Introduction
Osteosarcoma is the most common primary malignant
bone tumor with highly malignant and invasive growth
characteristics in adolescents and young adults (1). Osteosarcoma is associated with a poor
prognosis, which is a result of its resistance to chemotherapy and
tendency to metastasize to the lungs (2). Using traditional treatment methods,
including chemotherapy, wide tumor resection and amputation,
patients with osteosarcoma have a poor prognosis, with a five-year
survival rate of <20% (3). The
prognosis of patients with osteosarcoma has improved markedly,
primarily due to the introduction of the extensive application of
neoadjuvant chemotherapy and limb salvage surgery (4,5).
However, the prognosis of patients with advanced osteosarcoma
remains poor and advances in treatment are urgently required
(6). Effective prognostic factors
are important for clinicians to facilitate the selection of
appropriate treatments for patients with osteosarcoma.
The polymeric (p) immunoglobulin (Ig) receptor (R)
is a transporter of dimeric IgA and pentameric IgM, which are the
first-line antibodies produced in response to infection. pIgR is
widely expressed in epithelial cells and its expression is commonly
increased by proinflammatory cytokines in response to viral or
bacterial infection, linking innate and adaptive immunity (7–10).
Upregulation of pIgR has been identified in colon cancer (11), breast cancer (12,13),
endometrial carcinoma (14,15),
bladder carcinoma (16) and
hepatocellular carcinoma (HCC) (17,18).
High levels of the cleaved extracellular domain of pIgR, designated
as the secretory component, have also been detected in the sera of
patients with lung (19,20) and pancreatic cancer (21), as well as patients exhibiting colon
cancer with liver metastases (22). However, the clinical significance
of pIgR in osteosarcoma has yet to be elucidated.
The present study aimed to investigate the
association between pIgR expression and clinicopathological
features. In addition, the potential of pIgR as a novel prognostic
marker in patients with osteosarcoma following surgical resection
was investigated.
Materials and methods
Patients and tumor tissue samples
Fresh tumor samples were obtained from 22 patients
with osteosarcoma at initial surgery at the Department of
Orthopedics, the First Affiliated Hospital, Zhejiang University
School of Medicine (Hangzhou, China) between January 2010 and
December 2012 for quantitative polymerase chain reaction (qPCR)
analysis. Samples were snap-frozen and stored in liquid nitrogen
until use. Patients had received no treatment prior to surgery.
Paraffin-embedded osteosarcoma tissue samples were obtained from
136 patients undergoing surgical resection at the Department of
Orthopedics, the First Affiliated Hospital, Zhejiang University
School of Medicine between January 1998 and December 2007. None of
the 136 patients had received chemotherapy or radiotherapy prior to
resection. Following resection, patients were followed up every
three months and the sections were reviewed by two pathologists to
verify the histological assessment. Informed consent was obtained
from all patients and the study protocol was approved by the Ethics
Committee of the First Affiliated Hospital, Zhejiang University
School of Medicine. The location of the tumors and distant
metastases was determined using computed tomography (CT) and
magnetic resonance imaging (MRI). The patients with osteosarcoma
were staged according to the Enneking staging system (23). The staging workup involved CT scans
of the chest to assess for pulmonary metastases, MRI and X-ray
scans for local staging, and bone scans to assess for distant
skeletal metastases. Patients exhibiting secondary malignancies,
for which they had received prior chemoradiotherapy or surgery, or
patients with pulmonary or nonpulmonary distant metastases on
presentation to the First Affiliated Hospital, Zhejiang University
School of Medicine, were excluded from the present study.
