Introduction
Lung cancer has become one of the leading causes of
cancer-related mortality worldwide, and the most common form,
non-small cell lung cancer (NSCLC), accounts for 80–85% of these
cases (1). Clinical chemotherapy,
particularly with platinum-based chemotherapy regimens, can prolong
survival and improve the quality of life in patients with advanced
NSCLC; however, the overall prognosis remains unsatisfactory. With
the development of oncology, molecular targeted therapeutic drugs
have become widely used and are, at present, the clinical treatment
of choice for advanced NSCLC. The use of molecular selection
markers, other than epidermal growth factor receptor (EGFR)
mutation testing for EGFR-tyrosine kinase inhibitor (TKI) treatment
assignment, remains speculative at present, particularly for
patients who have already undergone first-line chemotherapy for
advanced stages of the disease (2). However, clinical experience, clinical
and metrological data and findings at the molecular biology level
all indicate that the occurrence of resistance is a constraint on
the further use of TKIs and represents a bottleneck (3).
Gefitinib is a small-molecule quinazoline derivative
that was developed as a TKI of the EGFR (4). The EGFR is known to promote cell
growth, functions as an oncogene and is expressed in up to 80–90%
of NSCLC cases (5). Gefitinib has
been shown to induce radiographic tumor regression in patients with
NSCLC that persisted following chemotherapy (6). However, despite the significant
responses to EGFR-TKIs in patients with NSCLC with EGFR-activating
mutations, de novo resistance to TKIs has been observed
(7). Additional treatments for
cases of NSCLC relapses following treatment with gefitinib are
urgently required (8). Salvage
chemotherapy has been shown to be less efficacious and is often at
the expense of severe residual chemotherapy-related side effects
(9). However, certain natural
products are suitable alternatives that could be used for
controlling cancer. In the past few decades, increasing attention
has been focused on finding biologically active cancer therapeutic
agents from natural resources (10).
Rhizoma paridis is the root of either Paris
polyphylla Smith var. chinensis (Franch.) Hara or
Paris polyphylla Smith var. yunnanensis (Franch.)
Hand-Mazz. Rhizoma paridis has been reported to exert numerous
pharmacological effects, including anti-inflammatory, hemostatic
and anti-cancer effects, and was shown to exhibit inhibitory
effects on tumor growth in numerous studies using hepatic, gastric
or nasopharyngeal carcinoma models (11–16).
Furthermore, Paris saponin II significantly inhibited tumor growth
by 70% in the human SKOV3 ovarian cancer xenograft model (17), and Paris saponin H showed a marked
cytotoxic activity on A549 cells with an IC50 value of
1.53±0.08 μg/ml (18). Paris
saponin D has been shown to overcome drug resistance in R-HepG2
cells, elicit programmed cell death via mitochondrial dysfunction,
inhibit endothelial cell functions in vitro and inhibit
angiogenesis in zebrafish embryos in vivo (19,20).
Preclinical studies have made Paris saponins emerge as promising
anti-cancer agents. Paris saponin I (PSI) has been demonstrated to
exert a wide range of pharmacological activities and cytotoxicity
against a number of malignancies, such as NSCLC, by increasing
levels of B-cell lymphoma 2-associated X protein (Bax) and
cytochrome c, activating caspase-3 and caspase-9, cleaving
polymerase, and by decreasing B-cell lymphoma 2 (Bcl-2) expression
levels and extracellular signal-regulated kinase-1/2 activity
(21).
PSI has been approved for cancer therapy due to its
potential involvement in the suppression of tumor growth. However,
the effects of PSI in gefitinib-resistant NSCLC, with regard to
increasing the Bax/Bcl-2 ratio and caspase-3 levels, have yet to be
demonstrated in vitro. The aim of the present study was to
focus on further investigating the effects of PSI on NSCLC with
acquired gefitinib resistance in vitro and in vivo.
The effects of PSI on a panel of gefitinib-resistant NSCLC cell
lines were examined in vitro, and tumor glucose metabolism
was evaluated in nude mice by micro-positron emission tomography
(microPET) scanning in vivo.
Materials and methods
Drugs and reagents
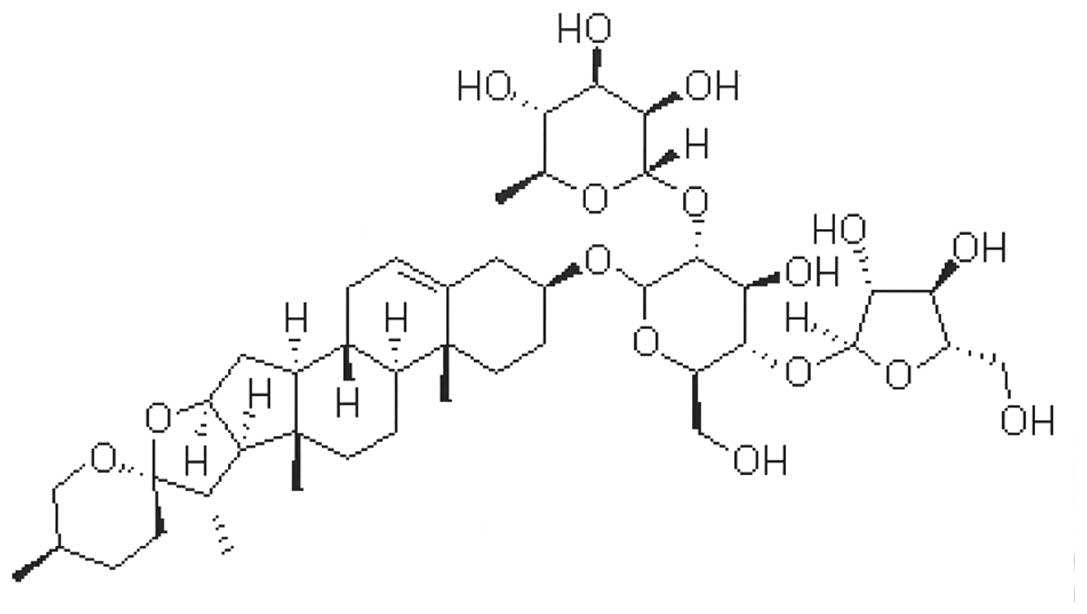
PSI, which has a molecular formula of
C44H70O16 (Fig. 1), was purchased at a purity of
>99% from the Zhejiang Institute for Food and Drug Control
(batch no. 111590, Hangzhou, China). PSI was dissolved in
dimethylsulfoxide (DMSO) as a 100 μg/μl stock solution and stored
at -20°C. This was subsequently diluted in Dulbecco’s Medium
Eagle’s medium (DMEM) to achieve the final concentration indicated
for each experiment. DMEM and fetal calf serum were obtained from
Hyclone Co. (Logan, UT, USA). Polyclonal rabbit anti-rat Bax
antibody (P-19) and monoclonal mouse anti-rat Bcl-2 antibody, both
at dilutions of 1:2,000, were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA) (22). Rabbit anti-rat caspase 3 polyclonal
antibody, at a dilution of 1:25, was purchased from Abcam
(Cambridge, MA, USA) (23). The
fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection kit
was from BD Biosciences (Franklin Lakes, NJ, USA). Cell culture
flasks, as well as six- and 96-well cell culture plates, were from
Corning Chemical Co. (New York, NY, USA). All other chemical
reagents were from Sigma Chemical Co. (St Louis, MO, USA).
