Introduction
The repair of large bone defects, caused by injury,
eradicated tumor masses or progressive periodontal diseases is
challenging. Autologous bone grafts, allografts and alloplasts have
been used for bone repair (1).
Autologous bone is considered the gold standard of graft materials
(2,3). However, it has a number of
shortcomings, including morbidity of the donor site and limitation
of the amount of bone that may be harvested (4,5).
Allografts and alloplasts may cause immunologic responses and
endemic risks. Therefore, it is necessary to identify different
types of bone substitutes.
Among the numerous bone substitutes, calcium-sulfate
(CS) is safe, highly biocompatible, bioresorbable and
osteoconductive (6,7). In addition, its potential as a good
carrier for local release of antibiotics and growth factors has
been demonstrated (8–12). The osteogenic properties of bone
substitutes may be enhanced when combined with osteoinductive
substances, including recombinant human bone morphogenetic protein
2 (rhBMP-2). However, rhBMP-2 is expensive, limiting its clinical
application. Notably, simvastatin, a cholesterol-lowering drug, has
been shown to stimulate new bone formation in murine calvaria and
also increase bone volume when administered orally to rats by
induction of BMP-2 (13).
Thus, simvastatin-loaded CS may be attractive as a
novel bone substitute enhancing bone regeneration. In the present
study, the release of simvastatin from simvastatin-loaded CS
scaffolds, the effect of simvastatin on the osteogenic
differentiation of bone marrow-derived mesenchymal stem cells
(MSCs) in vitro and the effects of simvastatin-loaded CS on
the regeneration of segmental bone defects in the ulna of rabbits
were investigated.
Materials and methods
Fabrication of simvastatin-loaded and
rhBMP-2-loaded CS scaffolds
Osteoset® (Wright Medical, Arlington, TN,
USA), a medical-grade CS powder, was used in the present study.
Simvastatin (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in
75% ethanol at a concentration of 100 mg/ml. rhBMP-2 (Genescript,
Piscataway, NJ, USA) was dissolved in phosphate-buffered saline
(PBS; Gibco Life Technologies, Grand Island, NY, USA) at the
concentration of 1 mg/ml. For the preparation of simvastatin-loaded
and rhBMP-2-loaded CS scaffolds, 0.48 g CS powder, 0.145 ml
distilled water and 5 μl simvastatin solution or 0.48 g CS powder,
0.14 ml distilled water and 10 μl rhBMP-2 solution were aseptically
mixed in a dish. The mixture was transferred into a circular mold
of 4-mm diameter and 12-mm thickness to create cylinders for
implantation. In addition, to compare the differences in
simvastatin release between CS scaffolds of different weights at
the same dose, 0.16 g CS powder, 0.045 ml distilled water and 5 μl
simvastatin solution were mixed, then simvastatin-loaded CS
scaffolds (0.16 g) of the same diameter were fabricated. Finally,
0.5 mg simvastatin was added to each scaffold.
In vitro assay of simvastatin release
from simvastatin-loaded CS scaffolds
The simvastatin-loaded CS scaffolds (160, 480 mg)
were placed in 5 ml PBS (Gibco-BRL) at 37°C and the PBS was changed
at 1, 3, 4, 6, 8, 11, 14 or 21 days, respectively. At every
time-point, the solution absorbance was measured at a wavelength of
238 nm using an ultraviolet-visible spectrophotometer, while the
simvastatin concentration was determined from a standard curve
prepared with various amounts of simvastatin.
MSC isolation from rabbit bone marrow and
culture
Rabbits heparinized bone marrow (BM) cells were
aspirated from the humerus with an 18-gauge needle. The mononuclear
cells were centrifuged at 1,000 × g for 10 min at room temperature.
The cells were collected and resuspended in low glucose Dulbecco’s
modified Eagle’s medium containing 10% fetal bovine serum
(Gibco-BRL, Carlsbad, CA, USA) and 1% antibiotics (100 U/ml
penicillin and 100 U/ml streptomycin; Gibco Life Technologies).
Following 48 h in culture, the medium was removed and fresh medium
was added to each flask. Cells were maintained at 37°C in a humid
atmosphere with 5% CO2 and the medium was changed every
two days. When adherent cells reached 80–90% confluency, they were
detached with 0.25% trypsin-EDTA (Gibco-BRL) and replated at a
ratio of 1:3 in regular growth medium to allow for continued
passaging. Only passage three cultures were used for the
experiments.
Osteogenic differentiation of MSCs
stimulated by simvastatin in vitro
The osteogenic differentiation of MSCs cultured with
simvastatin-loaded CS scaffolds was examined by measuring alkaline
phosphatase (ALP) activity expressed by the cells. Scaffolds were
first placed in a six-well plate and MSCs were seeded with samples
at a density of 5×105 cells/sample and cultured for 7 or
14 days in control medium. The medium was changed every two days.
At 7 and 14 days, the medium was removed and the samples were
transferred to a new plate, washed with PBS, followed by the
addition of a cell lysis solution. The samples were then processed
through two freeze-thaw cycles (−70°C and room temperature; 45 min
each) to rupture the cell membrane and extract the proteins. ALP
activity of the cell lysate was determined with a p-nitrophenyl
phosphate (pNPP) phosphatase assay kit (Nanjing Jiancheng
Bioengineering Co., Ltd., Nanjing, China). Total protein content of
the cell lysate was measured using a bicinchoninic acid (BCA)
protein assay kit (Nanjing Jiancheng Bioengineering), according to
the manufacturer’s instructions. Dividing the quantity of ALP by
the amount of total protein normalized the specific amount of ALP.
ALP activities of MSCs cultured on CS scaffolds were used as
controls.