qPCR analysis
Total RNA was extracted from the frozen tumor
tissues using TRIzol® Reagent according to the
manufacturer’s instructions (Invitrogen Life Technologies,
Carlsbad, CA, USA). Total RNA was reverse transcribed into single
stranded complementary (c)DNA using a moloney-murine leukemia virus
(M-MLV) reverse transcriptase (Promega Corporation, Madison, WI,
USA). Briefly, RNA was denatured by heating for 5 min at 70°C,
followed by rapid cooling on ice. The RNA was used for reverse
transcription in a 25-μl reaction volume containing 2 μg total RNA,
25 units RNase inhibitor, 0.5 mmol/l each deoxyribonucleotide
triphosphate, 1.5 μmol/l reverse primer and 200 units M-MLV reverse
transcriptase. For reverse transcription, the reactions were
incubated at 42°C for 60 min. The expression of pIgR was analyzed
using a fluorescence-based real-time detection method with the ABI
PRISM 7700 Sequence Detection System (PerkinElmer, Inc., Waltham,
MA, USA) as described previously (24,25).
The specific primer pairs and fluorescent probes for pIgR and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are shown in
Table I. GAPDH served as an
endogenous control. qPCR analysis was performed in triplicate for
each sample. The 25-μl qPCR reaction consisted of 1 μl cDNA
template, 1 μl each of sense and anti-sense primers, 0.75 μl 5′
FAM- and 3′ TAMARA-labeled oligonucleotide probes, 2 μl dNTP
mixture, 5 μl 5X reaction buffer and 0.125 μl Taq DNA polymerase.
The cycling conditions were as follows: 50°C for 2 min and 95°C for
10 min, followed by 46 cycles of 95°C for 15 sec and 60°C for 1
min. To determine the relative expression of pIgR mRNA in the
individual tissue samples the Ct values were normalized using the
Ct value for GAPDH mRNA (25).
 | Table ISequences of primers and probes used
for quantitative polymerase chain reaction analysis. |
Table I
Sequences of primers and probes used
for quantitative polymerase chain reaction analysis.
| Primer/probe | Sequence |
|---|
| Polymeric
immunoglobulin receptor |
| Forward primer |
5′-CTCTCTGGAGGACCACCGT-3′ |
| Reverse primer |
5′-CAGCCGTGACATTCCCTG-3′ |
| TaqMan probe |
6FAM-5′-AGATCAAGATTATCGAAGGAGAACCAAACCTC-3′-TAMRA |
|
Glyceraldehyde-3-phosphate
dehydrogenase |
| Forward primer |
5′-TCCATGACAACTTTGGTATCGTG-3′ |
| Reverse primer |
5′-ACAGTCTTCTGGGTGGCAGTG-3′ |
| TaqMan probe |
6FAM-5′-AAGGACTCATGACCACAGTCCATGCCA-3′-TAMRA |
Immunohistochemistry
Selected tumor samples were fixed in 10%
neutral-buffered formalin and embedded in paraffin. Sections (size,
5 μM) were cut, dewaxed, rehydrated and subjected to antigen
retrieval. Subsequent to blocking endogenous peroxidase activity,
the sections were incubated with the primary antibodies against
pIgR (1:100; Epitomics Inc., Burlingame, CA, USA) overnight at 4°C.
Immunohistochemistry was performed using the
streptavidin-biotin-peroxidase complex method (Lab Vision
Corporation, Fremont, CA, USA). The slides were analyzed and images
were captured using an Olympus BX60 microscope (Olympus
Corporation, Tokyo, Japan). Sections that are known to stain
positively were incubated in each batch and negative controls were
also established by replacing the primary antibody with pre-immune
serum.
