Introduction
To date, mature cardiac myocytes have been regarded
as terminally differentiated cells that are replaced with fibrous
scar tissue following injury and have no regenerative capability
(1). However, studies have
suggested that urodele amphibians and zebrafish possess the
capacity for cardiac regeneration (2,3).
Furthermore, accumulating evidence has revealed that the mammalian
heart possesses a measurable capacity for renewal (4). Mollova et al (5) demonstrated that cardiomyocyte
proliferation contributes to developmental heart growth in young
humans, which suggests that the heart has the capacity to
regenerate myocardium in children and adolescents (5). Neonatal mice also retain a capacity
for cardiac regeneration over a short time-frame (aged ≤6 days),
but this capacity is lost by 7 days of age (6). However, the specific biological
mechanisms underlying the process of cardiac regeneration have yet
to be elucidated. Furthermore, the mechanisms by which genes and
their proteins regulate this process remain unexplored.
A variety of different genes have been identified to
be involved in cardiac regeneration (7,8).
Kikuchi et al (9) reported
that GATA binding protein 4 (GATA4) expression increased to
stimulate cardiac regeneration following resection, prior to the
expression becoming localized to proliferating cardiomyocytes
surrounding and within the injury site. Therefore, GATA4 may be a
molecular marker of regeneration (9). Recently, Mahmoud et al
(10) found that overexpression of
Meis1 in cardiomyocytes decreased neonatal myocyte proliferation
and inhibited neonatal cardiac regeneration. Thus, Meis1 represents
a potential therapeutic target for cardiac regeneration (10). Although these general gene
expression patterns are meaningful, the global alterations in the
expression of genes involved in mammalian cardiac regeneration
during the relevant time-period have not been extensively
investigated. Therefore, in this study, three key time-points (1, 6
and 7 days old) were selected for the analysis of global gene
expression profiles in C57BL/6 mice using the Solexa/Illumina
digital gene expression (DGE) system.
Materials and methods
Experimental animals and tissue
collection
The study was approved by the Animal Care and Use
Committee of Nanjing Medical University (Nanjing, China). C57BL/6J
male and female mice were purchased from the Model Animal Research
Centre of Nanjing University (Nanjing, China) and raised under
pathogen-free conditions in individual cases in a
temperature-controlled room (18–24°C) with a 12-h light/dark cycle.
C57BL/6J females were mated with males at the age of 6 months. The
left ventricular apex was removed from neonates 1, 6 and 7 days
after birth (6). Specimens (n=5
per group) were snap-frozen in liquid nitrogen and subsequently
stored at −80°C. Total RNA was extracted using TRIzol®
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) in
accordance with the manufacturer’s instructions.
DGE-tag profiling
The main reagents and instruments used for RNA
library construction and deep-sequencing were the Illumina Gene
Expression Sample Prep kit, the Illumina Sequencing Chip (flow
cell), the Illumina Cluster Station and the Illumina HiSeq™ 2000
System (Illumina, Inc., San Diego, CA, USA). Sequence tags were
prepared using the Illumina DGE-Tag Profiling kit, in accordance
with the manufacturer’s instructions. In brief, mRNA was isolated
from 6 μg total RNA using magnetic oligo beads. First- and
second-strand cDNA was then synthesized using Oligo (dT) primers.
The bead-bound cDNA was subsequently digested with NlaIII,
which recognizes and cleaves CATG sites. The fragments [with the
exception of the 3′ cDNA fragments connected to the Oligo (dT)
beads] were washed away and the Illumina adaptor 1 was ligated to
the sticky 5′-end of the digested bead-bound cDNA fragments. The
junction of Illumina adaptor 1 and the CATG site is the recognition
site of MmeI, which has separated recognition and digestion
sites. This enzyme then cuts 17 bp downstream of the CATG site,
producing tags with adaptor 1. Following the removal of the 3′
fragments using magnetic bead precipitation, Illumina adaptor 2 was
ligated to the 3′-ends of the tags, thus producing tags with
different adaptors at either end to form a tag library. Following
15 cycles of linear polymerase chain reaction (PCR) amplification,
105-bp fragments were purified by 6% Tris-Borate-EDTA PAGE.
