Introduction
Brucella species are facultative
intracellular bacteria that cause brucellosis in humans and
animals. Brucella invades phagocytic and non-phagocytic
cells and then survives inside the host cells (1,2). The
control of the infection is managed by vaccination against animal
brucellosis. Human brucellosis has also been controlled by
vaccination as well as culling within animals (3,4).
The effective vaccines currently used for livestock
are B. abortus S19 and RB51 for cattle, and B.
melitensis Rev1 for small ruminants (5). However, these stems are infectious
for humans and cause abortion in pregnant animals. B.
abortus S19 has been widely used to prevent cattle brucellosis,
while it usually has low virulence (6). B. abortus S19 is able to
induce the production of antibodies to the O-polysaccharide (PS),
which is difficult to distinguish from that resulting from natural
infection (7–9). Developing a safe and efficacious
vaccine and overcoming the serological obstacle is likely to have a
broad impact on public health.
Lipopolysaccharides (LPS) provide bacterial
resistance to anti-microbial attacks and modulates the host immune
response, which makes it a significant virulence factor for its
survival and replication in the host cell (1,10).
Brucellae without O-PS are termed as a rough or ‘R’ strain.
R. brucella species or mutants lacking antigenic O-PS do not
reduce levels of anti-O-PS antibodies and do not react with
antibodies of this specificity (11,12).
Thus vaccination with rough strains is distinguished from wild
infection according to serological tests (13). It has been observed that
Brucella R mutants are attenuated, and therefore, they are
potential vaccines (14,15).
Several genes of Brucella melitensis 16M
associated with LPS synthesis were analyzed and the integral
membrane protein (wzm) and the adenosine triphosphatase (ATPase)
domain (wzt) of the ATP-binding cassette (ABC)-type transporters
were putative components of the ABC transporter system. Mutations
in the wzm/wzt genes was proved to lead to the
absence of the O-side-chains on the bacterial surface (16,17).
In order to investigate the virulence and
characteristics of the rough mutants of S19, mutants with partial
deletion of the wzm and wzt genes with no DNA marker
addition were constructed to estimate their effect on LPS
synthesis, survival in vivo and the serological response.
Finding were asperated to enhance the understanding of the effect
of the wzm and wzt genes on LPS synthesis and on the
virulence of the S19 vaccination strain, and provide valuable
information for the construction of a vaccine based on a Brucella
rough mutant.
Materials and methods
Bacterial strains and growth
conditions
The Escherichia coli DH5α strain was grown on
Luria-Bertani broth (LB) agar at 37°C. The Brucella strains
B. abortus S19, Δwzm and Δwzt were grown on
tryptic soy broth (TSB, Sigma Co., St. Louis MO, USA) agar at 37°C
(Table I). In total, 100 μg/ml
ampicillin and 50 μg/ml kanamycin were added for plasmid screening
if it was considered necessary. A total of 7% sucrose in TSB medium
was prepared for screening the allelic-exchange mutants.
 | Table IBacteria strains and plasmids. |
Table I
Bacteria strains and plasmids.
| Strain or
plasmid | Phenotype and/or
genotype | Source |
|---|
| Strain |
| Escherichia
coli DH5α | F-, decR,
recA1 | Takara Co.
