Introduction
Lung cancer is one of the leading causes of
cancer-related mortality worldwide. Cisplatin is an alkylating
agent, approved as a first-line chemotherapeutic drug for the
disease. However, improvement in the efficacy of conventional
chemotherapy on cancer is limited due to severe toxic side effects
and acquired resistance (1,2).
Certain studies have reported that radiation or chemotherapy
administered in combination with gene therapy may provide a greater
anticancer therapy response with enhanced antitumor effects and
reduced toxicity (3,4). Results from animal studies have
suggested that a combination of low-dose chemotherapy with gene
therapy for treatment of solid tumors results in more effective
inhibition of tumor growth than using gene therapy or traditional
chemotherapy alone (3,5).
Gene therapy, transferring therapeutic genes to
tumor cells using diverse delivery vehicles, is considered to have
potential advantages for treating intractable cancers. AAV is a
parvovirus with a 4.7 kb single-stranded DNA genome, and has
emerged as a potential vector for mediating gene transfer into
tumor cells, in that it is non-pathogenic, has a broad host range,
is capable of infecting nondividing and dividing cells, can stably
integrate into host DNA, is able to establish long-term transgene
expression, and presents low immunogenicity (6). These features render AAV a useful
alternative to other viral vectors for human gene therapy. To date,
AAV vectors have been utilized in numerous preclinical and clinical
studies for the treatment of a number of diseases (7–9).
Thus, in the present study, low-dose cisplatin was administered in
combination with an AAV2 vector encoding the human
pigment epithelium-derived factor (hPEDF) gene, to investigate the
antitumor effect in a mouse model of Lewis lung carcinoma.
PEDF, an endogenously produced 50-kDa glycoprotein,
is a member of the serpin family, which is widely expressed
throughout the body, including in the brain, eye, liver, bone,
heart and lung (10,11). PEDF was first identified as an
effective neurotrophic factor produced by cultured human fetal
retinal pigment epithelial cells (12). Recently however, more attention has
been paid to its antiangiogenic activity, far greater than that of
other known endogenous angiostatic molecules, such as endostatin,
angiostatin and thrombospondin-1 (13). The antivascular activity of PEDF
appears to be associated with two main mechanisms: Activation of
Fas-FasL-mediated apoptosis and downregulation of vascular
endothelial growth factor (VEGF) expression (14).
The effectiveness of combination treatment of
AAV-hPEDF and low-dose cisplatin on lung cancer has, to the best of
our knowledge, not been reported. The present study was designed to
evaluate the enhanced efficacy of AAV-hPEDF combined with low-dose
cisplatin on the growth of established Lewis lung carcinoma in
mice.
Materials and methods
Cell lines
LLC cell lines were obtained from the American Type
Culture Collection (Manassas, VA, USA). The cells were maintained
as monolayers in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum (FBS). Human umbilical
vein endothelial cells (HUVECs) were isolated from umbilical cords
by a standard procedure (15) and
then grown in Endothelial Basal Medium-2 (Lonza, Basel,
Switzerland) supplemented with VEGF, epidermal growth factor,
fibroblast growth factor and R3-IGF-1.
Construction of AAV-hPEDF and AAV-EGFP
vectors
AAV-hPEDF and AAV-enhanced green fluorescent protein
(EGFP) were constructed as previously described (16). rAAV viral particles were packaged
and purified as described previously (17).
Transduction with AAV-EGFP or AAV-hPEDF,
treatment with cisplatin and flow cytometry
LLC cells were grown in 6-well plates to 70–80%
confluence, then the cells were treated with serum-free DMEM in the
presence or absence of cisplatin (250 ng/ml; Haosen pharmaceutical
Co., Ltd, Jiangsu, China). Two hours after cisplatin
administration, the cells were transduced by AAV-hPEDF or AAV-EGFP
at a multiplicity of infection (MOI) of 1×105 particles
per cell in serum-free DMEM, with or without cisplatin treatment.
At 2 h post-infection, the medium was changed to complete medium,
with or without cisplatin, and the cells were cultured for 72
h.
Cells treated with AAV-EGFP were collected and
resuspended in phosphate-buffered saline (PBS) at a concentration
of ~106 cells/ml and fixed with ice-cold absolute
ethanol overnight. Subsequently, cellular expression of the EGFP
transgene was quantitatively assessed using a flow cytometer (BD
Biosciences, San Jose, CA, USA).
Western blot analysis
LLC cells treated with AAV-EGFP, cisplatin,
AAV-hPEDF or cisplatin plus AAV-hPEDF were lysed with
radio-immunoprecipitation assay solution, and protein
concentrations were then determined with a modified Lowry protein
assay kit (Thermo Fisher Scientific, Rockford, IL, USA). Proteins
(40 mg) from each sample were loaded onto SDS-PAGE gels and then
electrotransferred onto a polyvinylidine fluoride membrane, and
probed with anti-hPEDF monoclonal antibody (1:1000; R&D
Systems, Boston, MA, USA). Blots were incubated for 1 h with
horseradish peroxidase (HRP)-conjugated secondary antibody
(1:10,000; ZSJQ Biotechnology, Beijing, China). The proteins on the
blots were visualized using an enhanced chemoluminescence system
(Pierce Biotechnology, Inc., Rockford, IL, USA).