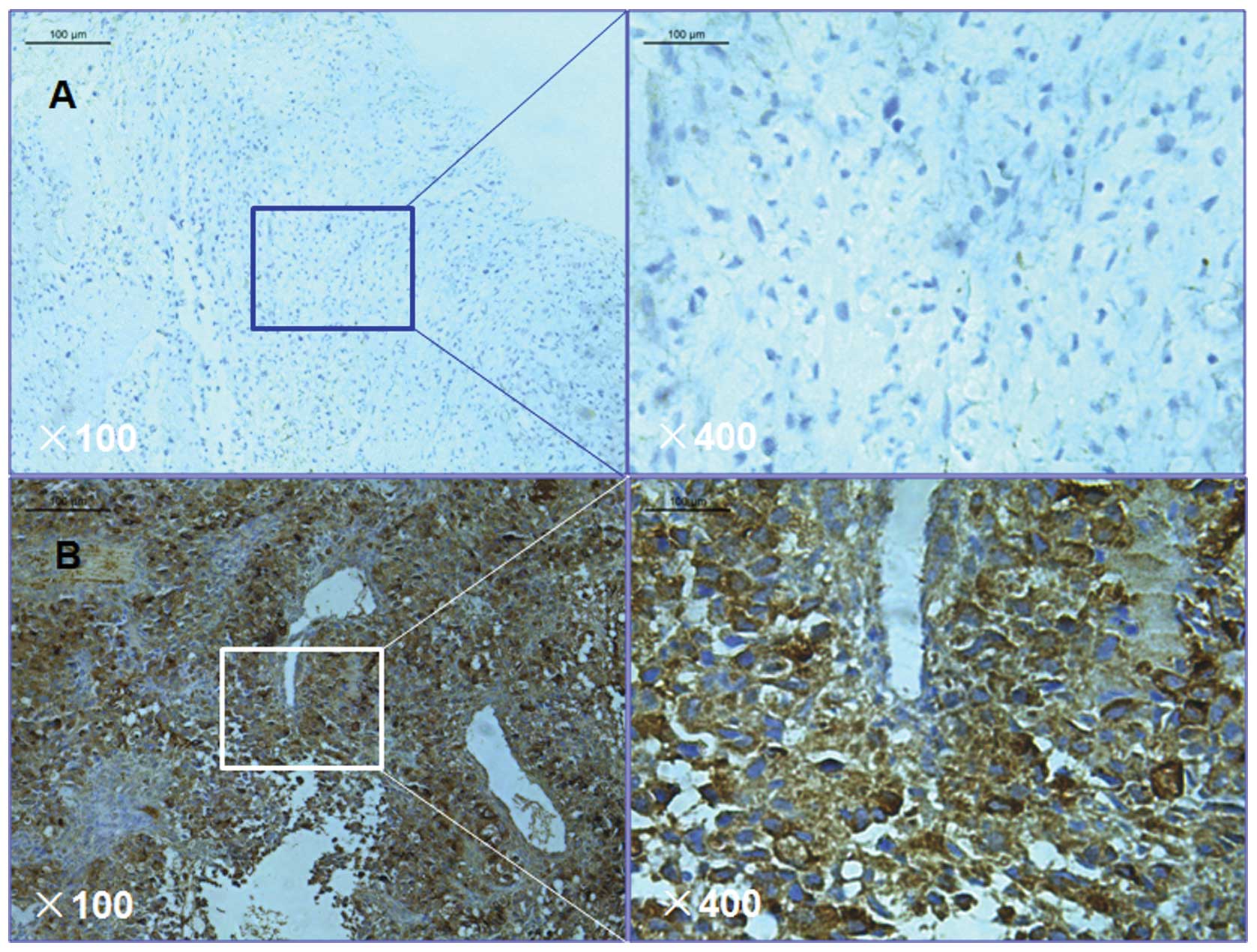
Expression analysis of pIgR in the tumor tissue was
performed by comparing the staining intensity with the percentage
of immunoreactive cells. Staining intensity was arbitrarily scored
on a scale of four grades: 0, No staining; 1, weak staining; 2,
moderate staining; and 3, strong staining. The percentage of
positive cells was scored according to the following grades: 0, 0%;
1, 1–25%; 2, 26–50%; and 3, >50%. pIgR staining positivity was
determined using the following formula: Overall score = positive
percentage score × staining intensity score. A score of 0 was
termed 0, a score >0 and ≤2 was termed 1, a score >2 and ≤6
was termed 2 and a score >6 and ≤9 was termed 3. Tumor samples
graded as level 0 or 1 were defined as negative for pIgR
expression, whereas samples graded as level 2 or 3 were defined as
positive for pIgR expression.