Cell lines and culture
The gefitinib-resistant PC-9-ZD cell line was
cultured in DMEM supplemented with 10% fetal bovine serum in a
humidified incubator (Fischer Scientific, Inc., Houston, TX, USA)
containing 5% CO2 at 37°C. PC-9-ZD cells were derived
from human NSCLC PC-9 cells (derived from a patient with
adenocarcinoma). The clone of a gefitinib-resistant cell line,
known as ‘PC-9-ZD’, was selected following its development in
gefitinib (200 nmol/l) for three months. PC-9-ZD cells were more
resistant to gefitinib than their parental PC-9 cells (8).
Animals
Eight-week-old nude male mice, weighing 20.13±0.98
g, were obtained from the Experimental Animal Center of Zhejiang
Chinese Medical University (Hangzhou, China). Mice were housed in
plastic cages with sterilized bedding in an air-conditioned room at
22±1°C and 51±4% humidity with a 12-h light/dark cycle and access
to filtered water and total nutrient feed. The experiments were
performed in accordance with the national guidelines for animal
care and use. The present study was approved by the ethics
committee of Zhejiang Hospital (Hangzhou, Zhejiang, China).
MTT assay
The MTT colorimetric assay was performed to detect
cell proliferation following exposure to PSI. Following harvesting
by trypsinization, the PC-9-ZD cells (100 μl/well) were seeded in
96-well plates at a density of 1×104 cells/ml. Each
group had three wells with a nontreated group as the control. When
the cells had attached to the plates, PSI was added at various
concentrations (0.5, 1, 2, 3, 4, 5, 6, 7, 8 and 9 μg/ml) and the
plates were incubated at 37°C in a humidified atmosphere containing
5% CO2. Following incubation for the indicated time
intervals, 20 μl 0.5% MTT was added to each well and cultured for a
further 4 h. The supernatant was discarded and the MTT formazan
precipitate was dissolved in 150 μl DMSO, agitated for 10 min and
then the absorbance (A) value was measured at 492 nm using a
multiscanner autoreader. The following formula was used: Inhibition
rate (%)=(1-average A value of the experimental samples)/average A
value of the control) ×100.
Cell cycle analysis by flow
cytometry
Following treatment with PSI (1, 2 and 4 μg/ml) and
incubation for 48 h, PC-9-ZD cells were collected. The cells were
then resuspended and fixed in 70% ice-cold ethanol overnight at
−20°C. The next day, the cells were incubated in 10 μg/ml RNase for
30 min at 37°C and then stained with 50 μg/ml propidium iodide (PI)
for 1 h at 4°C in the dark. Cell cycle analysis was performed on a
FACSCalibur flow cytometer (BD Biosciences) and the data were
analyzed using CellQuest™ software (BD Biosciences). The
experiments were repeated three times.
Assessment of apoptosis using flow
cytometry
Apoptosis was examined by the Annexin-V/PI method.
PC-9-ZD cells (2 ml/well) were seeded into a six-well plate at a
density of 1×104 cells/ml. Following treatment with PSI
(1,2 and 4 μg/ml) and incubation for 48 h, the PC-9-ZD cells were
collected and apoptosis was examined by using an Annexin V-FITC
apoptosis detection kit (BD Biosciences), which detects
phosphatidylserine exposed on the outer surface of the cell
membrane. The cells were harvested with trypsin and washed with
phosphate-buffered saline (PBS). Following centrifugation at 100 ×
g, the supernatant was removed and the cells were suspended in a
stain containing Annexin V-FITC and PI. The suspension was mixed
and incubated at room temperature for 15 min in the dark. The cells
were analyzed by FACSCalibur flow cytometry (BD Biosciences) within
1 h of staining. Data from 1×106 cells were collected
for each data file. Apoptotic cells were defined as Annexin
V-FITC-positive and PI-negative cells.
Morphology of apoptotic cells
Following incubation with 1, 2 and 4 μg/ml PSI for
48 h, PC-9-ZD cells were collected and centrifuged at 400 × g to
obtain a pellet. Pellets were fixed with 2.5% glutaraldehyde in
0.02 M PBS (pH 7.4) at 4°C for 4 h and post-fixed in 1% osmic acid
for 1 h, dehydrated in an ascending acetone series and subsequently
embedded in Epon816. Ultrathin 70-nm sections were stained with
uranyl acetate and lead citrate. The ultrastructural organization
was observed with a JEM-1200EX transmission electron microscope
(Jeol, Tokyo, Japan).
Western blot analysis
Following incubation with 4 μg/ml PSI for 48 h,
PC-9-ZD cells were collected. Samples containing equal amounts of
protein were electrophoresed using 10% SDS-PAGE, transferred onto
polyvinylidene fluoride membranes and then incubated with specific
primary antibodies. The blots were reacted with horseradish
peroxidase-conjugated secondary antibodies and were detected using
the enhanced chemiluminescence system (Santa Cruz Biotechnology,
Inc.). The density of the band was quantified by densitometry and
exposed to X-ray film (Eastman-Kodak, Rochester, NY, USA) using
GAPDH levels as a control.