Following denaturation, the single-chain molecules were fixed onto
the Illumina Sequencing Chip (flow cell). Each molecule then grew
into a single-molecule cluster sequencing template through in
situ amplification. The four types of nucleotides, which were
labeled using four different colors, were subsequently added and
sequencing was performed using the sequencing by synthesis method.
Sequencing-received raw image data were transformed by base calling
into sequence data. Raw sequences had 3′ adaptor fragments as well
as a few low-quality sequences and several types of impurities. Raw
sequences were transformed into clean tags following data
processing. All clean tags were aligned to the reference sequences
and unambiguous tags were annotated. The number of clean tags
corresponding to each gene was counted.
Determination of gene expression levels
and detection of differentially expressed genes (DEGs)
To compare the differential expression of genes
across samples, the number of raw clean tags in each library was
normalized to the transcripts per million clean tags to obtain
normalized gene expression levels. Differential expression of genes
across samples was performed using a rigorous algorithm method.
Genes were deemed to be significantly differentially expressed with
a P-value <0.005, a false discovery rate (FDR) <0.01 and a
two-fold relative change threshold in the sequence counts across
libraries.
Gene ontology (GO) and pathway functional
enrichment analysis
In gene expression profiling analysis, GO enrichment
analysis of functional significance involves the application of a
hypergeometric test to map all DEGs to terms in the GO database.
This allows the identification of significantly enriched GO terms
in DEGs compared with the genome background. Biological functions
require the cooperation of different genes. Pathway-based analysis
provides information regarding the biological functions of genes
and is usually conducted using the Kyoto Encyclopedia of Genes and
Genomes, which is the major public pathway-related database.
Pathway enrichment analysis identifies significantly enriched
metabolic pathways or signal transduction pathways in DEGs compared
with the whole genome background. Pathways with Q-values ≤0.05 are
considered significantly enriched in DEGs.
Quantitative PCR (qPCR) analysis
qPCR analysis was used to validate the DGE results.
Total RNA was extracted from neonatal mouse heart tissue using
TRIzol reagent (Invitrogen Life Technologies). First-strand cDNA
was generated with random primers using an AMV Reverse
Transcriptase kit (Invitrogen Life Technologies). Specific primers
used for PCR were as follows: Dopachrome tautomerase (Dct), forward
5′-GTCCTCCACTCTTTTACAGACG-3′, reverse 5′-ATTCGGTTGTGACCAATGGGT-3′;
Gck (glucokinase), forward 5′-ACTTCCCAGGGTCCCACTGCTG-3′, reverse
5′-GGCTTTGGAGGTGCCACGATAG-3′; δ-aminolevulinate synthase 2 (Alas2),
forward 5′-ACTCACCGTCTTGGTTCGTCCTC-3′, reverse
5′-TGAGAACAGGTTGGTCCTTGAGTGG-3′; matrix metallopeptidase 12
(Mmp12), forward 5′-CACAACAGTGGGAGAGAAAA-3′, reverse
5′-AGCTTGAATACCAGATGGGATG-3′; perilipin 2 (Plin2), forward
5′-CCCGCAACCTGACCCAGCAG-3′, reverse 5′-CGCCTGCCATCACCCCCAAG-3′.
qPCR was performed in an ABI 7300 Sequence Detection system
(Applied Biosystems, Foster City, CA, USA) in accordance with the
manufacturer’s instructions under the following conditions:
Denaturation at 95°C for 10 min, and 40 cycles of 95°C for 15 sec
and 60°C for 1 min. GAPDH was used as a normalizer (forward
5′-TTCACCACCATGGAGAAGGC-3′, reverse 5′-GGCATGGACTGTGGTCATGA-3′) and
the relative expression levels of genes were calculated according
to the 2−ΔΔCT method. Each sample was assayed at least
in triplicate and was reproduced at least three times.