Follow-up
Patient follow-up consisted of physical examination,
including CT, MRI and X-ray scans every three months for the first
five years, then annually thereafter. Patients were followed up
until mortality or until the date of the final follow-up. Follow-up
was terminated on December 31, 2012. The median follow-up was 41.7
months (range, 10–179 months).
Statistical analysis
All statistical analyses were performed using SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA). Data are expressed as
the mean ± standard error of the mean. Clinicopathological
parameters were analyzed via two-tailed χ2 and
two-tailed t-tests to assess the association between pIgR
expression and clinicopathological parameters. Overall survival
(OS) curves for patients with positive and negative pIgR expression
were estimated using the Kaplan-Meier method. Survival functions
were compared using the log-rank test. Univariate and multivariate
analyses were based on the Cox proportional-hazards regression
model. Factors that significantly influenced OS were used in the
Cox proportional-hazards regression model for multivariate
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
pIgR expression in osteosarcoma
qPCR analysis was performed to assess pIgR
gene expression in 22 fresh frozen osteosarcoma samples. The
housekeeping gene, GAPDH served as a control. pIgR
expression was found to be positive in 15/22 (68.2%) patients with
osteosarcoma (Table II).
 | Table IIpIgR mRNA expression in osteosarcoma
samples. |
Table II
pIgR mRNA expression in osteosarcoma
samples.
| pIgR mRNA
expression |
|---|
|
|
|---|
| Tumor tissue | Samples
(positive/total) | Level of expression
(mean ± SEM) |
|---|
| Osteosarcoma | 15/22 | 0.32±0.07 |
To determine the frequency of positive expression of
the pIgR gene in osteosarcoma, pIgR expression was analyzed
in 136 paraffin-embedded osteosarcoma tissue samples using
immunohistochemical staining. Among the 136 osteosarcoma samples,
pIgR was observed to be expressed in 93/136 (68.4%) samples