Animals and surgical procedure
A total of 18 New Zealand white rabbits (provided by
the Laboratory Animal Center of Zhejiang University, Hangzhou,
China) weighing 2.5–3.0 kg were used for the study. All animal
experimental instructions were approved by the Animal Care and Use
Committee of Zhejiang University (Hangzhou, Zhejiang, China) and
followed the ‘Principles of Laboratory Animal Care’ (NIH
publication No. 86–23, revised 1985), as well as specific national
laws (e.g., the current version of the German Law on the Protection
of Animals). The animals were anesthetized with an intravenous
injection of 3% sodium pentobarbital (30 mg/kg). A total of 24
complete bone defects of 1.2 cm were created with a high-speed saw
under irrigation with physiological saline and the periosteum was
removed. The radius was left intact for mechanical stability.
The 36 defects were randomized into 3 groups (n=6 in
each group) and treated with the CS scaffold (group A),
simvastatin-loaded CS scaffold (group B) or rhBMP-2-loaded CS
scaffold (group C, positive control). The rabbits of each group
were sacrificed at 4 or 8 weeks following surgery.
Gross observation
The status of bone repair and growth of callus were
observed in samples removed through the original incision following
animal sacrifice.
Radiological examination
Anterior and posterior radiographs of the bone
defects were obtained to observe bone healing four and eight weeks
following implantation. Bone formation was assessed in each group
following instructions with triple blinding according to the
Lane-Sandhu X-ray scores (14)
(Table I).
 | Table ILane-Sandhu X-ray scores. |
Table I
Lane-Sandhu X-ray scores.
| Indicator scores | X-ray scores |
|---|
| New bone
formation |
| None | 0 |
| <25% | 1 |
| 25–50% | 2 |
| 50–75% | 3 |
| >75% | 4 |
| Recreation of the
marrow cavity |
| No recreation | 0 |
| Partial recreation
of marrow cavity | 2 |
| Cortical bone
formation following recreation of marrow cavity | 4 |
Histological observation and
histomorphometrical analysis
Samples were fixed with 10% paraformaldehyde,
decalcified with formate-sodium formate and embedded with paraffin.
Sagittal plane sections (7-μm thick) from the interface region of
each implant were prepared and stained with hematoxylin and eosin
(H&E), then examined under a light microscope (Olympus, Tokyo,
Japan).
To quantitatively determine the amount of newly
formed bone, the histological sections were statistically analyzed
at four and eight weeks following implantation following the
procedures described previously (15). Three pieces of histological
sections of each sample were randomly selected. Following H&E
staining, each section was observed by light microscopy
(magnification, ×40) and at least 10 images were randomly obtained
per section. Using the image analytical software Image-Pro Plus 6.0
(Media Cybernetics Inc, Acton, MA, USA), the amount of newly formed
bone was expressed as the percentage of the newly formed bone area
within the original drill defect area.
Statistical analysis
Lane-Sandhu X-ray scores and newly formed bone areas
were examined by one-way analysis of variance. Data analysis was
performed using SPSS software (version 15.0; SPSS Inc., Chicago,
IL, USA). Fisher’s Least Significant Difference test was used for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
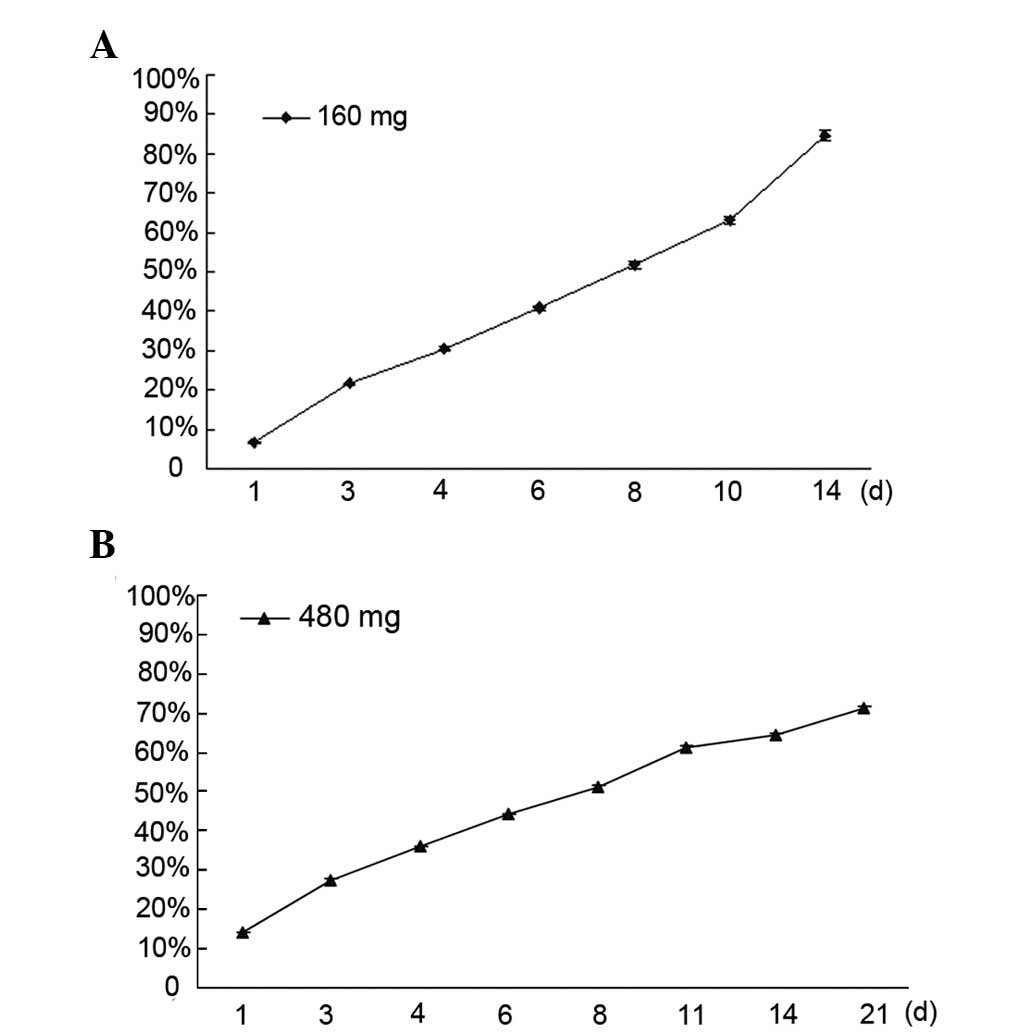
In vitro release behavior of simvastatin
from simvastatin-loaded CS
The in vitro release pattern of simvastatin
from simvastatin-loaded CS is shown in Fig. 1. On day 1, ~5.2% and 11.3% of
simvastatin was released from the simvastatin-loaded CS (160 and
480 mg), respectively, and a stable release was maintained. By day
14, ~85% of the loaded simvastatin was released from the
simvastatin-loaded CS (160 mg). However, in the simvastatin-loaded
CS (480 mg), ~65% of the loaded simvastatin was released by day 14
and 71% of the loaded simvastatin was released by day 21.