D9057A |
| Brucella
abortus S19 | Vaccine strain,
smooth | IVDC |
| Brucella
abortus Δwzm | Δwzm gene
partial deleted | Present study |
| Brucella
abortus Δwzt | Δwzt gene
partial deleted | Present study |
| Plasmid |
| pBKCMV | Kanamycin
resistance | Stratagene |
| pIBP279 | Provided
sacB gene | NJAU |
Construction of allelic exchange
plasmids
The allelic exchange plasmids were constructed by
pBKCMV (kanamycin resistance, kanr) with a sacB
gene and fragments upstream and downstream of the target genes. The
sacB gene along with its promoter was amplified from pIBP279
(presented by Nanjing Agricultural University) by polymerase chain
reaction (PCR; Thermal Cycler Px2 PCR amplifier, Thermo Fisher
Scientific Inc., Rockford, IL, USA) using the following primers:
sacB forward, 5′-gtcgacACTCAGTAC
ATAATAAAGGAGACAT-3′ and reverse, 3′-ggatccTGGGATTCACCTTTATGTTGATAA
G-5′. The PCR conditions were: 95°C for 3 min; 95°C for 30 sec,
56°C for 30 sec, 72°C for 90 sec, 30 cycles; 72°C for 10 min. Next,
it was ligated into pBKCMV for constructing plasmid pBKsacB using
the following primers: wzmf forward,
5′-ggatccTTTCATTTGAGGAGCCGGAGTA-3′ and reverse,
3′-ctcgagGCCCACGTAAATCAGACATTGAAAG-5′; wzmr forward,
5′-ctcgagGGCAGGGTGGATTGAATGCATTCG T-3′ and reverse,
3′-cccgggGCGTCGCAACCGCAATCTTAT CAAT-5′; wztf forward,
5′-ggatccGCGATGAAGTCATTGT ACCGACCTT-3′ and reverse,
3′-ctcgagGGCGTTTACTAG AGTTTTGACTGA GC-5′; wztr forward,
5′-ctcgagATAGGT GCAGGTGATGCGGCATTCA-3′ and antisense
3′-tctagaTGCCGAGTTCGCTCAGACAATCAAC-5′. The PCR conditions were:
95°C 3 min; 95°C 30 sec, 62°C 30 sec, 72°C 120 sec, 30 cycles; 72°C
10 min. These were amplified and ligated into the pBKsacB plasmid
to construct pBKsacBwzm and pBKsacBwzt.
Preparation of competent cells and
electroporation
B. abortus S19 was cultured in TSB for 24 h
until it reached ~108 cells/ml. The cells were prepared
for electroporation by pelleting and washing, first with 1/2 volume
of 10% ice-cold glycerol twice and then treated with 1/10 volume of
10% ice-cold glycerol. Finally, the sample was resuspended with
1/200 volume of ice-cold 10% glycerol and stored at −80°C until
further use.
In total, 30 ng/μl pBKsacBwzm or pBKsacBwzt plasmid
DNA was added to the competent cells (10–100 μl) and electroporated
at 1,500 kV (1 mm bottom; BTX, Holliston, MA, USA). Super Optimal
Broth (1ml; 2% tryptone, 0.5% yeast extract, 10 mM NaCl, 2.5 mM
KCl, 10 mM CaCl2, 10 mM MgSO4 and 20 mM
glucose) was added, the cells were grown with agitation at 28°C for
24 h and then plated on TSB agar (with 50 μg/ml kanamycin) and
cultured for 96 h at 28°C.
Mutant screening
The colonies on the TSB (Kanr) plates
were inoculated in TSB liquid medium separately and cultured for 36
h at 28°C, and then the cultures were plated on 7% sucrose TSB agar
medium and cultured at 37°C for 96 h. The colonies grown were
picked in 96-well plates with TSB medium and incubated for 48 h at
37°C and then detected by wzm and wzt primers as
follows: wzm forward, 5′-catatgGTGAGACGATTT CGTATGATATCGT-3′
and reverse, 3′-ctcgagTCATAGGTA AAAAATGGCTCTCTTCTCC-5′, wzt
forward, 5′-catatgATG ATCCAGCCATCGATTACC CTGT-3′ and reverse,
3′-ctcgag TCATGCTATAGCTCCCAT TCCCGAG-5′. The colonies in which the
wzm or wzt fragment was altered, termed Δwzm
and Δwzt positive mutants, were confirmed in TSB
(Kanr). The Kanr-negative strains were
inoculated on TSB agar medium as candidate mutant strains for
next-cycle screening.
Mutant detection and acriflavine
agglutination
The mutants were inoculated for 30 generations,
assessed by PCR and the sequences of the wzm, wzt,
wzmf, wzmr, wztf and wztr fragments
were analyzed. The phenotype of the mutants was further determined
by agglutination with acriflavine at 1:100 (18).
LPS extraction and analysis
The extraction process of LPS was performed using
the LPS Extraction Kit (no. 17141; iNtRON, Seongnam-Si, Korea)
according to the manufacturer’s instructions. The extracted LPSs
from S19, Δwzm and Δwzt were subjected to 12%
SDS-PAGE. Silver nitrate staining was processed following the
method described by Tsai and Frasch (19). New Zealand white rabbits were
immunized three times with Brucella abortus vaccine strain
S19 by multi-point injection, and the injection interval was 4
weeks. Immunization was detected by ELISA. The ear blood was
collected to prepare serum. The crude LPS samples were assayed by
western blotting using rabbit serum containing antibodies.