In vivo xenograft studies and PSI
administration
Nude mice were inoculated subcutaneously into the
left flank with ~2×106 cells. When the xenograft grew to
~15 mm in diameter for 1–2 weeks, the mice were randomly divided
into four groups (six mice/group): The mice in the control group
were intramuscularly injected with normal saline and those in the
PSI treatment groups received 2, 4 and 8 mg/kg PSI, respectively,
by gavage administration once a day. Tumor growth was measured by
18F-fludeoxyglucose (FDG) microPET scan at the 14th day
after administration. At the end of the experiments, the mice were
sacrificed.
microPET scanning and data analysis
The tracer 18F-FDG was synthesized
routinely using an automatic 18F-FDG synthesizer
(FDG-F100; Sumitomo Heavy Industries, Ltd., Shinagawa, Japan), and
the radiochemical purity of the 18F-FDG produced was
>99%. Following anesthesia with pentobarbital (35 mg/kg,
intraperitoneally), the mice were injected with 0.1 mCi
18F-FDG through the tail vein. Mice were placed in a
spread prone position on a dedicated holder for scanning. Static
acquisition was performed in a three dimensional mode using the
microPET imaging system (R4; Concorde Microsystems, Knoxville, TN,
USA). A 10-min data collection was performed for 18F-FDG
microPET with an uptake time of 30 min after tracer injection.
Image reconstruction was performed with attenuation by an ordered
subset of expectations of maxima using the posteriori algorithm.
Corrections for dead-time and random scattering were also
performed. Transaxial, coronal and sagittal computed tomographic
slices were then obtained. For semi-quantitative evaluation using
the standardized uptake value (SUV), the region of interest (ROI)
method was used to evaluate the regional uptake of
18F-FDG. ROIs were drawn around the tumor manually. The
mean uptake (percentage injection dose) in the ROI was recorded and
calculated automatically. The SUV was calculated as follows: SUV =
ROI activity × mouse weight / injected dose.
Statistical analysis
The experiments were performed in triplicate. Data
are presented as the mean ± standard deviation. Groups were
compared using one-way analysis of variance. Differences between
the treatment groups were considered significant at P<0.05 or
P<0.01.
Results
PSI inhibits the proliferation of PC-9-ZD
cells
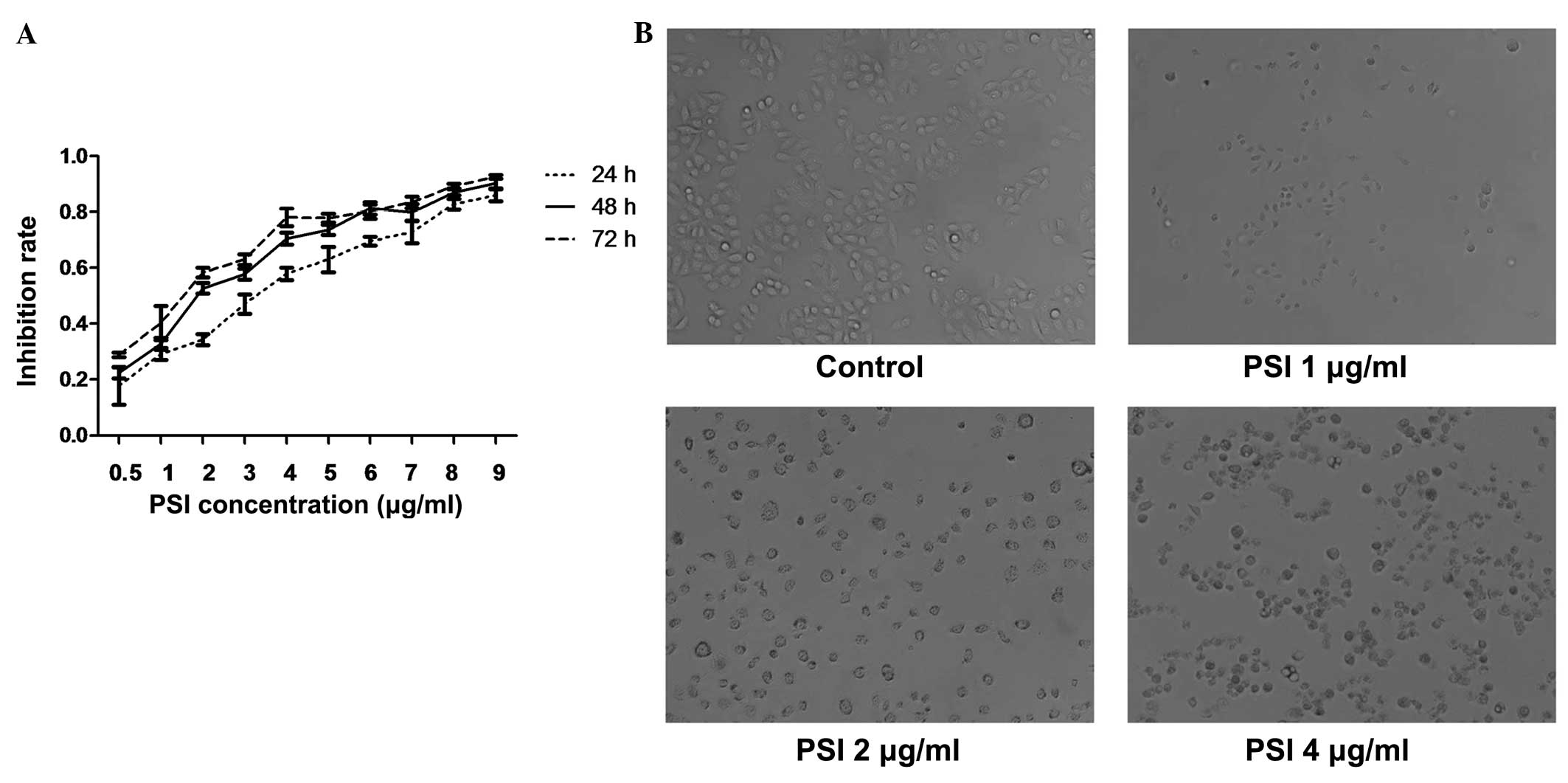
PSI inhibited the growth of PC-9-ZD cells in a time-
and dose-dependent manner with increasing concentrations from 0.5
to 9 μg/ml and incubation times of 24, 48 and 72 h. The
IC50 of PSI at 24, 48 and 72 h of incubation was 2.51,
2.07 and 1.53 μg/ml, respectively (Fig. 2A). As shown in Fig. 2B, MTT assay revealed that PSI
inhibited the growth of PC-9-ZD cells.