(Fig. 1). This finding indicates
that pIgR may be key in osteosarcoma. Table III demonstrates the association
between pIgR expression and clinicopathological characteristics of
the 136 osteosarcoma tissue samples, including age, gender, tumor
location, histological type and grade.
 | Table IIIAssociation between pIgR expression
and clinicopathological parameters in 136 patients with
osteosarcoma. |
Table III
Association between pIgR expression
and clinicopathological parameters in 136 patients with
osteosarcoma.
| pIgR expression |
|---|
|
|
|---|
| Parameter | Positive | Negative | P-value |
|---|
| Patients (n/%) | 93/68.4 | 43/31.6 | - |
| Age (years; mean ±
SEM) | 22.7±7.2 | 24.3±8.5 | 0.832 |
| Gender |
| Female (n/%) | 43/46.2 | 21/48.8 | 0.637 |
| Male (n/%) | 50/53.8 | 22/51.2 | - |
| Tumor location |
| Femur | 45/48.4 | 22/51.2 | 0.635 |
| Tibia | 23/24.7 | 11/25.6 | - |
| Humerus | 8/8.6 | 3/7.0 | - |
| Fibula | 6/6.5 | 2/4.7 | - |
| Pelvis | 5/5.4 | 2/4.7 | - |
| Other | 6/6.5 | 3/7.0 | - |
| Histological
type |
| Osteoblastic | 47/50.5 | 23/53.5 | 0.712 |
| Chondroblastic | 22/23.7 | 11/25.6 | - |
| Fibroblastic | 18/19.4 | 7/16.3 | - |
| Telangetatic | 6/6.5 | 2/4.7 | - |
| Histological
grade |
| Low | 22/23.7 | 9/20.9 | 0.563 |
| High | 71/76.3 | 34/79.1 | - |
pIgR expression is associated with poor
survival in patients with osteosarcoma
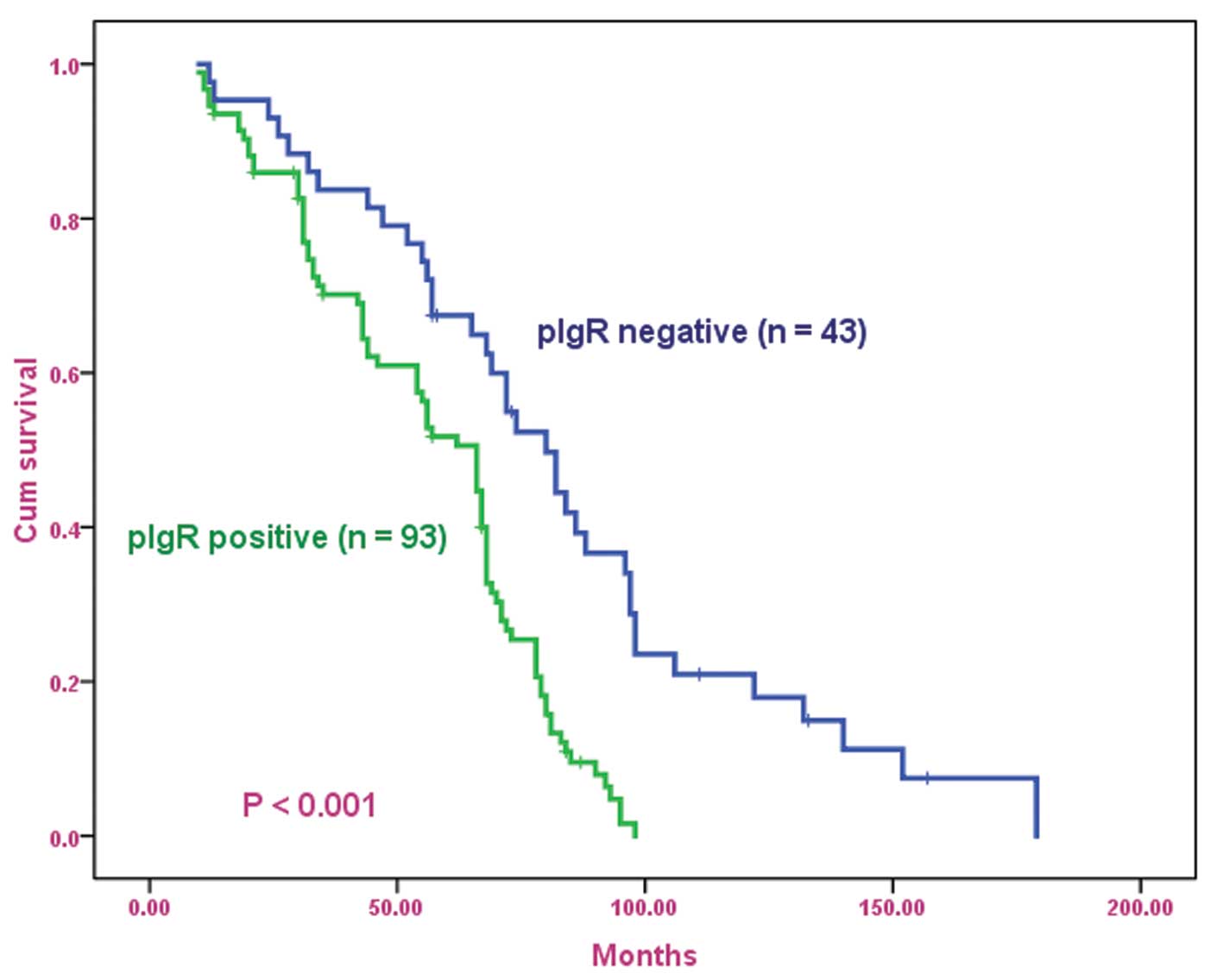
The OS curves for patients with osteosarcoma,
subdivided on the basis of pIgR expression, are shown in Fig. 2. Positive pIgR expression was found
to be associated with poor prognosis in patients with osteosarcoma
(log-rank test, P<0.001). Univariate analysis revealed that
patients who exhibited a positive expression for pIgR had a
significantly poorer prognosis compared with those who exhibited a
negative expression for pIgR (P<0.001; Table IV). Multivariate analysis
demonstrated that positive pIgR expression was an independent and
significant predictor in OS (Table
V).