Animals
The 4–6-week-old female specific pathogen-free
BALB/c mice were provided by The Animal Centre of Jilin University
(Changchun, China). Mice were bred in the animal facilities with
filtered air in a restricted-access room and under pathogen limited
conditions. Mice were acclimatized for a minimum of one week prior
to the experiments and water and food were provided ad
libitum (14). All animal
experiments were approved by the Center of Laboratory Animals in
Jilin University (Changchun, China).
Survival of Δwzm and Δwzt strains in
mice
Survival of the strains, Δwzm and
Δwzt, were determined by quantitating the number of
colony-forming units (CFU) of the strains in the spleens at
different time periods. Female BALB/c mice of 6–8 weeks of age were
housed with water and food. Animals were randomly allotted and
acclimated for one week prior to the start of the experiments. To
prepare the inoculated samples, bacteria were suspended in
phosphate-buffered saline (PBS) and adjusted to the appropriate
108 CFU/ml in the same buffer. In all the experiments
the number of CFU administered was determined by culturing
triplicate aliquots. At 1, 2, 4 and 8 weeks animals were
anaesthetized by ether inhalation and sacrificed; spleens were
removed and homogenized in 10 mM PBS with 1% Triton-100. Tissue
homogenates were serially diluted with PBS and plated onto TSB agar
to determine the number of CFU per spleen by incubating for 72 h at
37°C. The spleens were processed in order to calculate the mean and
standard deviation (n=5) of the log10 of CFU per spleen (known as
infection kinetics).
Serological test
Blood samples from BALB/c mice were collected and
allowed to clot for 12 h at 4°C and centrifuged. Serum was divided
into Eppendorf tubes (Eppendorf, Hamburg, Germany) and stored at
−80°C. The Rose Bengal plate agglutination test (RBPT) kit (Harbin
Pharmaceutical Group Bio-vaccine Co. Ltd, Harbin, China) was
performed with 30 μl serum and 30 μl antigen mixing and the
reaction was observed to occur within 4 min. The positive samples
were evaluated by a tube agglutination test. The sera were diluted
from 1:12.5 to 1:400 with 0.85% sodium chloride solution, and 0.5
ml inactivated standard B. abortus broth was added in a 1:1
ratio. Sodium chloride solution (0.85%) was used as the negative
control and standard positive serum and negative serum were from
the National Institute for Communicable Disease Control and
Prevention (Chinese Center for Disease Control and Prevention,
Beijing, China). The sample tubes were maintained at 37°C for 24 h.
The positive samples were defined by a titer >1:100.
Statistics
Data were analyzed using Original 7.5 software
(OriginLab Corporation, Northampton, MA, USA) and presented as the
mean ± standard deviation. Differences between groups were
identified by statistical tests using one-way analysis of variance,
with P<0.01 indicating a statistically significant
difference.
Results
Generation of mutant strains
In order to obtain partial mutants of the wzm
and wzt genes, the plasmids pBKsacBwzm and
pBKsacBwzt were constructed. The plasmids were
electroporated into B. abortus S19 cells and the transformed
samples were plated on TSB agar medium (Kanr) for the
first screening. The selected colonies were spread onto TSB medium
and detected by PCR with sacB primers for the second
screening. The positive culture was spread on 7% sucrose TSA medium
for allelic exchange screening (20). The colonies from the 7% sucrose TSB
agar medium were inoculated into TSB medium and screened by
pre-gene primers (wzm or wzt gene) for the fourth
screening. The pre-gene in the mutant cells was expected to be
shortened. The positive mutants had only one band at ~300 bp
subsequent to the screening process. The putative positive mutants
were inoculated into TSB medium (Kanr) to remove any
false positives.
The PCR results (Fig.
1A and B) of the third screening showed that the bands of 8.5%
(8/94) of colonies exhibited transformed pBKsacBwzm and the bands
of 19.6% (18/92) colonies exhibited transformed pBKsacBwzt
(Fig. 2A and B). The positive
mutant ratio of Δwzm was 1.0% (1/94) and that of Δwzt
was 3.3% (3/92) (Fig. 1C and
D).
 | Figure 1PCR detection of mutants. (A) Results
of the fourth screen of Δwzm, lane 6 is a false positive
mutant, and lane 9 is a putative positive mutant. (B) Results of
the fourth screen of Δwzt, lanes 4 and 5 are false positive
mutants and lane 12 is a putative positive mutant. (C) Second cycle
screen of Δwzm. Lanes 1, 6, 11 are from putative positive
mutants and lanes 2–5, 7–10 and 12 are false positive mutants. (D)
Second cycle screen of Δwzt of a putative mutant. (E) PCR
detection of target gene (lane 1, 280 bp) and upstream (lane 2,
2,000 bp) and downstream (lane 3, 1,900 bp ) fragments Δwzm.