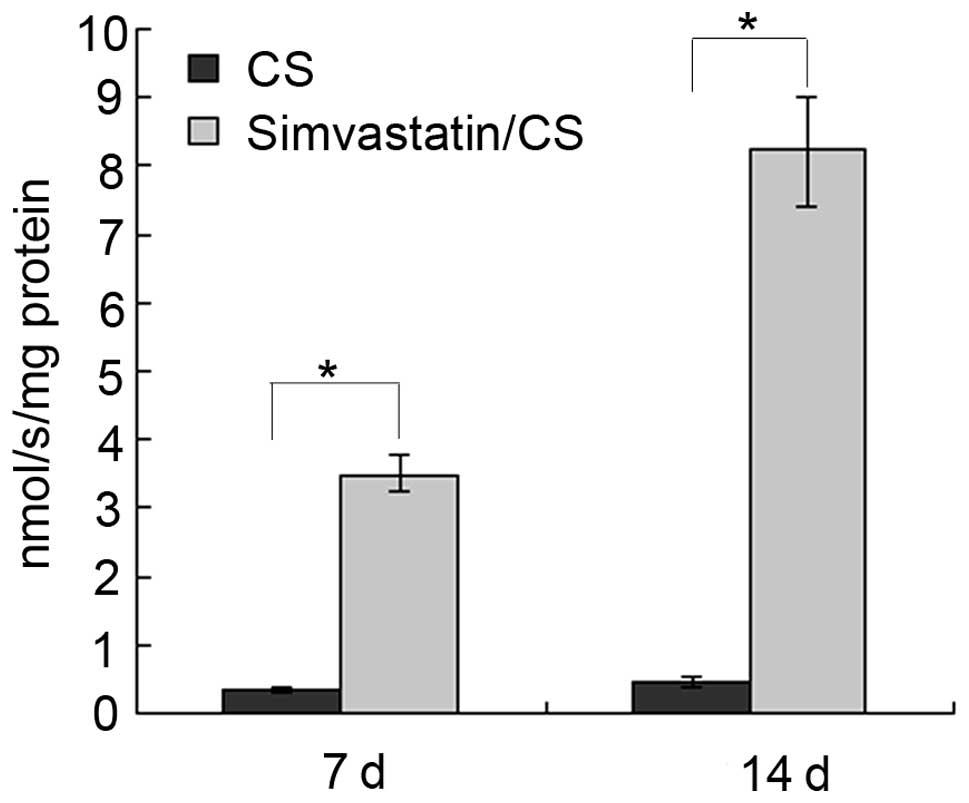
ALP measurement
Specific ALP expression increased on
simvastatin-loaded CS scaffolds between 7 and 14 days (Fig. 2). At 7 days, ALP (mean ± standard
deviation; n=5) levels were 3.51±0.28 nmol/sec/mg protein for MSCs
on simvastatin-loaded CS scaffolds, which was higher than 0.35±0.03
nmol/sec/mg protein on CS scaffolds (P<0.01). At 14 days, ALP
levels increased to 8.22±0.81 nmol/sec/mg protein in cells on the
simvastatin-loaded CS scaffolds, which was significantly higher
than 0.48±0.04 nmol/sec/mg protein for cells on CS scaffolds
(P<0.01).
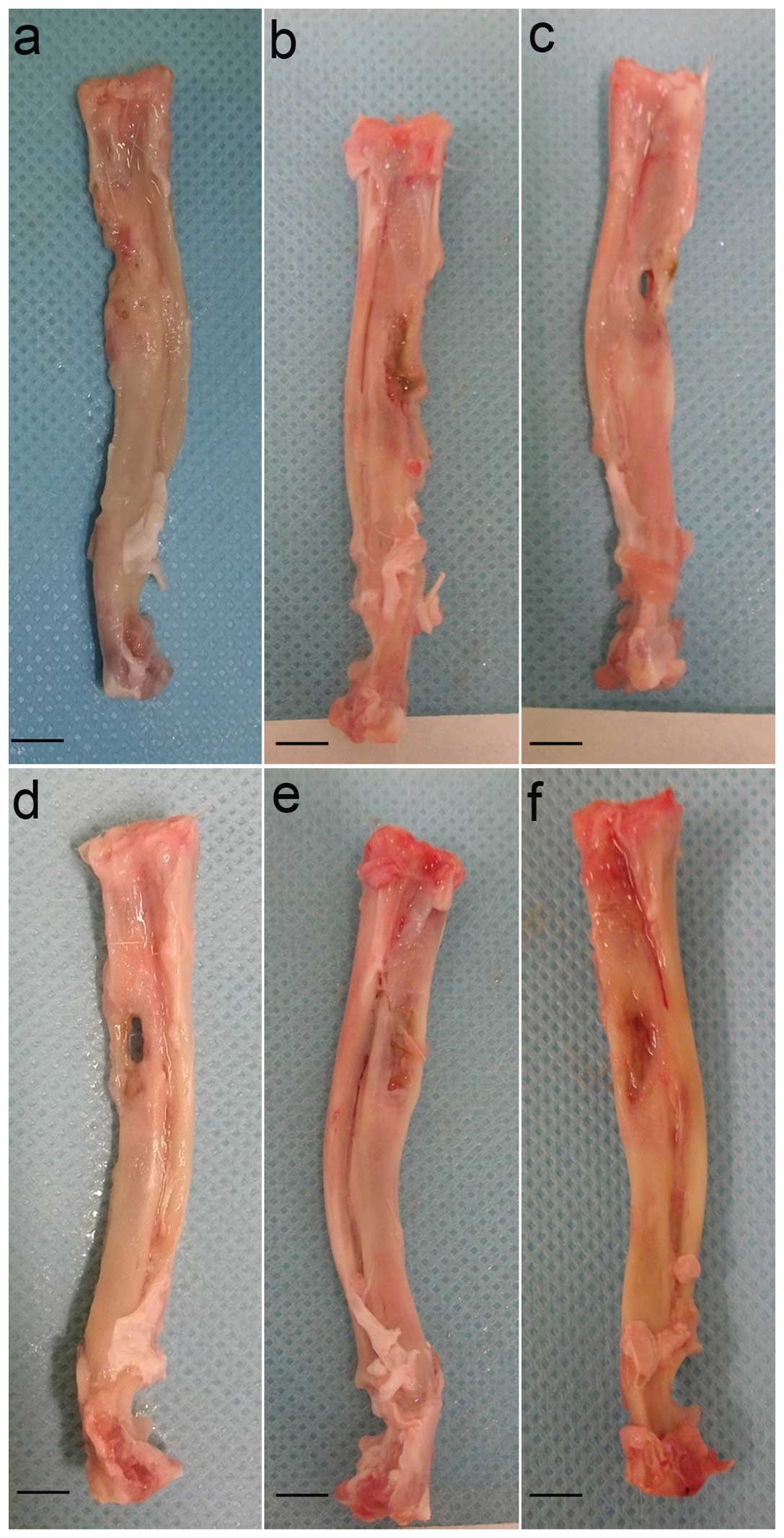
Gross observation
All rabbits had normal diets and movement following
surgery and survived until the scheduled date of sacrifice without
any apparent complications.