 | Table IVUnivariate analysis of OS in patients
with osteosarcoma following surgical resection. |
Table IV
Univariate analysis of OS in patients
with osteosarcoma following surgical resection.
| OS |
|---|
|
|
|---|
| pIgR expression | Patients (n) | P-value |
|---|
| Positive | 93 | <0.001 |
| Negative | 43 | - |
| Total | 136 | - |
 | Table VMultivariate analysis of overall
survival in patients with osteosarcoma following surgical
resection. |
Table V
Multivariate analysis of overall
survival in patients with osteosarcoma following surgical
resection.
| Parameter | HR (95% CI) | P-value |
|---|
| Positive pIgR
expression | 2.582
(1.763–3.585) | <0.001 |
Discussion
Osteosarcoma is the most common type of malignant
primary bone tumor (1).
Osteosarcoma has a high metastatic potential, most commonly
spreading to the lungs and bone (26). The relatively high mortality rate
associated with osteosarcoma is predominantly associated with
systemic metastasis, particularly pulmonary metastasis (27). The five-year survival rate for
patients with osteosarcoma metastases is 20% compared with 65% for
patients with localized disease and the majority of the mortalities
associated with osteosarcoma are the result of metastasis (5,28).
Despite aggressive treatment modalities, including high-dose
chemotherapy and wide tumor resection, the five-year survival rate
for patients with osteosarcoma is between 55 and 60% and <40%
for patients with pulmonary metastases (4,5).
Thus, the identification of biomarkers, which offer prognostic
insight and guide clinical treatment, is considered to be
important.
The present study aimed to investigate the
prognostic value of pIgR in patients with osteosarcoma following
surgical resection. pIgR is a glycoprotein present on glandular
epithelial cells that functions as a receptor for pIg. pIgR
transports pIgA into external secretions as secretory IgA, which is
critical for mucosal tissue defense (29). pIgR has been reported to be
overexpressed in colon (11) and
breast cancer (12,13), endometrial carcinoma (14,15),
bladder carcinoma (16), and HCC
(17,18); however, the clinical significance
of pIgR remains unknown. The prognostic value of pIgR in patients
with malignancy also remains unclear. Ai et al (18) were the first to report the clinical
significance of pIgR in HCC. pIgR was identified as a prognostic
biomarker for HCC and was shown to have a role in the hepatitis B
infection, chronic liver inflammation, the induction of the
epithelial-mesenchymal transition, HCC recurrence and metastatic
progression (18). The role of
pIgR in osteosarcoma required investigation, thus the present study
aimed to immunohistochemically assess pIgR expression in 136
pretherapeutic tumor samples and correlate the expression with
clinicopathological parameters in order to identify the potential
prognostic implications of pIgR in osteosarcoma.
In the present study, pIgR expression was analyzed
in cryopreserved osteosarcoma tissues from 22 patients using qPCR
analysis and was found to be expressed in 15 (68.2%) patients. pIgR
expression was subsequently assessed in paraffin-embedded
osteosarcoma tissue samples from 136 osteosarcoma patients with
clinical follow-up records; positive pIgR expression was identified
in 93 (68.4%) of the paraffin-embedded osteosarcoma tissue samples.
Univariate analysis revealed that OS for patients with a positive
pIgR expression in osteosarcoma tissues was significantly poorer
compared with patients with negative pIgR expression. Furthermore,
multivariate analysis showed that positive pIgR expression in
osteosarcoma tissues was an independent prognostic factor for OS
following surgical resection (P<0.001). To the best of our
knowledge, this is the first study to indicate that pIgR has a role
in osteosarcoma, however, this requires further investigation.