Statistical analysis
Differential mRNA expression was based on the
log2 ratios between the competing conditions. All data
are expressed as the mean ± standard deviation. Statistical
analysis was performed using one-way analysis of variance using the
SPSS 10.0 statistical software package (SPSS, Inc., Chicago, IL,
USA). The threshold of significance was defined as P<0.05.
Results
Sequencing quality evaluation
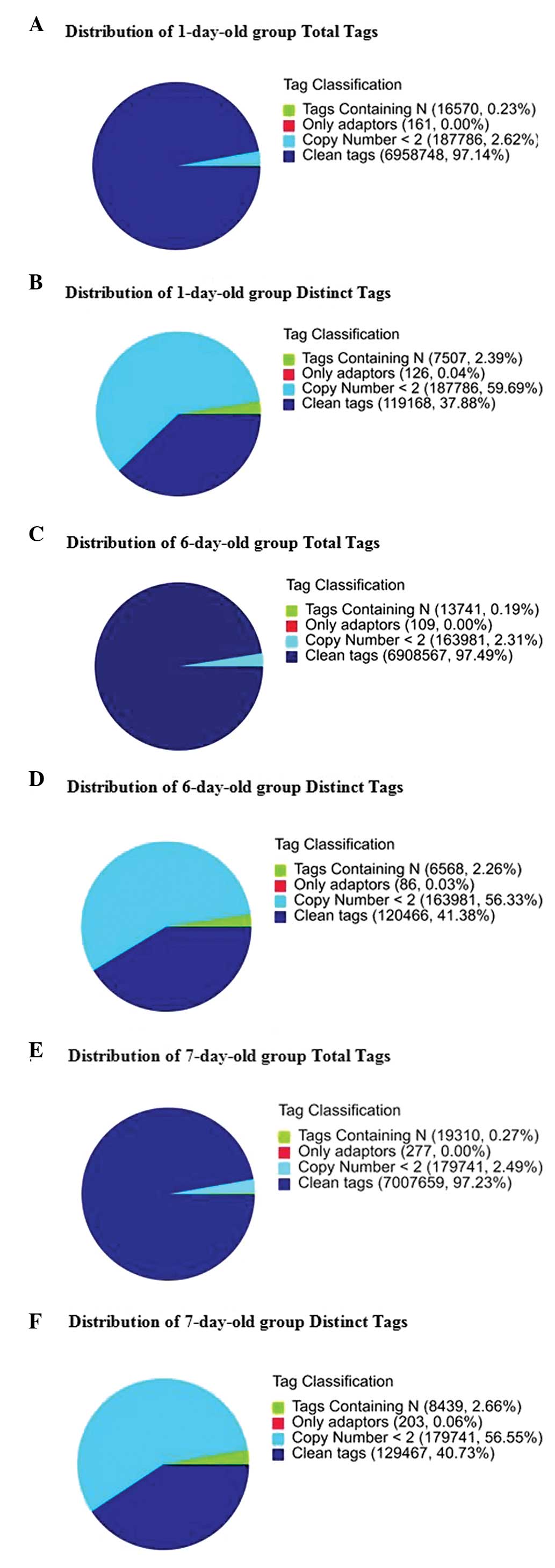
The number of tags containing clean tags represented
97.14% (6,958,748), 97.49% (6,908,567) and 97.23% (7,007,659) of
the total tags in the 1-, 6- and 7-day-old groups, respectively.