(F) PCR detection of target gene (lane 1, 300 bp) and upstream
(lane 2, 1,800 bp) and downstream (lane 3, 2,100 bp ) fragments
Δwzt. PCR, polymerase chain reaction. |
Mutant strains were rough mutants
Subsequent to a 30 generation culture for genetic
stability, the mutants were detected by PCR using target gene,
upstream and downstream fragment primers, and the sequences were
analyzed. The target gene contained only 300 bp. wzmf
contained a 2.0 fragment and wzmr 1.9, wztf 1.8 and wztr
contained 2.1 kb with stable sequences (Fig. 1E and F). The mutants were prepared
for acriflavine agglutination. The Δwzm and Δwzt
mutants were positive, and the S19 strain was negative for
acriflavine agglutination.
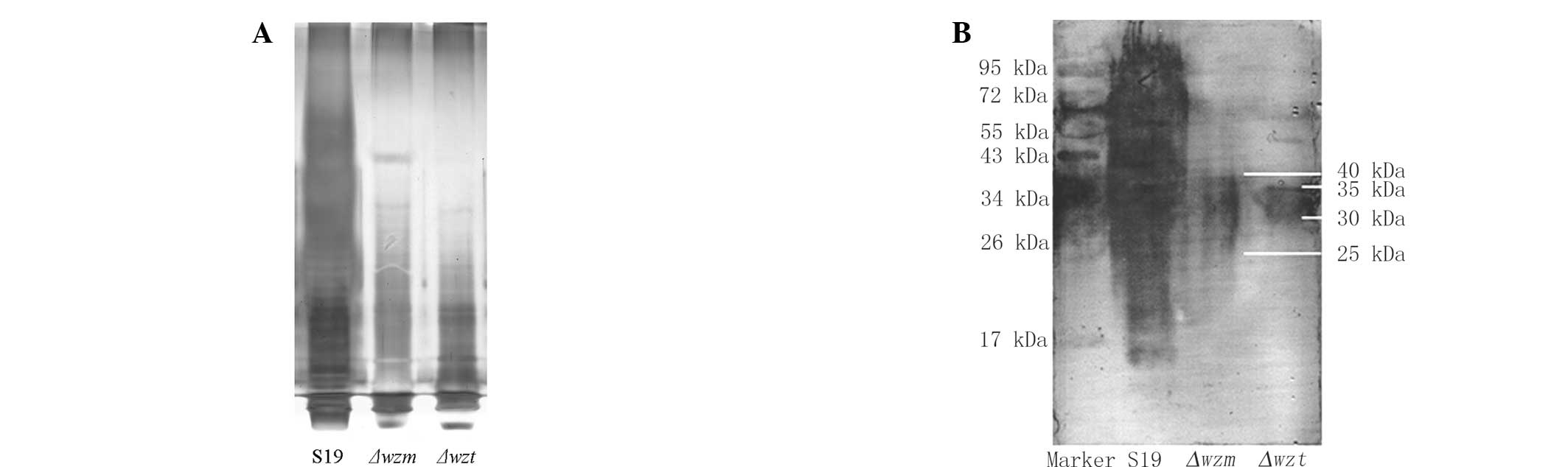
wzm and wzt mutation causes differences
in LPS
The LPS of S19, Δwzm and Δwzt was
extracted by kits. Fig. 2A shows
that the crude LPS of mutants was significantly changed compared
with the S19 strain. There was no detectable signal of extracted
LPS from the Δwzm and Δwzt mutants. There was no
difference between the Δwzm and Δwzt mutants. This
indicated that the mutants of Δwzm and Δwzt may be
able to interfere with LPS synthesis.
The western blotting results indicated that LPS in
Δwzm and Δwzt mutants was significantly different
from S19 (Fig. 2B). The molecular
weight of normal S19 LPS ranged from 10 to 100 kDa, whereas that of
Δwzm and Δwzt mutants was clustered between 25 and 40
kDa and 30 and 35 kDa separately.