PSI modifies the cell cycle in PC-9-ZD
cells
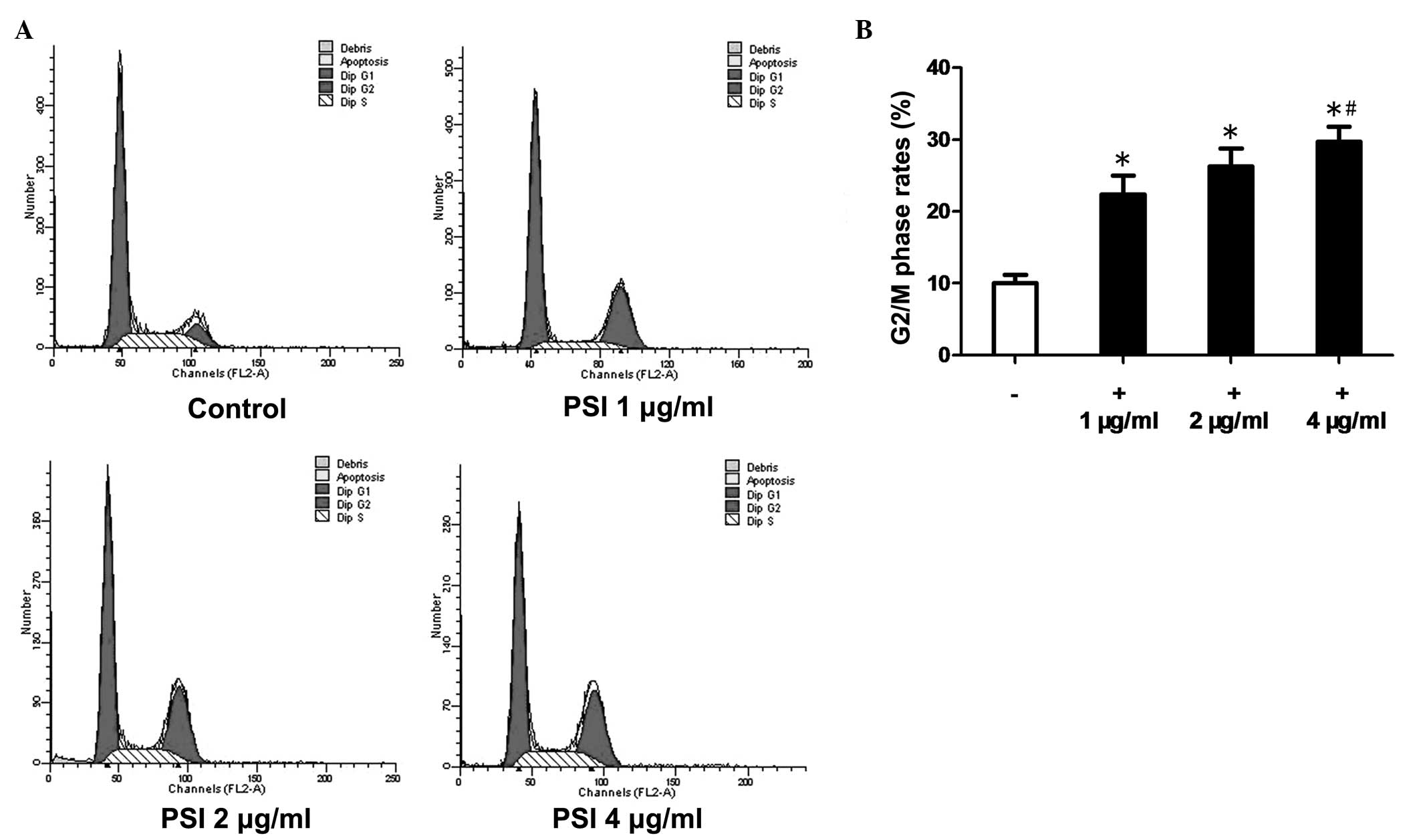
Flow cytometric analysis revealed that, after 48 h
of PSI treatment, the cell cycle progression in PC-9-ZD cells was
disrupted with an increasing percentage of cells in G2/M phase
(Fig. 3A). The G2/M phase rates
were 10.2% in the control group and 22.4, 26.3 and 29.7% in the
PSI-treated (1, 2 and 4 μg/ml) groups, respectively (Fig. 3B).
PSI induces apoptosis in PC-9-ZD
cells
The ability of PSI to induce apoptosis in PC-9-ZD
cells was assessed using the Annexin V/PI method. PSI induced
significant concentration-dependent levels of apoptosis in PC-9-ZD
cells (Fig. 4A). The apoptosis
rate was 0.3% in the control group as compared with 17.2, 20.3 and
32.2% in the PSI-treated (1, 2 and 4 μg/ml, respectively) groups
after 48 h of PSI treatment (Fig.
4B).
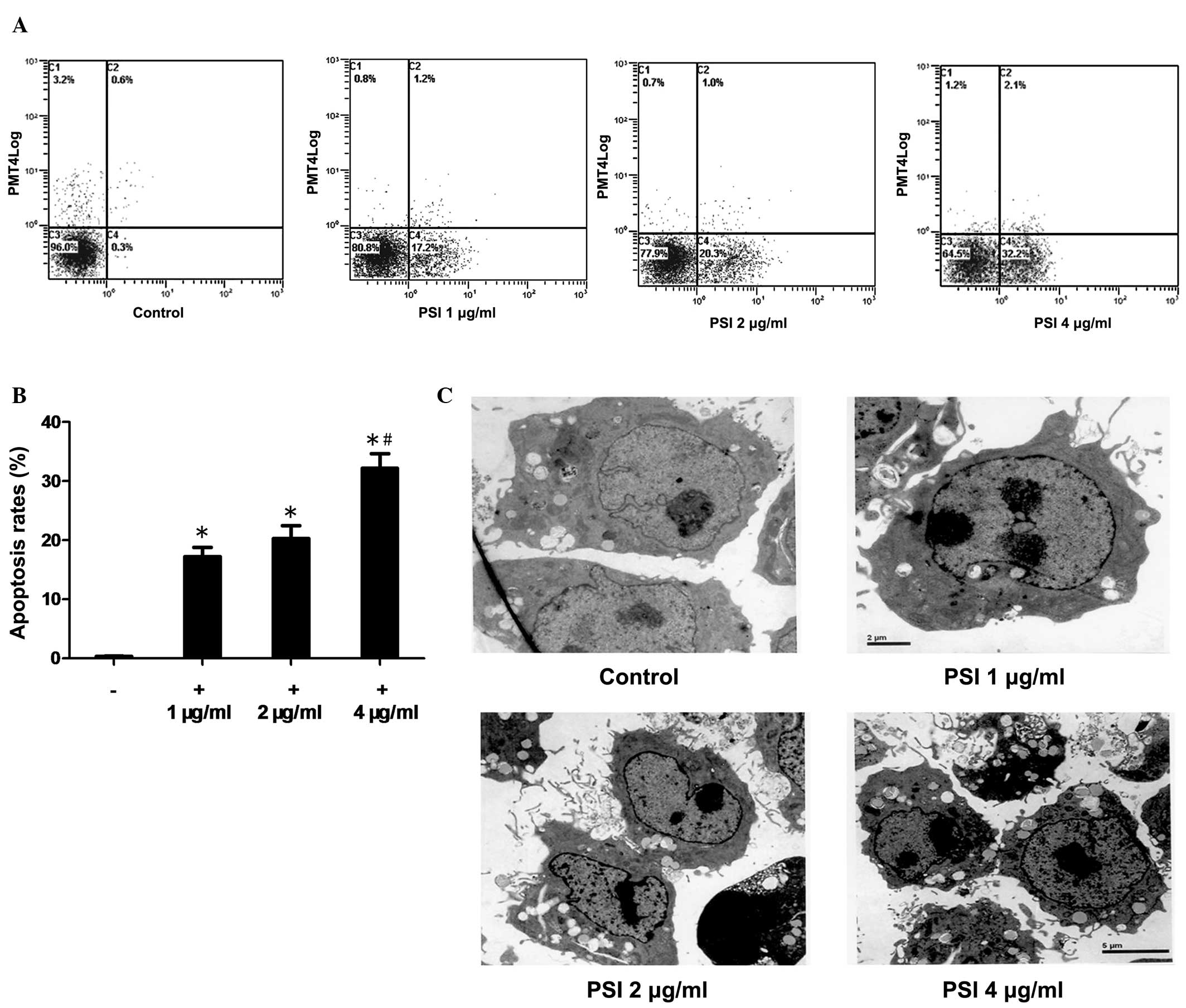 | Figure 4Apoptosis induced by PSI. (A) Flow
cytometric analysis of apoptosis in the PSI-treated (1, 2 and 4
μg/ml) and control groups. (B) Percentage of apoptotic cells at
different PSI concentrations. *Statistically significant
difference (P<0.01) between the treated groups and the control
group. #Significantly increased in the 4 μg/ml PSI group
compared with the 1 and 2 μg/ml PSI groups (all P<0.05). (C)
Transmission electron microscopy showing cross-sectional features