At four weeks, the majority of defects in group A
was filled with fibrous tissue (Fig.
3A). In groups B and C, new bone only formed in the extremities
of defects and the areas next to the radius (Fig. 3B and C). The outside of the defects
linked together in group C (Fig.
3C). At eight weeks, new bone formed in the inside of the
defects in group A and the outside of the defects was filled with
fibrous tissue. In addition, there was a cavity in the middle of
the defect (Fig. 3D). In groups B
and C, the ulna achieved bone union. However, they were not
completely regenerated; only the inside and outside cortical bone
was regenerated (Fig. 3E and
F).
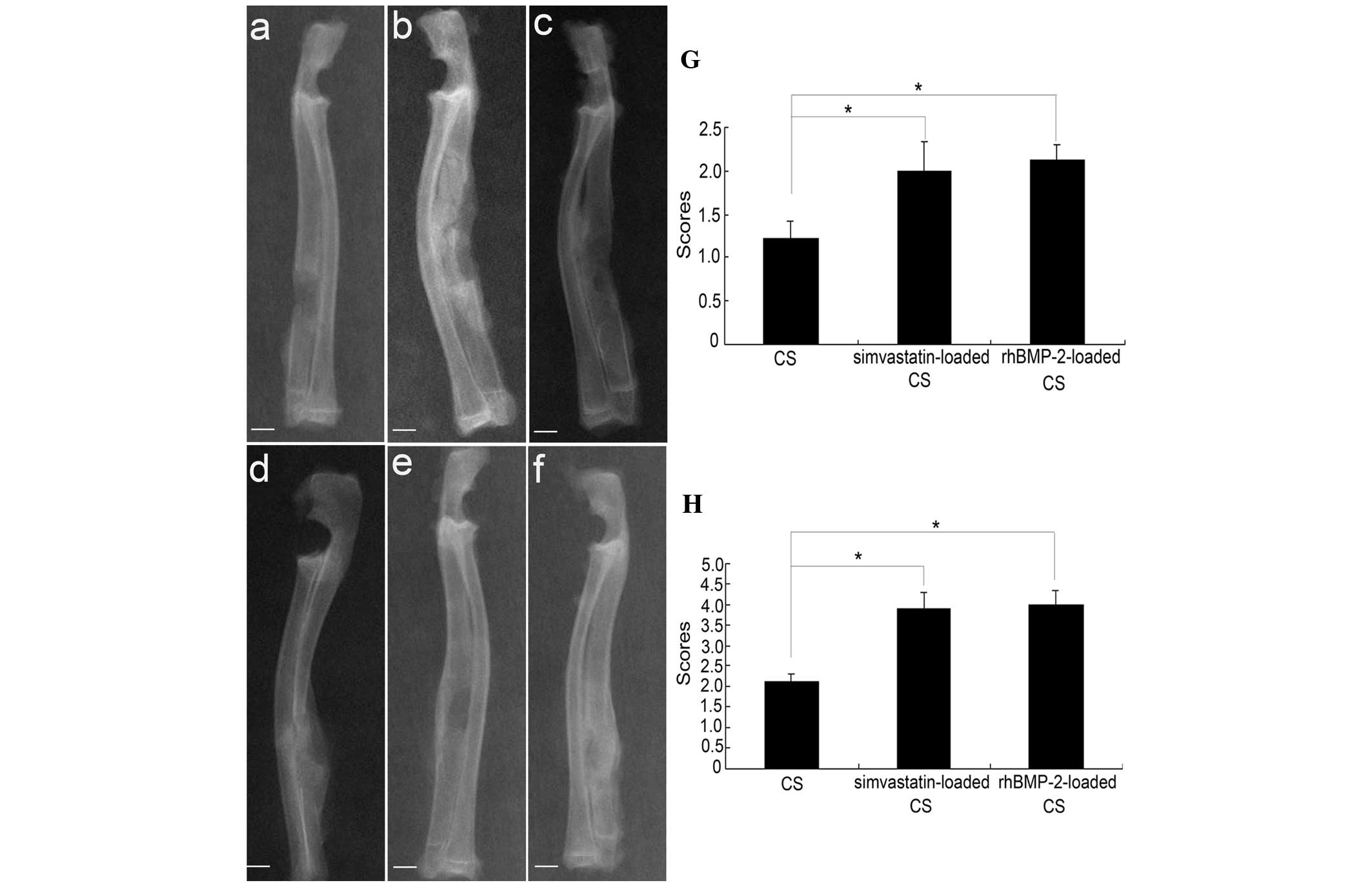
X-ray examinations
The X-ray images of each group are shown in Fig. 4. In group A, the bone defects were
not repaired at four weeks (Fig.