In conclusion, to the best of our knowledge, this is
the first study to show that positive expression of pIgR is
significantly associated with a poor prognosis in osteosarcoma
patients. Therefore, pIgR may be a novel predictor for poor
prognosis in osteosarcoma patients following surgical resection and
may be a promising candidate for targeted osteosarcoma therapy.
References
|
1
|
Ma O, Cai WW, Zender L, Dayaram T, Shen J,
Herron AJ, Lowe SW, Man TK, Lau CC and Donehower LA: MMP13, Birc2
(cIAP1), and Birc3 (cIAP2), amplified on chromosome 9, collaborate
with p53 deficiency in mouse osteosarcoma progression. Cancer Res.
69:2559–2567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Janeway KA and Grier HE: Sequelae of
osteosarcoma medical therapy: a review of rare acute toxicities and
late effects. Lancet Oncol. 11:670–678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caudill JS and Arndt CA: Diagnosis and
management of bone malignancy in adolescence. Adolesc Med State Art
Rev. 18:62–78. 2007.
|
|
4
|
Bacci G, Rocca M, Salone M, Balladelli A,
Ferrari S, Palmerini E, Forni C and Briccoli A: High grade
osteosarcoma of the extremities with lung metastases at
presentation: treatment with neoadjuvant chemotherapy and
simultaneous resection of primary and metastatic lesions. J Surg
Oncol. 98:415–420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: an analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar
|
|
6
|
Errani C, Longhi A, Rossi G, Rimondi E,
Biazzo A, Toscano A, Alì N, Ruggieri P, Alberghini M, Picci P, et
al: Palliative therapy for osteosarcoma. Expert Rev Anticancer
Ther. 11:217–227. 2011. View Article : Google Scholar
|
|
7
|
Denning GM: IL-4 and IFN-gamma
synergistically increase total polymeric IgA receptor levels in
human intestinal epithelial cells. Role of protein tyrosine
kinases. J Immunol. 156:4807–4814. 1996.
|
|
8
|
Kvale D, Løvhaug D, Sollid LM and
Brandtzaeg P: Tumor necrosis factor-alpha up-regulates expression
of secretory component, the epithelial receptor for polymeric Ig. J
Immunol. 140:3086–3089. 1988.PubMed/NCBI
|
|
9
|
Rojas R and Apodaca G: Immunoglobulin
transport across polarized epithelial cells. Nat Rev Mol Cell Biol.
3:944–955. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaetzel CS: The polymeric immunoglobulin
receptor: bridging innate and adaptive immune responses at mucosal
surfaces. Immunol Rev. 206:83–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poger ME, Hirsch BR and Lamm ME: Synthesis
of secretory component by colonic neoplasms. Am J Pathol.
82:327–338. 1976.PubMed/NCBI
|
|
12
|
Harris JP, Caleb MH and South MA:
Secretory component in human mammary carcinoma. Cancer Res.
35:1861–1864. 1975.PubMed/NCBI
|
|
13
|
Harris JP and South MA: Secretory
component: a glandular epithelial cell marker. Am J Pathol.
105:47–53. 1981.PubMed/NCBI
|
|
14
|
DeSouza LV, Krakovska O, Darfler MM,
Krizman DB, Romaschin AD, Colgan TJ and Siu KW: mTRAQ-based
quantification of potential endometrial carcinoma biomarkers from
archived formalin-fixed paraffin-embedded tissues. Proteomics.
10:3108–3116. 2010. View Article : Google Scholar
|
|
15
|
DeSouza LV, Romaschin AD, Colgan TJ and
Siu KW: Absolute quantification of potential cancer markers in
clinical tissue homogenates using multiple reaction monitoring on a
hybrid triple quadrupole/linear ion trap tandem mass spectrometer.