of apoptosis, including cell shrinkage, chromatin condensation,
integrity of the plasma membrane, increased cellular granularity,
nuclear collapse, continual blebbing and the formation of apoptotic
bodies, in the PSI (1, 2 and 4 μg/ml) groups compared with the
control group. PSI, Paris saponin I. |
Morphology of apoptotic cells
The morphological observation of PSI-induced
apoptosis in PC-9-ZD cells using transmission electron microscopy
showed cross-sectional features of apoptosis: Cell shrinkage,
chromatin condensation, integrity of the plasma membrane, increased
cellular granularity, nuclear collapse and continual blebbing and
the formation of apoptotic bodies (Fig. 4C). The morphology of apoptotic
PC-9-ZD cells was assessed following treatment with 1, 2 and 4
μg/ml PSI for 48 h, respectively.
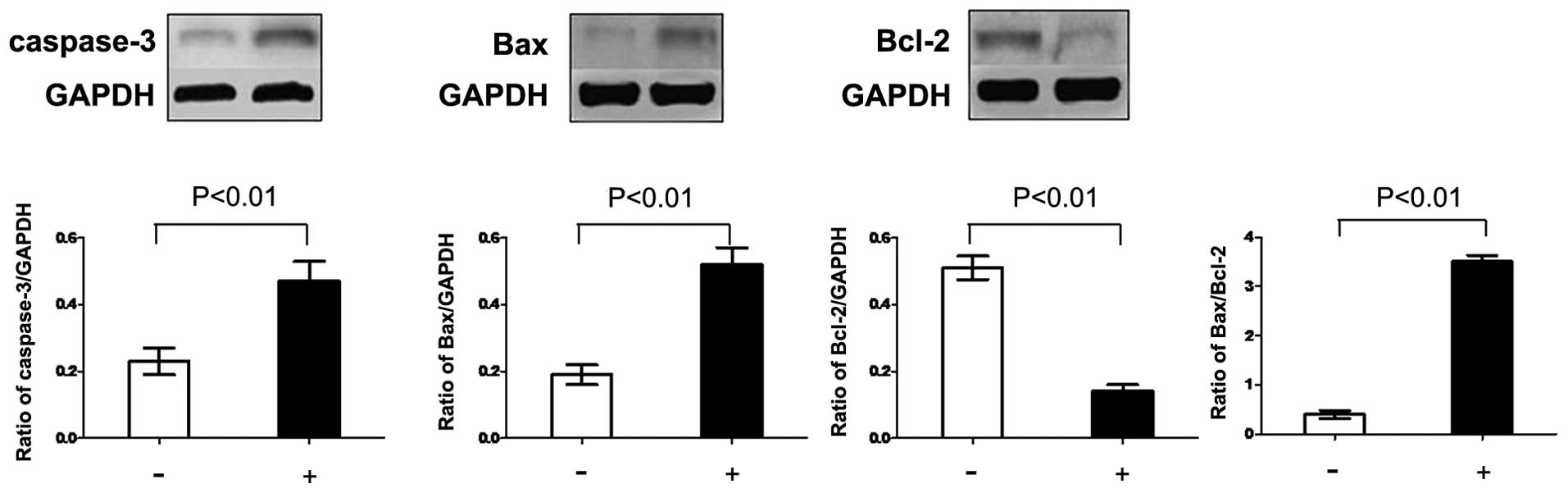
Effects of PSI on the levels of Bcl-2,
Bax and caspase-3 in PC-9-ZD cells
In order to explore potential signaling pathways by
which PSI induced apoptosis, western blot analysis was used to
evaluate the expression of the Bcl-2 family and caspase-3 protein.
The level of Bcl-2 protein decreased, while the level of Bax
protein increased following treatment with PSI for 48 h. The ratio
of Bax to Bcl-2 was significantly enhanced. Furthermore, the
expression of caspase-3 protein was significantly enhanced
(Fig. 5).