The number of the distinct tags containing clean tags represented
37.88% (119,168), 41.38% (120,466) and 40.73% (129,467) of the
total distinct tags in the three groups, respectively (Fig. 1). The distribution of the total and
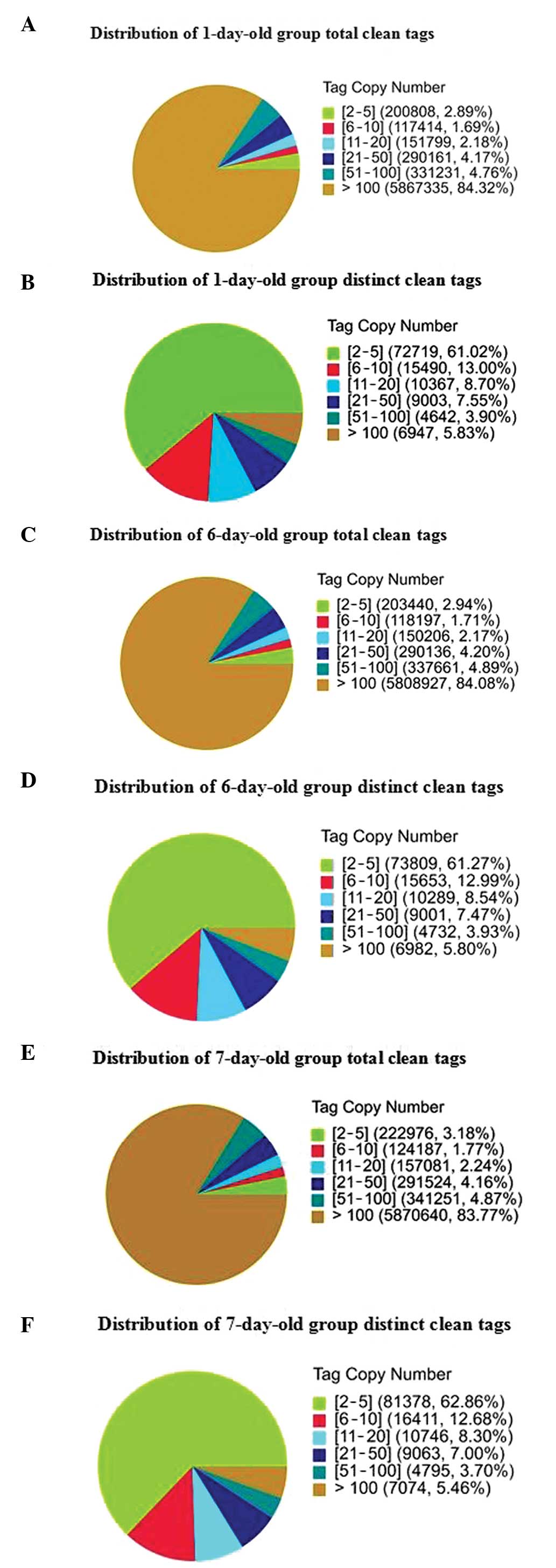
distinct clean tag copy numbers is shown in Fig. 2. Among the distribution of total
clean tags, >80% had copy numbers >100 and <4% had copy
numbers between two and five in the three groups. However, the low
expression tags (<5 copies) had the highest ratio of the number
of distinct to total tags and the lowest percentage of distinct
clean high copy number tags. Heterogeneity and redundancy are two
significant characteristics of mRNA expression. Certain categories
of mRNA have very high abundance, while the majority remain at very
low levels of expression. The distribution of clean tag expression
may be used to evaluate the normality of the whole data.
Comparison of gene expression levels
among the three groups
The rigorous algorithm method was applied to
identify DEGs from the normalized DGE data using pairwise
comparisons across all time-points. FDRs were ≤0.01 and estimated
absolute |log2Ratio| values were ≥1 in at least one of
the pairwise comparisons. In detail, 635 upregulated and 738
downregulated genes were identified in 6-day-old mice cardiac
tissue samples when the samples were compared with those from
1-day-old mice. Compared with samples from 1-day-old mice, 783
genes were upregulated and 775 genes were downregulated in
7-day-old mice cardiac samples. A total of 902 DEGs were identified
in the 7- and 6-day-old mice cardiac tissue samples, including 521
upregulated and 381 downregulated genes. In addition, a total of
306 DEGs, including 115 upregulated and 191 downregulated genes,
were detected in 7-day-old cardiac tissue samples compared with
samples from 1- and 6-day-old mice, respectively (Table I).