4A). In groups B and C, the CS was mostly degraded at 4 weeks
and osteoid tissue had formed at the extremities and areas next to
the radius (Fig. 4B and C). At
eight weeks, the CS was completely degraded. Osteoid tissue had
formed in the areas next to the radius in group A (Fig. 4D). In groups B and C, the CS and
bone tissue connected. The medullary cavity achieved partial
recanalization; the inside and outside cortical bone was
regenerated (Fig. 4E and F).
The bone formation scores were evaluated according
to the Lane-Sandhu score standard. The X-ray scores increased with
time and the score of group A was significantly lower compared with
groups B and C at postoperative weeks four and eight (P<0.05).
The scores of group C were slightly higher compared with group B.
However, there was no significant difference between groups B and C
both at four and eight weeks (P>0.05; Fig. 4G and H).
Histological analysis
At four weeks following implantation the CS was
mostly degraded. In group A, small amounts of woven bone were
observed to form in the extremities of the defects. The outsides of
the defects were filled with fibrous tissue (Fig. 5A). In group B, abundant woven bone
formed in the extremities of the defect areas next to the radius
and marrow cavities (Fig. 5B).
Compared with group B, the woven bone of group C was increased and
denser, which was observed in the majority of defects, even on the
outside (Fig. 5C).
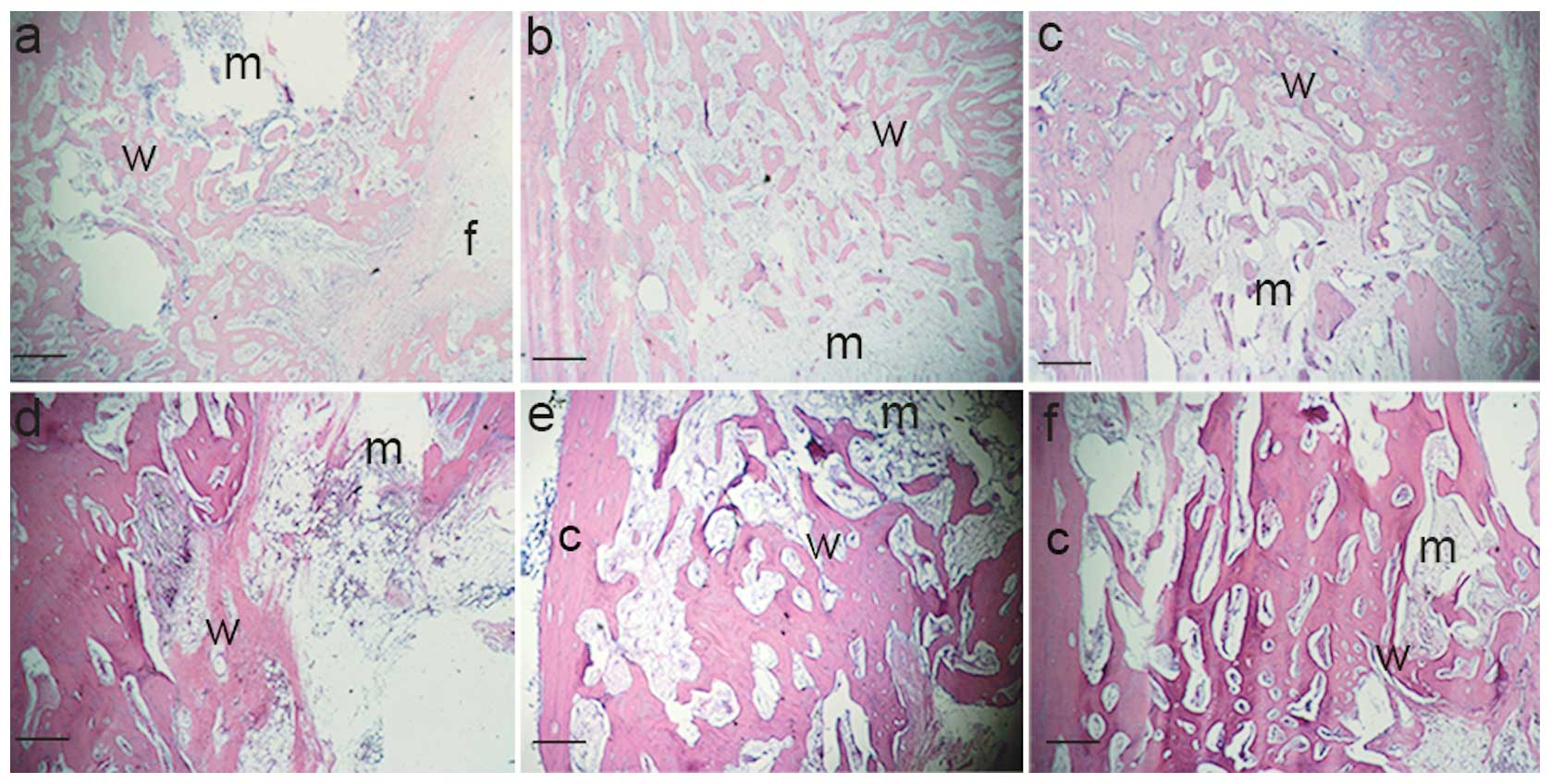 | Figure 5Histological examination of the
repaired bone tissue at (A–C) 4 weeks and (D–F) 8 weeks following
implantation. A and D, CS group; B and E simvastatin-loaded CS
group; C and F, rhBMP-2-loaded CS group. H&E staining;
magnification, ×20; scale bar, 200 μm. w, woven bone; c, cortical
bone; f, fibrous tissue; m, medullary cavity; CS, calcium sulphate;
rhBMP-2, recombinant human bone morphogenetic protein 2; H&E,
hematoxylin and eosin. |
At eight weeks, the two ends of the original ulna
were united with the regenerated new bone. The CS was completely
degraded. Compared with four weeks, the newly formed bone resembled
normal cortical bone. In group A, dense woven bone formed in the
extremities and inside the defect. However, the outside was not
regenerated with bone and the marrow cavity was not recanalized
(Fig. 5D). In groups B and C, the
cortical bone regenerated on the inside of the defects and the
outside was filled with abundant dense woven bone. In addition, the
medullary cavity formed and achieved slight recanalization
(Fig. 5E and F).