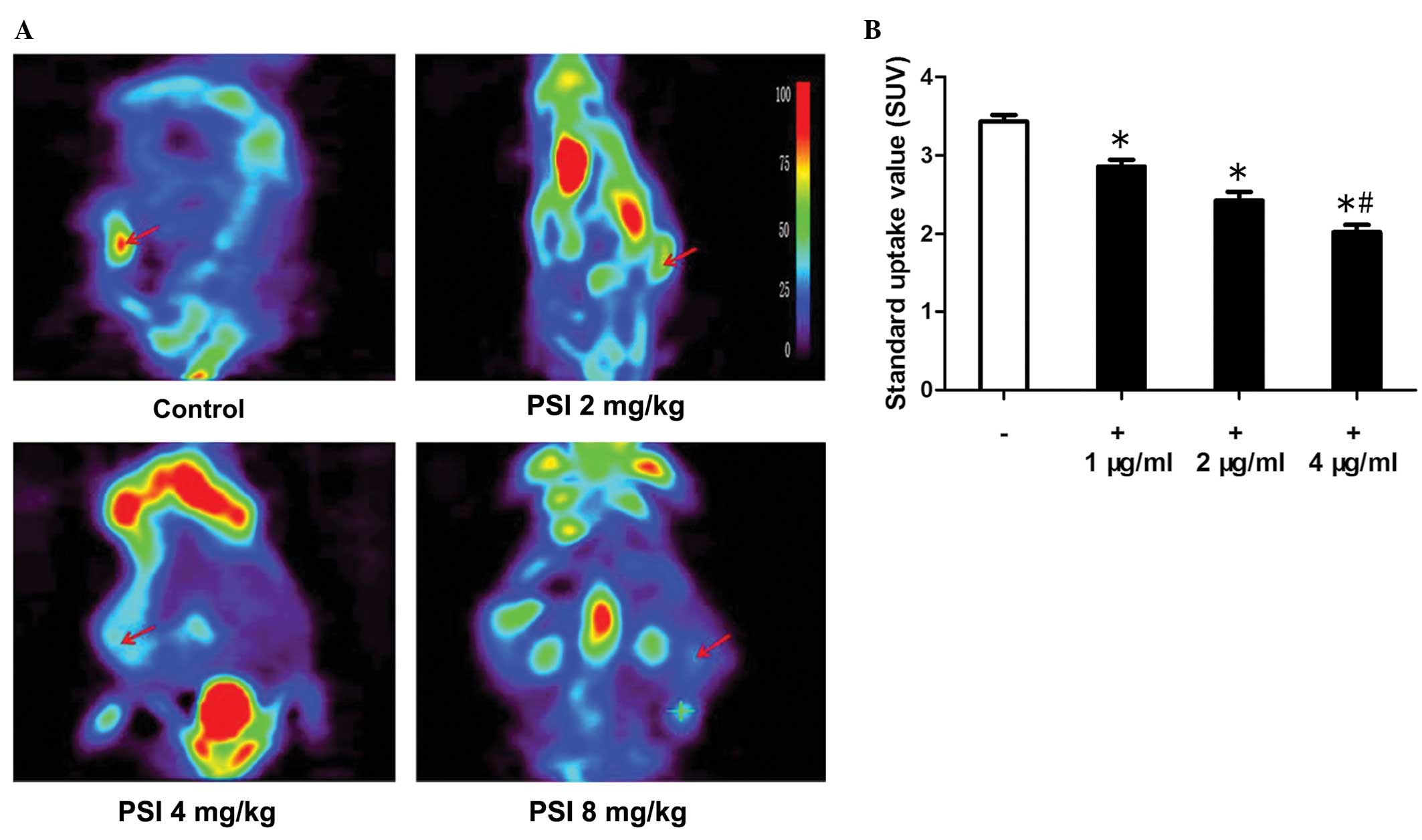
PC-9-ZD tumor xenograft glucose
metabolism variation and data analysis
18F-FDG-microPET imaging has been
extensively used for diagnosing tumors that exhibit higher
metabolic activity than normal tissue. To visualize glucose uptake
in tumor xenografts following treatment,
18F-FDG-microPET imaging was performed. Compared with
muscle tissue with endogenous 18F-FDG signals, the
uptake by tumors was significantly higher, as shown in Fig. 6A. The microPET imaging data showed
that the 18F-FDG uptake in the control group was higher
than that in the PSI-treated groups; the data are quantitatively
summarized in Fig. 6B. This
indicates that 18F-FDG-microPET imaging may not be able
to demonstrate the metabolic outcome of tumor growth
inhibition.
Discussion
Lung cancer is by far the leading cause of
cancer-related mortality within the United States and throughout
the world (24), with a global
incidence. There has been much progress in the treatment of the
disease, which is associated with improved surgical techniques,
combined modality treatment of limited-stage NSCLC, improved
symptom palliation and moderate but significant improvements in the
survival of stage IV of the disease (25). However, further research is
required in a field in which only 15–17% of patients live five
years. The role of chemotherapy in the last decade has expanded
substantially, with evidence for increases in the median survival
at stage IV from four months to 8–10 months, as well as
improvements in symptom control (26). Furthermore, progress has been
observed in the use of concomitant chemoradiotherapy, which has
become the mainstream approach for treating patients with locally
advanced NSCLC (27). A trial with
cisplatin-based chemotherapy suggested that, for resectable NSCLC
(28), the improvements in
disease-free and overall survival at five years were by 4–5%
(29). However, with the
development and progress of multiple small molecules and monoclonal
antibodies targeting important growth factor receptors, oncogenes
and tumor-suppressor genes known to be aberrant in lung cancer,
there is hope for further incremental improvements in the treatment
of this deadly disease.
Despite the continued decline in cancer mortality
rates over recent years, the total number of recorded
cancer-related mortalities worldwide continues to slightly increase
due to drug resistance (30). Due
to the genetic instability of cancer cells, genetic modifications
can enable them to acquire a phenotype with resistance to anti-EGFR
therapies. In combination, these findings support the importance of
understanding the molecular mechanisms affecting cancer cell
sensitivity or resistance to such inhibitors. The
gefitinib-resistant variant (PC-9-ZD) has been widely investigated
(31), and the most relevant
mechanisms contributing to the acquisition of
sensitivity/resistance to EGFR inhibitors include EGFR gene somatic
mutations in exons 18–21 in the EGFR kinase domain and loss of the
target, Akt, and inactivating mutations or loss of phosphatase and
tensin homolog function. Other mechanisms involve cyclin D1 and
cyclin-dependent kinase inhibitor 1B, which are commonly
deregulated in various types of cancer (32).
Preclinical studies have made Paris saponins emerge
as promising anti-cancer agents (33–37).
PSI exerts a wide range of pharmacological activities, including
cytotoxic activity against a number of malignancies, such as NSCLC
(38–42). PSI has been approved for cancer
therapy as a result of its potential involvement in the suppression
of tumor growth.
In the present study, the potential therapeutic
effects of PSI were evaluated in a gefitinib-resistant cell line.
Cell cycle regulation is important for cell proliferation, and the
present study showed that PSI changed the cycle distribution of
PC-9-ZD cells, leading to cell cycle arrest in G2/M phase. The
G2/M-phase rates were 10.2% in the control group and 22.4, 26.3 and
29.7% in the PSI treatment groups (1, 2 and 4 μg/ml, respectively).