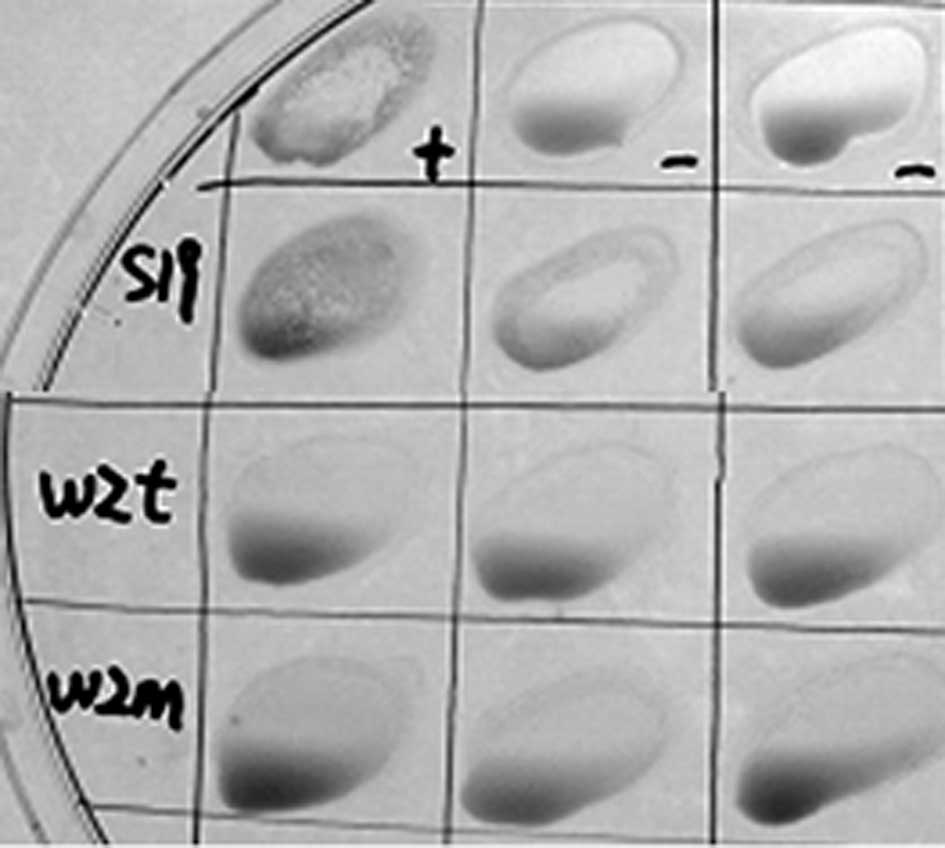
wzm and wzt mutants lack antigenicity to
LPS antibodies
Smooth strains of brucella present O-polysaccharides
on their surface. B. abortus S19 as a vaccine maintains the
O-antigen, which causes difficulties in its diagnosis. The Rose
Bengal plate agglutination test (RBPT) was performed (21,22).
The results revealed that the serum of S19 was positive and
Δwzm and Δwzt were negative compared with the
positive and negative serum in regard to agglutination (Fig. 3). The tube agglutination tests
revealed that the titer of the S19 serum was over 1:100 (++,
positive ratio was 100%), while the titers of Δwzm and
Δwzt were not detected (negative ratio was 100%). These
results indicated that there were no effective LPS antibodies
formed by Δwzm and Δwzt mutant infection in BALB/c
mice.