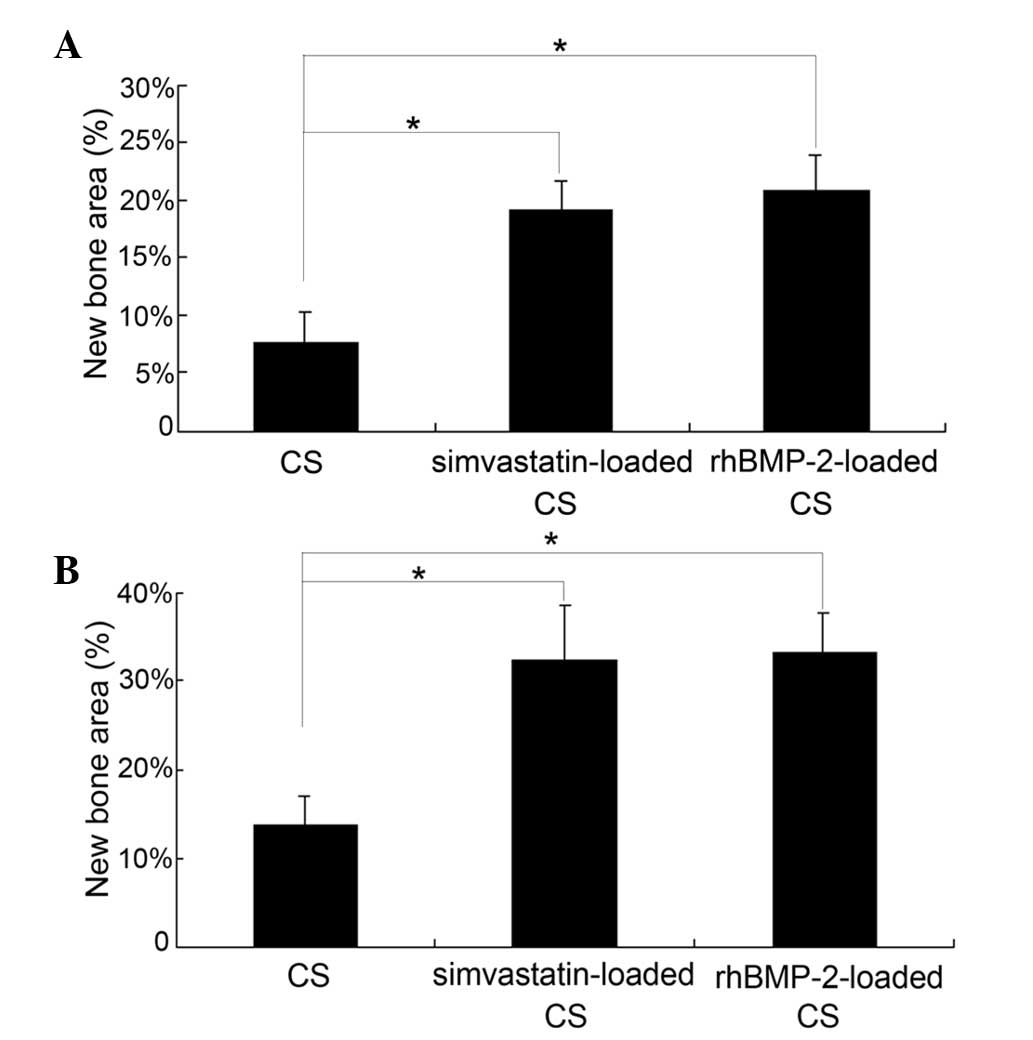
Bone area analysis
The area of newly formed bone in groups B and C was
significantly higher compared with group A at four and eight weeks
following implantation (P<0.05). No significant difference in
the area of the newly formed bone was observed between groups B and
C at each time-point (Fig. 6).
Discussion
The present study demonstrated that simvastatin was
highly efficiently released from simvastatin-loaded CS, promoted
the osteogenic differentiation of MSCs and stimulated bone
regeneration when it was locally released from CS scaffolds into
bone defects. The beneficial effect of simvastatin, locally applied
from CS scaffolds, was similar to those of rhBMP-2.
In the present study, simvastatin was incorporated
into CS scaffolds and was gradually released in a sustained manner.
The release assay showed that the release efficiency correlated
with the volume of simvastatin-loaded CS. Simvastatin-loaded CS
(480 mg) showed a more efficient release of simvastatin on day 1 as
compared with simvastatin-loaded CS (160 mg), which had a lower
volume. On day 1, ~11.3% of the simvastatin was released from
simvastatin-loaded CS (480 mg). However, from the
simvastatin-loaded CS (160 mg), only 5.2% of the simvastatin was
released. In addition, the release of simvastatin from
simvastatin-loaded CS (480 mg) was extended over a longer time
period as compared with the 160 mg sample. At day 21, 71% of
simvastatin was released. In the simvastatin-loaded CS (160 mg),
~85% of the loaded simvastatin was released at day 14. The results
suggested that the simvastatin-loaded CS (480 mg) may provide a
longer period of simvastatin release, as well as a highly efficient
release of simvastatin at the onset.