PSI further increased the rate of apoptosis in PC-9-ZD cells. Rates
of apoptosis were 0.3% in the control group, while they were 17.2,
20.3 and 32.2% in the PSI treatment groups (1, 2 and 4 μg/ml,
respectively). This was further verified by transmission electron
microscopy, which showed cross-sectional features of apoptosis:
Cell shrinkage, chromatin condensation, integrity of plasma
membrane, increased cellular granularity, nuclear collapse,
continual blebbing and the formation of apoptotic bodies. Caspases
are crucial mediators of apoptosis and, among them, caspase-3 is a
frequently activated death protease catalyzing the specific
cleavage of numerous cellular key proteins (43). The Bcl-2 family, which comprises
both anti-apoptotic (including Bcl-2 and B-cell lymphoma extra
large) and pro-apoptotic (including Bax and Bcl-2-homologous
antagonist/killer) members, is the main controller and mediator of
apoptosis (44,45). In particular, a high Bcl-2/Bax
ratio is considered to be a crucial factor of cellular resistance
to apoptosis (46,47). Reduced levels of proliferation and
enhanced levels of apoptosis are associated with the upregulation
of the pro-apoptotic protein Bax (48). In the present study, the protein
levels of Bcl-2 were decreased, while those of Bax were increased
following treatment with PSI. Furthermore, the expression levels of
caspase-3 protein and the ratio of Bax to Bcl-2 were significantly
enhanced following PSI treatment. 18F-FDG-PET is a
pharmacodynamic biomarker for the early assessment of the treatment
response to drugs in NSCLC xenograft models (49). In the present study,
18F-FDG uptake in PSI treatment groups was lower
compared with that in the control group.
In conclusion, PSI is a potent antitumor agent that
acts by inhibiting Bcl-2 and enhancing the expression of caspase-3
protein, and it should be developed as a natural drug for the
therapy of gefitinib-resistant NSCLC.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81303274),
Wujieping Foundation of China (grant no. 320.6700.09035) and
Zhejiang Traditional Medicine Project (grant no. 2011ZZ011). The
authors would like to thank Dr Lijinhui from the Department of
Chinese Medicine and Rehabilitation at the Second Affiliated
Hospital of Zhejiang University School of Medicine for assistance
with the analysis of in vivo data.
References
|
1
|
Peters S, Adjei AA, Gridelli C, et al;
ESMO Guidelines Working Group. Metastatic non-small-cell lung
cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis,
treatment and follow-up. Ann Oncol. (Suppl 7): vii56–vii64.
2012.
|
|
2
|
Yang JJ, Chen HJ, Yan HH, et al: Clinical
modes of EGFR tyrosine kinase inhibitor failure and subsequent
management in advanced non-small cell lung cancer. Lung Cancer.
79:33–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Milella M, Nuzzo C, Bria E, et al: EGFR
molecular profiling in advanced NSCLC: a prospective phase II study
in molecularly/clinically selected patients pretreated with
chemotherapy. J Thorac Oncol. 7:672–680. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maemondo M, Inoue A, Kobayashi K, et al;
North-East Japan Study Group. Gefitinib or chemotherapy for
non-small-cell lung cancer with mutated EGFR. N Engl J Med.
362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukuoka M, Yano S, Giaccone G, et al:
Multi-institutional randomized phase II trial of gefitinib for
previously treated patients with advanced non-small-cell lung
cancer (The IDEAL 1 Trial). J Clin Oncol. 21:2237–2246. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kris MG, Natale RB, Herbst RS, et al:
Efficacy of gefitinib, an inhibitor of the epidermal growth factor
receptor tyrosine kinase, in symptomatic patients with non-small
cell lung cancer: a randomized trial. JAMA. 290:2149–2158. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin L and Bivona TG: Mechanisms of
resistance to epidermal growth factor receptor inhibitors and novel
therapeutic strategies to overcome resistance in NSCLC patients.
Chemother Res Pract. 2012:8172972012.PubMed/NCBI
|
|
8
|
Ji Y, Ma SL, Zhang YP, et al: Combined
treatment with TNF-alpha/gefitinib alleviates the resistance to
gefitinib in PC-9 cells. Anticancer Drugs. 20:832–837. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramalingam S and Sandler AB: Salvage
therapy for advanced non-small cell lung cancer: factors
influencing treatment selection. Oncologist. 11:655–665. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grabley S and Thiericke R: Bioactive
agents from natural sources: trends in discovery and application.
Adv Biochem Eng Biotechnol. 64:101–154. 1999.PubMed/NCBI
|
|
11
|
Xiao X, Bai P, Bui Nguyen TM, et al: The
antitumoral effect of Paris Saponin I associated with the induction
of apoptosis through the mitochondrial pathway. Mol Cancer Ther.
8:1179–1188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun J, Liu BR, Hu WJ, et al: In vitro
anticancer activity of aqueous extracts and ethanol extracts of
fifteen traditional Chinese medicines on human digestive tumor cell
lines. Phytother Res. 21:1102–1104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao M, Dai X, He X, et al: Paris saponin
I induces G2/M cell cycle arrest and apoptosis in human gastric
carcinoma SGC7901 cells. J Huazhong Univ Sci Technolog Med Sci.
31:768–772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Sun Y, Fan L, et al: Paris saponin
VII inhibits growth of colorectal cancer cells through Ras
signaling pathway. Biochem Pharmacol. 88:150–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
GuangLie C, WeiShi G, GaiLing H, et al:
Effect of paris saponin on antitumor and immune function in U14
tumor-bearing mice. Afr J Tradit Complement Altern Med. 10:503–507.
2013.PubMed/NCBI
|
|
16
|
Li Y, Gu JF, Zou X, et al: The anti-lung
cancer activities of steroidal saponins of P. polyphylla Smith var.
chinensis (Franch) Hara through enhanced immunostimulation in
experimental Lewis tumor-bearing C57BL/6 mice and induction of
apoptosis in the A549 cell line. Molecules. 18:12916–12936. 2013.