 | Table IDifferentially expressed genes in
neonatal mouse cardiac tissue. |
Table I
Differentially expressed genes in
neonatal mouse cardiac tissue.
| Age |
|---|
|
|
|---|
| Genes | 1 vs. 6 days (n) | 1 vs. 7 days (n) | 6 vs. 7 days (n) |
|---|
| Total | 1,373 | 1,558 | 902 |
| Upregulated | 635 | 783 | 521 |
| Downregulated | 738 | 775 | 381 |
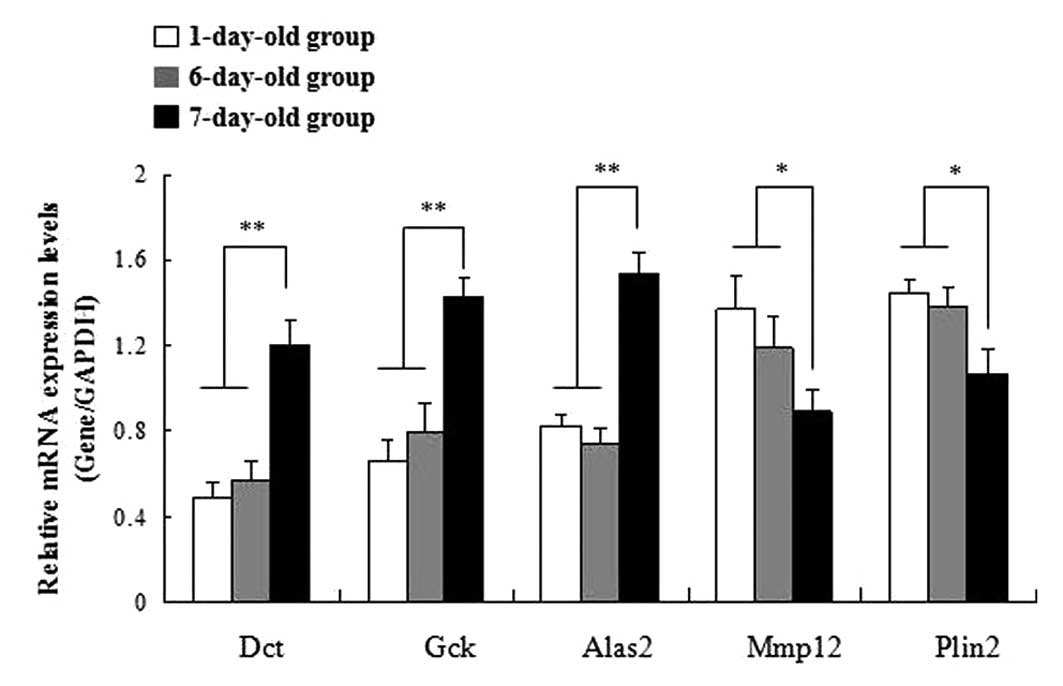
Validation of DEGs using qPCR
In order to confirm the DGE results, five genes were
selected for qPCR analysis. Among these genes, three (Dct, Gck and
Alas2) were upregulated and two (Mmp12 and Plin2) were
downregulated in 7-day-old cardiac tissue compared with tissue from
1- and 6-day-old mice. The results of the qPCR were consistent with
the DGE analysis (Fig. 3).