Anal Chem. 81:3462–3470. 2009. View Article : Google Scholar
|
|
16
|
Rossel M, Billerey C, Bittard H, Ksiazek
P, Alber D, Revillard JP and Vuitton DA: Alterations in polymeric
immunoglobulin receptor expression and secretory component levels
in bladder carcinoma. Urol Res. 19:361–366. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rossel M, Seilles E, Voigt JJ, Vuitton D,
Legait N and Revillard JP: Polymeric Ig receptor expression in
hepatocellular carcinoma. Eur J Cancer. 28A:1120–1124. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ai J, Tang Q, Wu Y, Xu Y, Feng T, Zhou R,
Chen Y, Gao X, Zhu Q, Yue X, et al: The role of polymeric
immunoglobulin receptor in inflammation-induced tumor metastasis of
human hepatocellular carcinoma. J Natl Cancer Inst. 103:1696–1712.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiao T, Ying W, Li L, Hu Z, Ma Y, Jiao L,
Ma J, Cai Y, Lin D, Guo S, et al: An approach to studying lung
cancer-related proteins in human blood. Mol Cell Proteomics.
4:1480–1486. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rossel M, Brambilla E, Billaud M, Vuitton
DA, Blanc-Jouvan F, Biichle S and Revillard JP: Nonspecific
increased serum levels of secretory component in lung tumors:
relationship to the gene expression of the transmembrane receptor
form. Am J Respir Cell Mol Biol. 9:341–346. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Makawita S, Smith C, Batruch I, Zheng Y,
Rückert F, Grützmann R, Pilarsky C, Gallinger S and Diamandis EP:
Integrated proteomic profiling of cell line conditioned media and
pancreatic juice for the identification of pancreatic cancer
biomarkers. Mol Cell Proteomics. 10:M111.0085992011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kvale D, Norstein J, Meling GI, Børmer OP,
Brandtzaeg P, Langmark F and Rognum TO: Circulating secretory
component in relation to early diagnosis and treatment of liver
metastasis from colorectal carcinomas. J Clin Pathol. 45:568–571.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Enneking WF, Spanier SS and Goodman MA: A
system for the surgical staging of musculoskeletal sarcoma. 1980.
Clin Orthop Relat Res. 415:4–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bruno ME and Kaetzel CS: Long-term
exposure of the HT-29 human intestinal epithelial cell line to TNF
causes sustained up-regulation of the polymeric Ig receptor and
proinflammatory genes through transcriptional and
posttranscriptional mechanisms. J Immunol. 174:7278–7284. 2005.
View Article : Google Scholar
|
|
25
|
Khattar NH, Lele SM and Kaetzel CS:
Down-regulation of the polymeric immunoglobulin receptor in
non-small cell lung carcinoma: correlation with dysregulated
expression of the transcription factors USF and AP2. J Biomed Sci.
12:65–77. 2005. View Article : Google Scholar
|
|
26
|
Laverdiere C, Hoang BH, Yang R, Sowers R,
Qin J, Meyers PA, Huvos AG, Healey JH and Gorlick R: Messenger RNA
expression levels of CXCR4 correlate with metastatic behavior and
outcome in patients with osteosarcoma. Clin Cancer Res.
11:2561–2567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Urakawa H, Nishida Y, Nakashima H,
Shimoyama Y, Nakamura S and Ishiguro N: Prognostic value of
indoleamine 2,3-dioxygenase expression in high grade osteosarcoma.
Clin Exp Metastasis. 26:1005–1012. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eccles SA and Welch DR: Metastasis: recent
discoveries and novel treatment strategies. Lancet. 369:1742–1757.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mestecky J and McGhee JR: Immunoglobulin A
(IgA): molecular and cellular interactions involved in IgA
biosynthesis and immune response. Adv Immunol. 40:153–245. 1987.
View Article : Google Scholar : PubMed/NCBI
|