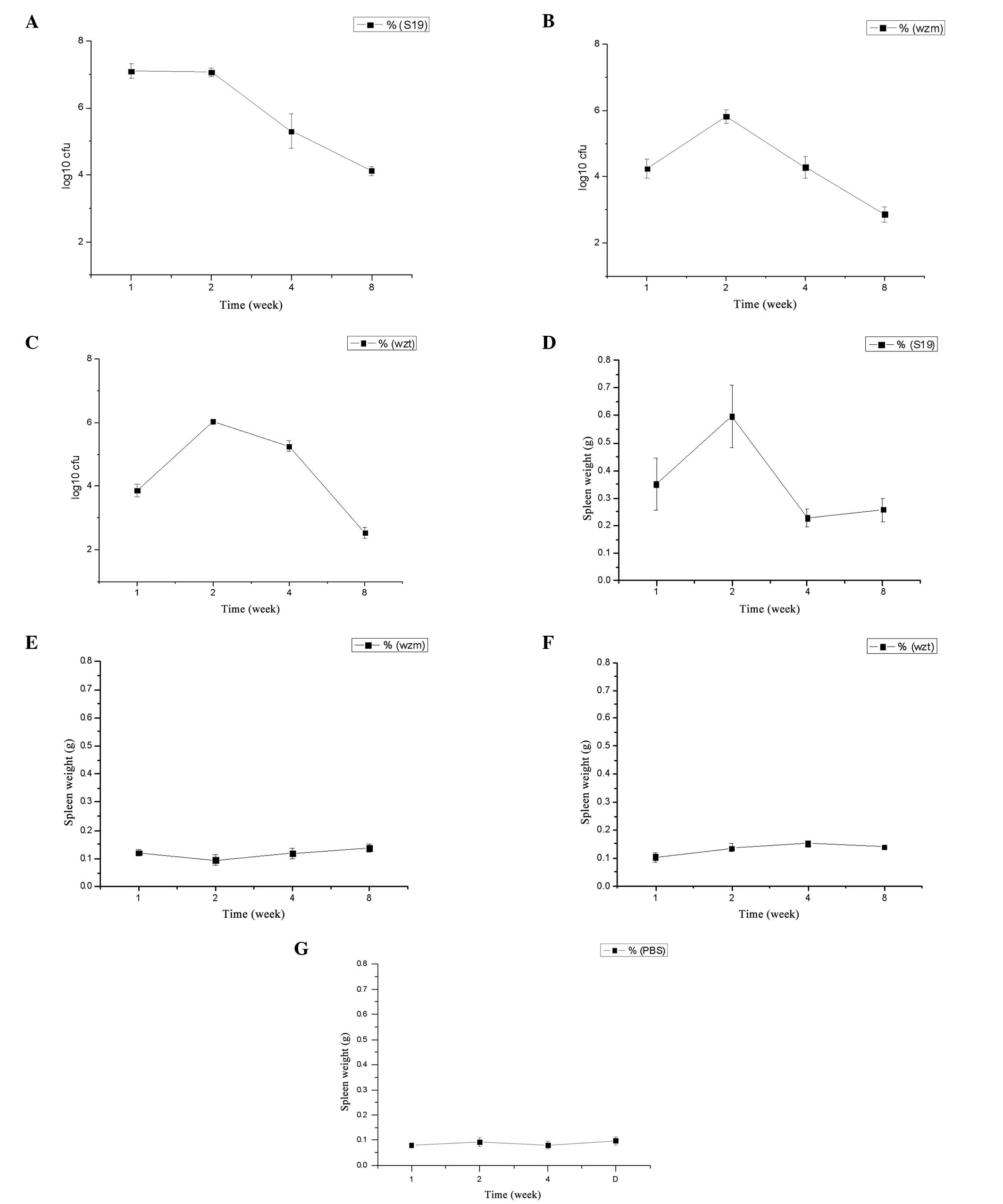
wzm and wzt mutation reduces
virulence
The infection kinetics in the spleens and the spleen
weight of BALB/c mice inoculated with the mutants and B.
abortus S19 are presented in Fig.
4A–C. The infection kinetics show that the log10 of
the number of colony-forming units (CFU) of S19 was maintained at
~7.1 prior to the second week and after two weeks it was decreased
to 4.1 until the eighth week. While the infection kinetics of
Δwzm and Δwzt mutants were just about half of that of
S19 following the first week, there was an increasing phase at the
second week prior to a decrease in the log10 of CFU to
2.9 for Δwzm, and 2.5 for Δwzt (Fig. 4A–C). The survival rate of the
mutants in vivo was lower than that for S19, which indicates
that the virulence was decreased. The virulence of Δwzm and
Δwzt was almost identical.
The spleen weight in mice injected with the S19
strain was significantly higher compared with that in mice injected
with the mutants and the PBS-negative control (P<0.01) (Fig. 4D–F). In particular, after two
weeks, the spleen weight showed a maximum following a decrease to a
relatively stable weight. The weight of the Δwzm and
Δwzt mutants was similar, and was increased compared with
that in the PBS group; however, the difference was not
significant.