A previous study suggested that the appropriate
concentration of simvastatin was 0.5–1 μM for in vitro
culture and MSCs could not proliferate in a medium containing
>2.5 μM simvastatin (16).
Therefore, in the present study, MSCs were co-cultured with 1 μM
simvastatin for 14 days and the osteogenic differentiation of MSCs
was investigated through detection of ALP expression. The ALP
expression of MSCs co-cultured with simvastatin was significantly
higher, which meant that simvastatin was able to induce the
osteogenic differentiation of MSCs. Similar results were observed
in a previous study where human adipose tissue-derived stromal
cells were treated with 0.01, 0.1 and 1 μM simvastatin (17).
The mechanism of sustained release of simvastatin
occurs through degradation of CS. In vivo, through the
degradation of CS, simvastatin was persistently and locally
released to induce bone formation. Local application has a
therapeutic advantage by preventing systemic side-effects. Previous
studies have investigated the effects of locally applied
simvastatin. In these studies, the doses of simvastatin used have
been variable: 2.2 mg (18), 0.5
mg (19,20); 0.1, 0.5, 1.0, 1.5 and 2.2 mg
(21) showed positive or negative
effects on bone repair. In a study by Wong and Rabie (19), 0.5 mg simvastatin in an aqueous
solution was added to a collagen matrix in calvarial defects in
rabbits and a 308% increase in new bone was present in the
simvastatin-collagen group compared with the collagen group alone
at 14 days. Stein et al (21) found that 0.5 mg simvastatin
appeared to be the optimal dose for single local application and a
dose of 0.5 mg produced the best bone growth/inflammation ratio.
Based on these findings, 0.5 mg simvastatin was selected and
incorporated into the CS scaffolds for segmental bone
regeneration.
Simvastatin could stimulate the BMP-2 expression in
osteoblasts and inhibit the osteoclastic activity (22,23).
Furthermore, a number of experimental animal studies have
demonstrated a beneficial effect of simvastatin on bone formation
(19,20,24,25),
which is in agreement with the results of the present study. The
simvastatin-loaded CS group had a significantly larger bone area
compared with the CS group at four and eight weeks. CS not only
worked as an osteoconductive scaffold for bone regeneration, but
also as a carrier for releasing simvastatin. The released
simvastatin could promote osteoblastic differentiation of bone
marrow-derived MSCs, which was confirmed in a previous study
(26). Another possible reason for
the effects of simvastatin on bone regeneration may be their effect
on angiogenesis and VEGF expression (27). In a study by Wong et al
(28), simvastatin triggered the
early expression of growth factors, including VEGF and BMP-2, and
induced and accelerated formation of bone locally (28).
Notably, the present study revealed that simvastatin
stimulated bone formation later than rhBMP-2. This was observed
from the quality of repaired bone tissue at four weeks following
implantation. rhBMP-2 may directly stimulate the progenitor cells
and osteoblasts; however, simvastatin needs to stimulate endogenous
expression of BMP-2 first (13).
Therefore, the simvastatin-loaded CS group showed delayed effects
compared with the rhBMP-2-loaded CS group at four weeks following
implantation, which was consistent with a previous study (29). However, no significant difference
of new bone area between rhBMP-2-loaded CS group and
simvastatin-loaded CS group was found at four and eight weeks
following implantation. Furthermore, in accordance with a study by
Mundy et al (13), no
serious side effects were observed in the present study. Thus, the
effect of simvastatin on bone repair was comparable with that of
rhBMP-2, which may provide important information on its
application. rhBMP-2 is an expensive substance, while simvastatin
is inexpensive, approved worldwide, well tolerated and has been
demonstrated to have a convenient side-effect profile (30). Thus, simvastatin may be used in the
clinic to improve bone regeneration instead of, or in combination
with rhBMP-2.
In conclusion, simvastatin may be efficiently
released from simvastatin-loaded CS and induce osteogenic
differentiation of MSCs. In addition, the advantages of simvastatin
and desirable effects of rhBMP-2 on segmental bone repair were
successfully combined with an efficient local application. Compared
with rhBMP-2, simvastatin may be considered a cheap, well-tested
drug with a beneficial side effect profile, and therefore, may be a
promising substance in terms of bone regeneration. The
simvastatin-loaded CS scaffold may have great potential in bone
tissue engineering.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (no. 81201414), the Natural Science
Foundation of Zhejiang Province (no. Y2090440) and the Doctoral
fund of Ministry of Education of China (no. 20100101120132).
References
|
1
|
John HD and Wenz B: Histomorphometric
analysis of natural bone mineral for maxillary sinus augmentation.
Int J Oral Maxillofac Implants. 19:199–207. 2004.PubMed/NCBI
|
|
2
|
Boyne PJ and James RA: Grafting of the
maxillary sinus floor with autogenous marrow and bone. J Oral Surg.
38:613–616. 1980.PubMed/NCBI
|
|
3
|
Moy PK, Lundgren S and Holmes RE:
Maxillary sinus augmentation: histomorphometric analysis of graft
materials for maxillary sinus floor augmentation. J Oral Maxillofac
Surg. 51:857–862. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Younger EM and Chapman MW: Morbidity at
bone graft donor sites. J Orthop Trauma. 3:192–195. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan H, Yang Z, De Bruij JD, De Groot K
and Zhang X: Material-dependent bone induction by calcium phosphate
ceramics: a 2.5-year study in dog. Biomaterials. 22:2617–2623.