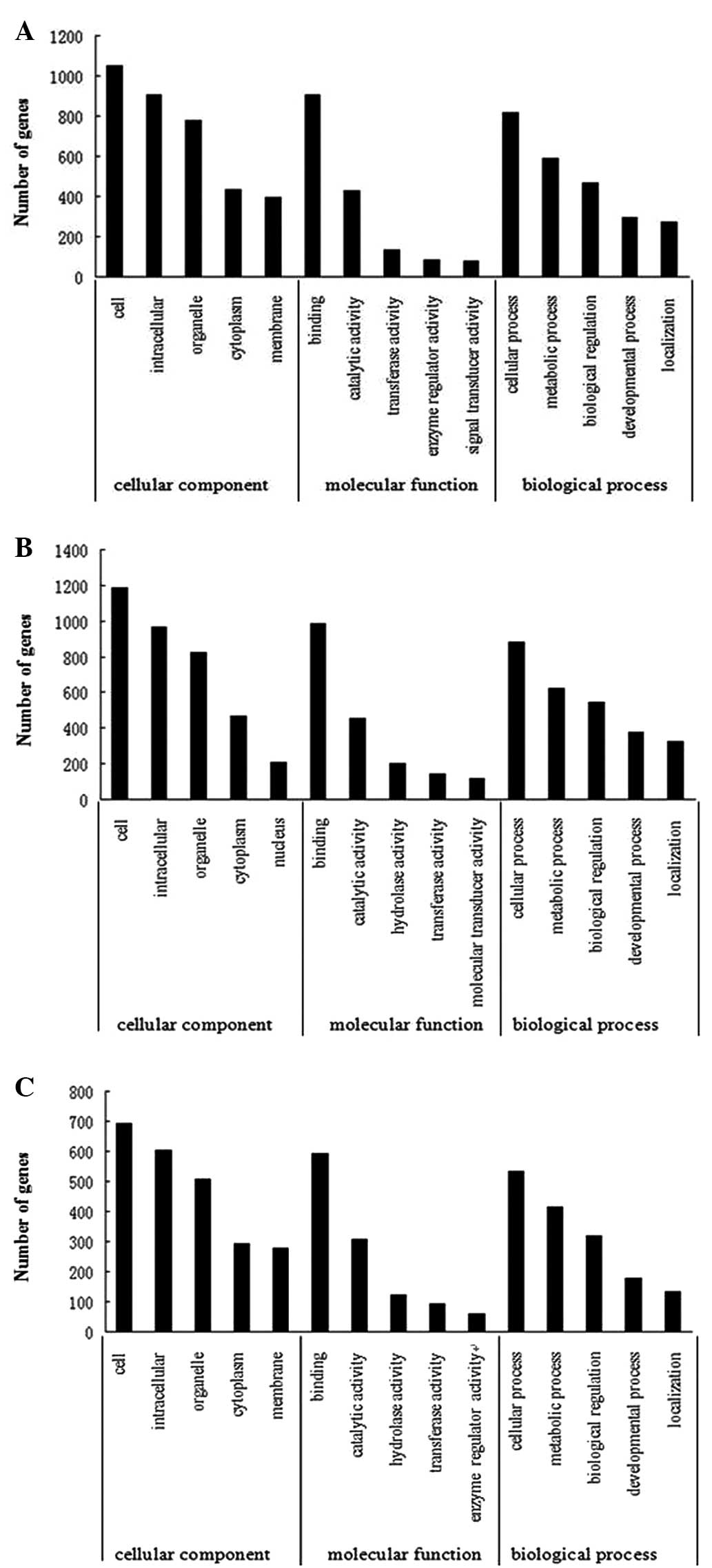
GO and pathway enrichment analysis of
DEGs
GO has three structured vocabularies: Cellular
components, molecular function and biological processes. The basic
unit of GO is the GO term. The top five GO terms in this study are
shown in Fig. 4. Pathway
enrichment analysis revealed the oxidative phosphorylation pathway
to be the process that was most significantly putatively affected
by the differential expression of these genes in 7-day-old cardiac
tissue compared with tissue from 1- and 6-day-old mice (Table II).
 | Table IIResults from the pathway enrichment
analysis. |
Table II
Results from the pathway enrichment
analysis.