View Article : Google Scholar
|
|
17
|
Xiao X, Zou J, Bui-Nguyen TM, et al: Paris
saponin II of Rhizoma Paridis - a novel inducer of apoptosis in
human ovarian cancer cells. Biosci Trends. 6:201–211. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wen F, Yin H, Chen C, et al: Chemical
characteristics of saponins from Paris fargesii var.
brevipetala and cytotoxic activity of its main ingredient,
paris saponin H. Fitoterapia. 83:627–635. 2012.PubMed/NCBI
|
|
19
|
Cheung JY, Ong RC, Suen YK, et al:
Polyphyllin D is a potent apoptosis inducer in drug-resistant HepG2
cells. Cancer Lett. 217:203–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan JY, Koon JC, Liu X, et al:
Polyphyllin D, a steroidal saponin from Paris polyphylla,
inhibits endothelial cell functions in vitro and angiogenesis in
zebrafish embryos in vivo. J Ethnopharmacol. 137:64–69.
2011.PubMed/NCBI
|
|
21
|
Xiao M, Dai X, He X, et al: Paris saponin
I induces G2/M cell cycle arrest and apoptosis in human
gastric carcinoma SGC7901 cells. J Huazhong Univ Sci Technolog Med
Sci. 31:768–772. 2011.PubMed/NCBI
|
|
22
|
Yang B, Johnson TS, Thomas GL, et al: A
shift in the Bax/Bcl-2 balance may activate caspase-3 and modulate
apoptosis in experimental glomerulonephritis. Kidney Int.
62:1301–1313. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marneros AG, Grossman ME, Silvers DN, et
al: Pralatrexate-induced tumor cell apoptosis in the epidermis of a
patient with HTLV-1 adult T-cell lymphoma/leukemia causing skin
erosions. Blood. 113:6338–6341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smith W and Khuri FR: The care of the lung
cancer patient in the 21st century: a new age. Semin Oncol. 31(2
Suppl 4): 11–15. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pfister DG, Johnson DH, Azzoli CG, et al;
American Society of Clinical Oncology. American Society of Clinical
Oncology treatment of unresectable non-small-cell lung cancer
guideline: update 2003. J Clin Oncol. 22:330–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gerber DE and Schiller JH: Maintenance
chemotherapy for advanced non-small-cell lung cancer: new life for
an old idea. J Clin Oncol. 31:1009–1020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Videtic GM: Locally advanced non-small
cell lung cancer: what is the optimal concurrent chemoradiation
regimen? Cleve Clin J Med. 79(Suppl 1): eS32–eS37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bonomi M, Pilotto S, Milella M, et al:
Adjuvant chemotherapy for resected non-small-cell lung cancer:
future perspectives for clinical research. J Exp Clin Canc Res.
30:1152011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saintigny P and Burger JA: Recent advances
in non-small cell lung cancer biology and clinical management.
Discov Med. 13:287–297. 2012.PubMed/NCBI
|
|
30
|
Jemal A, Murray T, Samuels A, et al:
Cancer statistics, 2003. CA Cancer J Clin. 53:5–26. 2003.
View Article : Google Scholar
|
|
31
|
Morgillo F, Bareschino MA, Bianco R, et
al: Primary and acquired resistance to anti-EGFR targeted drugs in
cancer therapy. Differentiation. 75:788–799. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morgillo F, Cantile F, Fasano M, et al:
Resistance mechanisms of tumour cells to EGFR inhibitors. Clin
Transl Oncol. 11:270–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Zhang YJ, Gao WY and Yan LL:
Anti-tumor constituents from Paris polyphylla var.
yunnanensis. Zhongguo Zhong Yao Za Zhi. 32:1425–1428.
2007.(In Chinese).
|
|
34
|
Sun J, Liu BR, Hu WJ, et al: In vitro
anticancer activity of aqueous extracts and ethanol extracts of
fifteen traditional Chinese medicines on human digestive tumor cell
lines. Phytother Res. 21:1102–1104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee MS, Yuet-Wa JC, Kong SK, et al:
Effects of polyphyllin D, a steroidal saponin in Paris
polyphylla, in growth inhibition of human breast cancer cells
and in xenograft. Cancer Biol Ther. 4:1248–1254. 2005.PubMed/NCBI
|
|
36
|
Cheung JY, Ong RC, Suen YK, et al:
Polyphyllin D is a potent apoptosis inducer in drug-resistant HepG2
cells. Cancer Lett. 217:203–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Siu FM, Ma DL, Cheung YW, et al: Proteomic
and transcriptomic study on the action of a cytotoxic saponin
(Polyphyllin D): induction of endoplasmic reticulum stress and
mitochondria-mediated apoptotic pathways. Proteomics. 8:3105–3117.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang H, Su D and Ma SL: The effect of
Chonglou Saponin I on proliferation and apoptosis in lung
adenocarcinoma cell line PC9. J Chin Oncol. 18:166–169. 2012.(In
Chinese).
|
|
39
|
Hua YH, Ma SL, Fu ZF, et al: Effect of
Polyphyllin I on radiosensitivity in nasopharyngeal carcinoma cell
line CNE-2 in vitro. Chin Arch Trad Chin Med. 29:1387–1390.
2011.(In Chinese).
|
|
40
|
Xiao M, Dai X, He X, et al: Paris saponin
I induces G2/M cell cycle arrest and apoptosis in human gastric
carcinoma SGC7901 cells. J Huazhong Univ Sci Technolog Med Sci.
31:768–772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiao X, Bai P, Bui Nguyen TM, et al: The
antitumoral effect of Paris Saponin I associated with the induction
of apoptosis through the mitochondrial pathway. Mol Cancer Ther.
8:1179–1188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yan LL, Zhang YJ, Gao WY, et al: In vitro
and in vivo anticancer activity of steroid saponins of Paris
polyphylla var. yunnanensis . Exp Oncol. 31:27–32.
2009.PubMed/NCBI
|
|
43
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shroff EH, Snyder C and Chandel NS: Bcl-2
family members regulate anoxia-induced cell death. Antioxid Redox
Signal. 9:1405–1409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Reed JC, Miyashita T, Takayama S, et al:
BCL-2 family proteins: regulators of cell death involved in the
pathogenesis of cancer and resistance to therapy. J Cell Biochem.
60:23–32. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sedlak TW, Oltvai ZN, Yang E, et al:
Multiple Bcl-2 family members demonstrate selective dimerizations
with Bax. Proc Natl Acad Sci USA. 92:7834–7838. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu F, Tian Y, Huang Y, et al: EGFR
inhibitors sensitize non-small cell lung cancer cells to
TRAIL-induced apoptosis. Chin J Cancer. 30:701–711. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mudd SR, Voorbach MJ, Reuter DR, et al:
FDG-PET as a pharmacodynamic biomarker for early assessment of
treatment response to linifanib (ABT-869) in a non-small cell lung
cancer xenograft model. Cancer Chemother Pharmacol. 69:1669–1672.
2012. View Article : Google Scholar : PubMed/NCBI
|