2001.PubMed/NCBI
|
|
6
|
Borrelli J Jr, Prickett WD and Ricci WM:
Treatment of nonunions and osseous defects with bone graft and
calcium sulfate. Clin Orthop Relat Res. 245–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gitelis S, Piasecki P, Turner T, Haggard
W, Charters J and Urban R: Use of a calcium sulfate-based bone
graft substitute for benign bone lesions. Orthopedics. 24:162–166.
2001.PubMed/NCBI
|
|
8
|
Turner TM, Urban RM, Gitelis S, Kuo KN and
Andersson GB: Radiographic and histologic assessment of calcium
sulfate in experimental animal models and clinical use as a
resorbable bone-graft substitute, a bone-graft expander, and a
method for local antibiotic delivery. One institution’s experience.
J Bone Joint Surg Am. 83-A(Suppl 2): S8–S18. 2001.PubMed/NCBI
|
|
9
|
McKee MD, Wild LM, Schemitsch EH and
Waddell JP: The use of an antibiotic-impregnated, osteoconductive,
bioabsorbable bone substitute in the treatment of infected long
bone defects: early results of a prospective trial. J Orthop
Trauma. 16:622–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mousset B, Benoit MA, Delloye C, Bouillet
R and Gillard J: Biodegradable implants for potential use in bone
infection. An in vitro study of antibiotic-loaded calcium sulphate.
Int Orthop. 19:157–161. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dacquet V, Varlet A, Tandogan RN, Tahon
MM, Fournier L, Jehl F, Monteil H and Bascoulergue G:
Antibiotic-impregnated plaster of Paris beads. Trials with
teicoplanin. Clin Orthop Relat Res. 241–249. 1992.PubMed/NCBI
|
|
12
|
Benoit MA, Mousset B, Delloye C, Bouillet
R and Gillard J: Antibiotic-loaded plaster of Paris implants coated
with poly lactide-co-glycolide as a controlled release delivery
system for the treatment of bone infections. Int Orthop.
21:403–408. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mundy G, Garrett R, Harris S, Chan J, Chen
D, Rossini G, Boyce B, Zhao M and Gutierrez G: Stimulation of bone
formation in vitro and in rodents by statins. Science.
286:1946–1949. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lane JM and Sandhu HS: Current approaches
to experimental bone grafting. Orthop Clin North Am. 18:213–225.
1987.PubMed/NCBI
|
|
15
|
Wu F, Wei J, Guo H, Chen F, Hong H and Liu
C: Self-setting bioactive calcium-magnesium phosphate cement with
high strength and degradability for bone regeneration. Acta
Biomater. 4:1873–1884. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wadagaki R, Mizuno D, Yamawaki-Ogata A,
Satake M, Kaneko H, Hagiwara S, Yamamoto N, Narita Y, Hibi H and
Ueda M: Osteogenic induction of bone marrow-derived stromal cells
on simvastatin-releasing, biodegradable, nano- to microscale fiber
scaffolds. Ann Biomed Eng. 39:1872–1881. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou Y, Ni Y, Liu Y, Zeng B, Xu Y and Ge
W: The role of simvastatin in the osteogenesis of injectable
tissue-engineered bone based on human adipose-derived stromal cells
and platelet-rich plasma. Biomaterials. 31:5325–5335. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thylin MR, McConnell JC, Schmid MJ,
Reckling RR, Ojha J, Bhattacharyya I, Marx DB and Reinhardt RA:
Effects of simvastatin gels on murine calvarial bone. J
Periodontol. 73:1141–1148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wong RW and Rabie AB: Statin collagen
grafts used to repair defects in the parietal bone of rabbits. Br J
Oral Maxillofac Surg. 41:244–248. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ozeç I, Kiliç E, Gümüs C and Göze F:
Effect of local simvastatin application on mandibular defects. J
Craniofac Surg. 18:546–550. 2007.PubMed/NCBI
|
|
21
|
Stein D, Lee Y, Schmid MJ, Killpack B,
Genrich MA, Narayana N, Marx DB, Cullen DM and Reinhardt RA: Local
simvastatin effects on mandibular bone growth and inflammation. J
Periodontol. 76:1861–1870. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nyan M, Miyahara T, Noritake K, Hao J,
Rodriguez R, Kuroda S and Kasugai S: Molecular and tissue responses
in the healing of rat calvarial defects after local application of
simvastatin combined with alpha tricalcium phosphate. J Biomed
Mater Res B Appl Biomater. 93:65–73. 2010.PubMed/NCBI
|
|
23
|
Giannoudis PV: Fracture healing and bone
regeneration: autologous bone grafting or BMPs? Injury.
40:1243–1244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ayukawa Y, Okamura A and Koyano K:
Simvastatin promotes osteogenesis around titanium implants. Clin
Oral Implants Res. 15:346–350. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee Y, Schmid MJ, Marx DB, Beatty MW,
Cullen DM, Collins ME and Reinhardt RA: The effect of local
simvastatin delivery strategies on mandibular bone formation in
vivo. Biomaterials. 29:1940–1949. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baek KH, Lee WY, Oh KW, Tae HJ, Lee JM,
Lee EJ, Han JH, Kang MI, Cha BY, Lee KW, Son HY and Kang SK: The
effect of simvastatin on the proliferation and differentiation of
human bone marrow stromal cells. J Korean Med Sci. 20:438–444.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weis M, Heeschen C, Glassford AJ and Cooke
JP: Statins have biphasic effects on angiogenesis. Circulation.
105:739–745. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong RW and Rabie AB: Early healing
pattern of statin-induced osteogenesis. Br J Oral Maxillofac Surg.
43:46–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pauly S, Luttosch F, Morawski M, Haas NP,
Schmidmaier G and Wildemann B: Simvastatin locally applied from a
biodegradable coating of osteosynthetic implants improves fracture
healing comparable to BMP-2 application. Bone. 45:505–511. 2009.
View Article : Google Scholar
|
|
30
|
Armitage J: The safety of statins in
clinical practice. Lancet. 370:1781–1790. 2007. View Article : Google Scholar : PubMed/NCBI
|