| Age | Pathway | DEGs with pathway
annotation, n=1,041 (n) | P-value | Q value |
|---|
| 1 vs. 6 days | Cell cycle | 23 | 0.0002558345 | 0.02855462 |
| Proteasome | 11 | 0.0002693832 | 0.02855462 |
| 1 vs. 7 days | Oxidative
phosphorylation | 25 |
1.098219×10−5 | 0.001646858 |
| Parkinson’s
disease | 28 |
1.546346×10−5 | 0.001646858 |
| 6 vs. 7 days | Alzheimer’s
disease | 26 |
8.94506×10−6 | 0.001789012 |
| Parkinson’s
disease | 18 | 0.0001735654 | 0.013760740 |
| Metabolic
pathways | 100 | 0.0002064111 | 0.013760740 |
| Oxidative
phosphorylation | 15 | 0.0005363196 | 0.026815980 |
| Huntington’s
disease | 23 | 0.0009825545 | 0.039302180 |
Discussion
In the present study, the global gene expression
profiles in regenerative hearts from neonatal mice were analyzed
using the Solexa/Illumina DGE system, a tag-based novel
high-throughput transcriptome deep-sequencing method. Significant
differences in gene expression profiles were observed in cardiac
tissues among the three time-points analyzed (1, 6 and 7 days). In
this study, tag-mapped genes were confirmed using qPCR. Although
the differences in gene expression did not match the magnitude of
those detected by the Solexa-based sequencing method, the trends of
up- and downregulation were similar. Functional roles for DEGs
identified in this study were categorized according to cellular
process, regulation of biological processes and metabolic process,
thus indicating that multiple genes are involved in the complex
biological alterations that occur during cardiac regeneration.
Pathway-Express analysis identified that the
oxidative phosphorylation pathway was most significantly affected
by the differential expression of genes in 7-day-old cardiac tissue
compared with tissue from 1- and 6-day-old mice. Oxidative
phosphorylation is a metabolic pathway that uses energy released by
the oxidation of nutrients to produce adenosine triphosphate (ATP).
Myocardial energy is generated mainly through mitochondrial
oxidative phosphorylation (11,12).
Various studies have investigated the role of oxidative
phosphorylation in liver regeneration, and it has been proposed
that oxidative phosphorylation is enhanced following partial
hepatectomy in order to compensate for the loss of tissue (13,14).
Neonatal mice retain the capacity for cardiac regeneration, but
this ability is lost after 7 days. The active biosynthesis of
components such as DNA and protein requires energy; therefore, the
demand for ATP production in the regenerating heart is increased
during the 6 days after birth. It is apparent that the oxidative
phosphorylation pathway also has an important role in cardiac
development, although the collaborative network of
mitochondria-related functions and genes requires further
investigation.
Further analysis of the DEGs revealed that MAP
kinase phosphatase, Tau, Deltex and Notch were upregulated, and p21
protein (Cdc42/Rac)-activated kinase 1/2, filamin A, α, Dv1 and
Delta were downregulated in 7-day-old cardiac tissue compared with
tissue from 1- and 6-day-old mice. These are key genes in the
mitogen-activated protein kinase (MAPK) and Notch signaling
pathways. Previous studies have shown that signaling pathways,
including MAPK and Notch signaling pathways, have non-redundant
roles in the regulation of zebrafish cardiac regeneration (15–17).
It was proposed in the present study that these two signaling
pathways participate in mammalian cardiac regeneration, although
the collaborative network requires further investigation.
In conclusion, the results of the present study
identified changes in the expression of a large number of genes
associated with a variety of molecular functions during the first
seven days after birth in the neonatal mouse heart. Numerous genes
of known and unknown function are involved in mammalian cardiac
regeneration, and biochemical and physiological investigations of
these genes are likely to be performed in the future.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81070138 and 81200126).
The authors would like to thank the Beijing Genomics
Institute-Shenzhen Co., Ltd. (Shenzhen, China) for their technical
assistance.