Discussion
Knockout of the wzm and wzt genes
resulted in rough mutants. The wzm and wzt genes are
membrane-spanning and the associated ATP-binding homologues of
ABC-transporters are involved in transmembrane export for
O-polysaccharide biosynthesis (23). Mutantion of the wzm and
wzt genes is expected to result in rough mutants, such as
B. melitensis 16M (16,17,24).
Acriflavine agglutination indicated that the wzm and
wzt mutants are likely to be rough mutants (14). Smooth strains were not able to
induce agglutination of acriflavine. Analysis of crude LPS extracts
using western blot analysis with multiple antibodies and
serological test results provided more evidence that the
Δwzm and Δwzt mutants were rough mutants. They were
able to be distinguished from S19.
The molecular weights of crude LPS profiles were
evaluated using western blot analysis. The results revealed that
the molecular weight of LPS in the mutants was significantly
different compared with that in S19. This result may provide
information on the O-LPS synthesis mechanism. The wzm and
wzt genes are components of ABC transporters and are
expected to have a similar function in LPS synthesis (16,17,24).
The difference in the obtained results may be caused by the
different effects of the wzm and wzt gene disruption
process; however, more evidence is required.
Knockout of the wzm and wzt genes
caused a reduction in virulence. LPS is one of the predominant
virulence factors, which provides bacterial resistance to
anti-microbial attacks and modulates the host immune response,
making it a significant virulence factor for the survival and
replication in the host cells. LPS may be the dominating factor of
S19 virulence (10). Wzm
and wzt genes are the putative genes of the ABC transporter
system. Although there is no evidence in regard to Wzm and Wzt
proteins structure and function in cells, evidence of exogenous
transporter insertion in wzm and wzt genes causing
B. melitensis virulence attenuation has been reported
(23). Attenuation or optimization
of S19 are likely to be required in order to develop a human
vaccine strain (25). The
virulence results indicated that the infectious ability of
Δwzm and Δwzt mutants was lower, while there was no
difference between them, and the knockout of wzm and
wzt reduced the virulence of S19 in a similar manner to that
reported for RB 51 and other rough mutant strains (12–14,24).
These strains may be applicable for studies on the mechanism of LPS
on S19 virulence.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 31302062), the
National Science and Technology Ministry (no. 2010BAD04B03) and the
Key Project of Chinese National Programs for Fundamental Research
and Development (no. 2012CB722501).
References
|
1
|
Cardoso PG, Macedo GC, Azevedo V and
Oliveira SC: Brucella spp noncanonical LPS: structure,
biosynthesis, and interaction with host immune system. Microb Cell
Fact. 5:132006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rambow-Larsen AA, Petersen EM, Gourley CR
and Splitter GA: Brucella regulators: self-control in hostile
environment. Trends Microbiol. 17:371–377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pappas G, Papadimitriou P, Akritidis N,
Christou L and Tsianos EV: The new global map of human brucellosis.
Lancet Infect Dis. 6:91–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ficht TA, Kahl-McDonagh MM, Arenas-Gamboa
AM and Rice-Ficht AC: Brucellosis: the case for live, attenuated
vaccines. Vaccine. 27:D40–D43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spink WW, Hall JW, Finstad J and Mallet E:
Immunization with viable Brucella organisms. Results of a safety
test in humans. Bull World Health Organ. 26:409–419.
1962.PubMed/NCBI
|
|
6
|
Fugier E, Pappas G and Gorvel JP:
Virulence factors in brucellosis: implications for
aetiopathogenesis and treatment. Expert Rev Mol Med. 9:1–10. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bundle DR, Cherwonogrodzky JW, Gidney MA,
Meikle PJ, Perry MB and Peters T: Definition of Brucella A and M
epitopes by monoclonal typing reagents and synthetic
oligosaccharides. Infect Immun. 57:2829–2836. 1987.PubMed/NCBI
|
|
8
|
Weynants V, Gilson D, Cloeckaert A, Tibor
A, Denoel PA, Godfroid F, Limet JN and Letesson JJ:
Characterization of smooth lipopolysaccharides and O
polysaccharides of Brucella species by competition binding assays
with monoclonal antibodies. Infect Immun. 65:1939–1943.
1997.PubMed/NCBI
|
|
9
|
Ugalde JE, Comerci DJ, Leguizamón MS and
Ugalde RA: Evaluation of Brucella abortus phosphoglucomutase
(pgm) mutant as a new live rough-phenotype vaccine. Infect Immun.
