5A–C is cleaved by
caspase-3. In addition, a caspase inhibitor or point mutation of
the caspase locus can prevent the apoptosis induction (16). However, the specific mechanism
downstream from the cell surface, which triggers p53 activation,
requires further investigation in order to be clarified.
p53 is a tumor suppressor gene and the most frequent
site of genetic alteration found in human cancers. p53 is activated
in response to various cellular stresses. The activated p53 binds
to specific sequences in the apoptotic-target genes and activates
their transcription. Several p53-regulated apoptotic-target genes
have been identified and are divided into two major pathways: The
mitochondrial and death-receptor pathways. Bax, Noxa and p53
upregulated modulator of apoptosis are involved in the
mitochondrial pathway, and Killer/death-inducing receptor 5 and
failed axon connections are involved in the death-receptor pathways
(17). In the present study, Unc5D
is found to be involved in p53-regulated apoptotic pathways,
implying the involvement of dependence receptors in a third
pathway. Notably Unc5D appears to form a type of feedback to induce
p53-dependent apoptosis by transcriptional induction of p53 and
phosphorylation at ser-15.
Unc5 receptors are involved in vasculogenesis and
apoptosis. Unc5B and Unc5D were identified to interact with
high-affinity fibronectin leucine rich transmembrane protein 3
(FLRT3) (18). FLRT3 and Unc5B
functionally interact in modulating cell adhesion during early
Xenopus development, and the effect of Unc5B on adhesion is
mediated by the Rho family GTPase 1. Additionally, it has been
reported that subventricular expressed transcript 1 (Svet1)
contains a high proportion of repetitive sequences and maps in the
first intron of Unc5D. The previously reported ‘SVZ-specific
expression of the Svet1 RNA’ indicates putative involvement of
Unc5D signaling in the multipolar migrating cells (19). Therefore, certain effects observed
in these studies may be due to Svet1, and Svet1 may be upregulated
by p53 alone with Unc5D. Additionally, Unc5s can regulate the
hepatocyte growth factor/methoprene-tolerant (MET) signaling
pathway via an interaction with the intracellular domain of the MET
receptor. The MET receptor has a dual anti-apoptotic and
pro-apoptotic role in different cell types. While no ligand is
bound to MET, the activated MET induces phosphatidylinositol
3-kinase-Akt-dependent signaling leading to the anti-apoptotic
response. When no ligand is bound to MET, the receptor is subjected
to caspase-dependent cleavage leading to the formation of a
pro-apoptotic fragment of MET (20). However, the reason the cells
require redundant functions of the different Unc5 proteins,
requires further investigation and the identification of other
associated proteins in order to elucidate how this transmembrane
receptor exerts its cellular functions.
Neuroblastoma treatment is a clinical challenge.
Although there have been improvements in chemotherapy, radiotherapy
and drug-induced differentiation, even with transplantation, the
long-term survival rate of neuroblastoma remains low. Therefore,
the identification of novel genes is a prospective way for
targeting treatment. Unc5D is a newly identified dependence
receptor for netrin-1, and a direct target of p53. Targeting at the
Unc5D gene and p53-dependent apoptosis may provide a novel strategy
for neuroblastoma treatment.
Acknowledgements
The authors would like to thank Professor Nakagawara
from the Chiba Cancer Center Research Institute, Japan for their
technical assistance. This study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81101528).
References
|
1
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hwang SK, Paek SH, Kim DG, Jeon YK, Chi JG
and Jung HW: Olfactory neuroblastomas: survival rate and prognostic
factor. J Neurooncol. 59:217–226. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okamura S, Arakawa H, Tanaka T, et al:
p53DINP1, a p53-inducible gene, regulates p53-dependent apoptosis.
Mol Cell. 8:85–94. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McKenzie PP, Danks MK, Kriwacki RW and
Harris LC: P21Waf1/Cip1 dysfunction in neuroblastoma: a novel
mechanism of attenuating G0–G1 cell cycle arrest. Cancer Res.
63:3840–3844. 2003.PubMed/NCBI
|
|
5
|
Tanaka H, Arakawa H, Yamaguchi T, et al: A
ribonucleotide reductase gene involved in a p53-dependent
cell-cycle checkpoint for DNA damage. Nature. 404:42–49. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamaguchi T, Matsuda K, Sagiya Y, et al:
p53R2-dependent pathway for DNA synthesis in a p53-regulated cell
cycle checkpoint. Cancer Res. 61:8256–8262. 2001.PubMed/NCBI
|
|
7
|
Miyashita T and Reed JC: Tumor suppressor
p53 is a direct transcriptional activator of the human bax gene.
Cell. 80:293–299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oda E, Ohki R, Murasawa H, et al: Noxa, a
BH3-only member of the Bcl-2 family and candidate mediator of
p53-induced apoptosis. Science. 288:1053–1058. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barak Y, Juven T, Haffner R and Oren M:
mdm2 expression is induced by wild type p53 activity. EMBO J.
12:461–468. 1993.PubMed/NCBI
|
|
10
|
Vousden KH: p53: death star. Cell.
103:691–694. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanikawa C, Matsuda K, Fukuda S, Nakamura
Y and Arakawa H: p53RDL1 regulates p53-dependent apoptosis. Nat
Cell Biol. 5:216–223. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leonardo ED, Hinck L, Masu M, Keino-Masu
K, Ackerman SL and Tessier-Lavigne M: Vertebrate homologues of
C. elegans UNC-5 are candidate netrin receptors. Nature.
386:833–838. 1997.PubMed/NCBI
|
|
13
|
Hong K, Hinck L, Nishiyama M, Poo MM,
Tessier-Lavigne M and Stein E: A ligand-gated association between
cytoplasmic domains of UNC5 and DCC family receptors converts
netrin-induced growth cone attraction to repulsion. Cell.
97:927–941. 1999. View Article : Google Scholar
|
|
14
|
Llambi F, Causeret F, Bloch-Gallego E and
Mehlen P: Netrin-1 acts as a survival factor via its receptors
UNC5H and DCC. EMBO J. 20:2715–2722. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oda K, Arakawa H, Tanaka T, et al:
p53AIP1, a potential mediator of p53-dependent apoptosis, and its
regulation by Ser-46-phosphorylated p53. Cell. 102:849–862. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thiebault K, Mazelin L, Pays L, et al: The
netrin-1 receptors UNC5H are putative tumor suppressors controlling
cell death commitment. Proc Natl Acad Sci USA. 100:4173–4178. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arakawa H: p53, apoptosis and
axon-guidance molecules. Cell Death Differ. 12:1057–1065. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karaulanov E, Böttcher RT, Stannek P, et
al: Unc5B interacts with FLRT3 and Rnd1 to modulate cell adhesion
in Xenopus embryos. PLoS One. 4:e57422009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sasaki S, Tabata H, Tachikawa K and
Nakajima K: The cortical subventricular zone-specific molecule
Svet1 is part of the nuclear RNA coded by the putative netrin
receptor gene Unc5d and is expressed in multipolar migrating cells.
Mol Cell Neurosci. 38:474–483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kokoszyńska K, Kryński J, Rychlewski L and
Wyrwicz LS: Unexpected domain composition of MACC1 links MET
signaling and apoptosis. Acta Biochim Pol. 56:317–323.
2009.PubMed/NCBI
|