References
|
1
|
Anversa P, Leri A and Kajstura J: Cardiac
regeneration. J Am Coll Cardiol. 47:1769–1776. 2006. View Article : Google Scholar
|
|
2
|
Jopling C, Sleep E, Raya M, Martí M, Raya
A and Izpisúa Belmonte JC: Zebrafish heart regeneration occurs by
cardiomyocyte dedifferentiation and proliferation. Nature.
464:606–609. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neff AW, Dent AE and Armstrong JB: Heart
development and regeneration in urodeles. Int J Dev Biol.
40:719–725. 1996.PubMed/NCBI
|
|
4
|
Kikuchi K and Poss KD: Cardiac
regenerative capacity and mechanisms. Annu Rev Cell Dev Biol.
28:719–741. 2012. View Article : Google Scholar
|
|
5
|
Mollova M, Bersell K, Walsh S, Savla J,
Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S
and Kühn B: Cardiomyocyte proliferation contributes to heart growth
in young humans. Proc Natl Acad Sci USA. 110:1446–1451. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Porrello ER, Mahmoud AI, Simpson E, Hill
JA, Richardson JA, Olson EN and Sadek HA: Transient regenerative
potential of the neonatal mouse heart. Science. 331:1078–1080.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lien CL, Schebesta M, Makino S, Weber GJ
and Keating MT: Gene expression analysis of zebrafish heart
regeneration. PLoS Biol. 4:e2602006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Forough R, Scarcello C and Perkins M:
Cardiac biomarkers: a focus on cardiac regeneration. J Tehran Heart
Cent. 6:179–186. 2011.PubMed/NCBI
|
|
9
|
Kikuchi K, Holdway JE, Werdich AA,
Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY
and Poss KD: Primary contribution to zebrafish heart regeneration
by gata4(+) cardiomyocytes. Nature. 464:601–605. 2010.PubMed/NCBI
|
|
10
|
Mahmoud AI, Kocabas F, Muralidhar SA,
Kimura W, Koura AS, Thet S, Porrello ER and Sadek HA: Meis1
regulates postnatal cardiomyocyte cell cycle arrest. Nature.
497:249–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mootha VK, Arai AE and Balaban RS: Maximum
oxidative phosphorylation capacity of the mammalian heart. Am J
Physiol. 272:H769–H775. 1997.PubMed/NCBI
|
|
12
|
Balaban RS: Regulation of oxidative
phosphorylation in the mammalian cell. Am J Physiol. 258:C377–C389.
1990.PubMed/NCBI
|
|
13
|
Chang YJ: Mitochondrial calcium ion and
oxidative phosphorylation in regenerating rat liver. J Formos Med
Assoc. 101:189–194. 2002.PubMed/NCBI
|
|
14
|
Mastrodonato M, Portincasa P, Mentino D,
Rossi R, Resta L, Ferri D and Liquori GE: Caveolin-1 and
mitochondrial alterations in regenerating rat liver. Microsc Res
Tech. 75:1026–1032. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jopling C, Suñe G, Morera C and Izpisua
Belmonte JC: p38α MAPK regulates myocardial regeneration in
zebrafish. Cell Cycle. 11:1195–1201. 2012.
|
|
16
|
Raya A, Koth CM, Büscher D, Kawakami Y,
Itoh T, Raya RM, Sternik G, Tsai HJ, Rodríguez-Esteban C and
Izpisúa-Belmonte JC: Activation of Notch signaling pathway precedes
heart regeneration in zebrafish. Proc Natl Acad Sci USA. 100(Suppl
1): 11889–11895. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lepilina A, Coon AN, Kikuchi K, Holdway
JE, Roberts RW, Burns CG and Poss KD: A dynamic epicardial injury
response supports progenitor cell activity during zebrafish heart
regeneration. Cell. 127:607–619. 2006. View Article : Google Scholar : PubMed/NCBI
|