71:6264–6269. 2003.PubMed/NCBI
|
|
10
|
Lapaque N, Moriyon I, Moreno E and Gorvel
JP: Brucella lipopolysaccharide acts as a virulence factor. Curr
Opin Microbiol. 8:60–66. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fernandez-Prada CM, Zelazowska EB,
Nikolich M, Hadfield TL, Roop RM 2nd, Robertson GL and Hoover DL:
Interactions between Brucella melitensis and human phagocytes:
bacterial surface O-Polysaccharide inhibits phagocytosis, bacterial
killing, and subsequent host cell apoptosis. Infect Immun.
71:2110–2119. 2003. View Article : Google Scholar
|
|
12
|
Jiménez de Bagüés MP, Terraza A, Gross A
and Dornand J: Different responses of macrophages to smooth and
rough Brucella spp: relationship to virulence. Infect Immun.
72:2429–2433. 2004.PubMed/NCBI
|
|
13
|
Moriyón I, Grilló MJ, Monreal D, González
D, Marín C, López-Goñi I, Mainar-Jaime RC, Moreno E and Blasco JM:
Rough vaccines in animals brucellosis: structural and genetic basis
and present status. Vet Res. 35:1–38. 2004.PubMed/NCBI
|
|
14
|
Adone R, Ciuchini F, Marianelli C,
Tarantino M, Pistoia C, Marcon G, Petrucci P, Francia M, Riccardi G
and Pasquali P: Protective properties of rifampin-resistant rough
mutants of Brucella melitensis. Infect Immun. 73:4198–4204. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haag AF, Myka KK, Arnold MF,
Caro-Hernández P and Ferguson GP: Importance of lipopolysaccharide
and cyclic β-1,2-glucans in Brucella-mammalian infections. Int J
Microbiol. 2010:1245092010.
|
|
16
|
Cloeckaert A, Grayon M, Verger JM,
Letesson JJ and Godfroid F: Conservation of seven genes involved in
the biosynthesis of the lipopolysaccharide O-side chain in Brucella
spp. Res Microbiol. 151:209–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Godfroid F, Cloeckaert A, Taminiau B,
Danese I, Tibor A, de Bolle X, Mertens P and Letesson JJ: Genetic
organization of the lipopolysaccharide O-antigen biosynthesis
region of Brucella melitensis 16M (wbk). Res Microbiol.
151:655–668. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allen CA, Adams LG and Ficht TA:
Transposon-derived Brucella abortus rough mutants are
attenuated and exhibit reduced intracellular survival. Infect
Immun. 66:1008–1016. 1998.PubMed/NCBI
|
|
19
|
Tsai CM and Frasch CE: A sensitive silver
stain for detecting lipopolysaccharides in polyacrylamide gels.
Anal Biochem. 119:115–119. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ried JL and Collmer A: An nptI-sacB-sacR
cartridge for constructing directed, unmarked mutations in
gram-negative bacteria by marker exchange-eviction mutagenesis.
Gene. 57:239–246. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Orduna A, Almaraz A, Prado A, Gutierrez
MP, Garcia-Pascual A, Dueñas A, Cuervo M, Abad R, Hernández B,
Lorenzo B, Bratos MA and Torres AR: Evaluation of an
immunocapture-agglutination test (Brucellacapt) for serodiagnosis
of human brucellosis. J Clin Microbiol. 38:4000–4005.
2000.PubMed/NCBI
|
|
22
|
Seleem MN, Boyle SM and Sriranganathan N:
Brucellosis: a re-emerging zoonosis. Vet Microbiol. 140:392–398.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raetz CR and Whitfield C:
Lipopolysaccharide endotoxins. Annu Rev Biochem. 71:635–700. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
González D, Grilló MJ, De Miguel MJ, Ali
T, Arce-Gorvel V, Delrue RM, Conde-Alvarez R, Muñoz P, López-Goñi
I, Iriarte M, Marín CM, Weintraub A, Widmalm G, Zygmunt M, Letesson
JJ, Gorvel JP, Blasco JM and Moriyón I: Brucellosis vaccines:
assessment of Brucella melitensis lipopolysaccharide rough mutants
defective in core and O-polysaccharide synthesis and export. PLoS
One. 3:e27602008.
|
|
25
|
Langford MJ and Myers RC: Difficulties
associated with the development and licensing of vaccines for
protection against bio-warfare and bio-terrorism. Dev Biol (Basel).
110:107–112. 2002.PubMed/NCBI
|