Tube formation assay
Tube formation assays were performed as previously
described (18). HUVECs were
seeded into a Matrigel-coated (BD Biosciences, Franklin Lakes, NJ,
USA) 96-well plate, and then treated with conditioned media from
LLC cells treated with normal saline (NS), AAV-EGFP, cisplatin,
AAV-hPEDF or cisplatin plus AAV-hPEDF, respectively. Six hours
later, the tubule branches were photographed (Olympus BX53;
Olympus, Tokyo, Japan).
Animal studies
All mouse experiments were approved by the Animal
Care and Use Committee of Sichuan University (Chengdu, Sichuan,
China).
Male C57BL/6 mice (age, 8 weeks) were obtained from
the Experimental Animal Center of Sichuan University, (Sichuan,
China). The LLC cells were inoculated subcutaneously into the back
right side of the animals. Seven days after tumor cell injection,
when the tumor nodule had reached an average size of 4×5 mm, the
animals were randomized into five groups. The groups were
administered either AAV-hPEDF (2×1010 viral genome
copies per 50 μl) intratumorally, cisplatin at a dosage of 2 mg/kg
intraperitoneally every three days (a total of six doses), or
cisplatin plus AAV-hPEDF simultaneously. The remaining two groups
of mice were either treated with AAV-EGFP intratumorally and
intraperitoneally injected with NS, or were treated with an
intratumoral injection of NS. Tumor volume was measured and
calculated using the formula: Tumor volume = 0.52 × length ×
width2.
The mice were sacrificed at the end of experiment;
solid tumor tissues were then surgically resected, weighed and
processed for routine histological analysis and
immunohistochemistry.
For the survival studies, another five groups of
mice (n=10) were treated as described and the survival time was
recorded.
Immunohistochemistry
Tumor microvessel density and human
PEDF expression
Prepared frozen sections of tumors were respectively
incubated with anti-mouse CD31 antibody (BD Biosciences, Franklin
Lakes, NJ, USA) and anti-human PEDF antibody (R&D Systems,
Minneapolis, MN, USA) overnight, and subsequently with a
fluorescence-conjugated secondary antibody (1:100; Abcam,
Cambridge, MA, USA) for 45 min. The CD31-positive vessels and the
PEDF-positive reaction were visualized with 3,3′-diaminobenzidine
(DAB; ZSJQ Biotechnology).
Caspase-3 staining and VEGF
staining
The primary anti-caspase-3 (Cell Signaling
Technology, Inc., Danvers, MA, USA) and anti-VEGF (R&D Systems)
monoclonal antibodies were applied to paraffin sections of tumor
tissues at 4°C overnight. After two washes with PBS, the
HRP-conjugated secondary antibody was applied at room temperature
for 40 min. Subsequently, DAB was used for signal amplification.
The VEGF staining intensity was quantified with Carl Zeiss
AxioImager microscope Image M1 Software (Carl Zeiss AG, Jena,
Germany).
In situ transferase-mediated dUTP nick
end labelling (TUNEL) assay
Apoptotic cells in tumor tissues from each group
were detected by in situ TUNEL analysis, following the
instructions of an In situ Apoptosis kit (Promega Corporation,
Madison, WI, USA). TUNEL-positive cells were quantified using a
fluorescence microscope (Carl Zeiss Microimaging Inc., Thornwood,
NY, USA).
Cell proliferation assay
LLC cells were seeded into a 96-well plate at a
density of 1×104 cells per well overnight, and then
treated with different doses of cisplatin (Haosen Pharmaceutical
Co., Ltd., Lianyungang, Jiangsu, China). After 72 hours of
incubation, cell proliferation was measured using an MTT assay.
Statistical analysis
Statistical analysis was conducted using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard deviation. The differences among the five groups
were evaluated by one-way analysis of variance. Survival data were
analyzed using the log-rank test and P<0.05 was considered to
indicate a statistically significant difference.
Results
Cisplatin enhances AAV-EGFP expression
and AAV-hPEDF expression in LLC cells in vitro
Previous studies have indicated that infection with
AAV was enhanced by irradiation, UV light and various
chemotherapeutic agents (19,20).
In the present study, the effect of cisplatin on transgene
expression following AAV vector delivery was investigated. MTT
assays were performed using different doses of cisplatin to
quantify cisplatin-induced cytotoxicity and to determine a
sub-toxic dosage that results in <25% cell death in LLC cells
(data not shown). It was found that 72 h treatment with cisplatin
inhibited cell proliferation in a dose-dependent manner in LLC
cells, and that 250 ng/ml cisplatin administered to the cells for
72 h led to 20% inhibition of cell proliferation, indicating that
this was an optimal concentration to be used in combination therapy
with AAV-hPEDF.
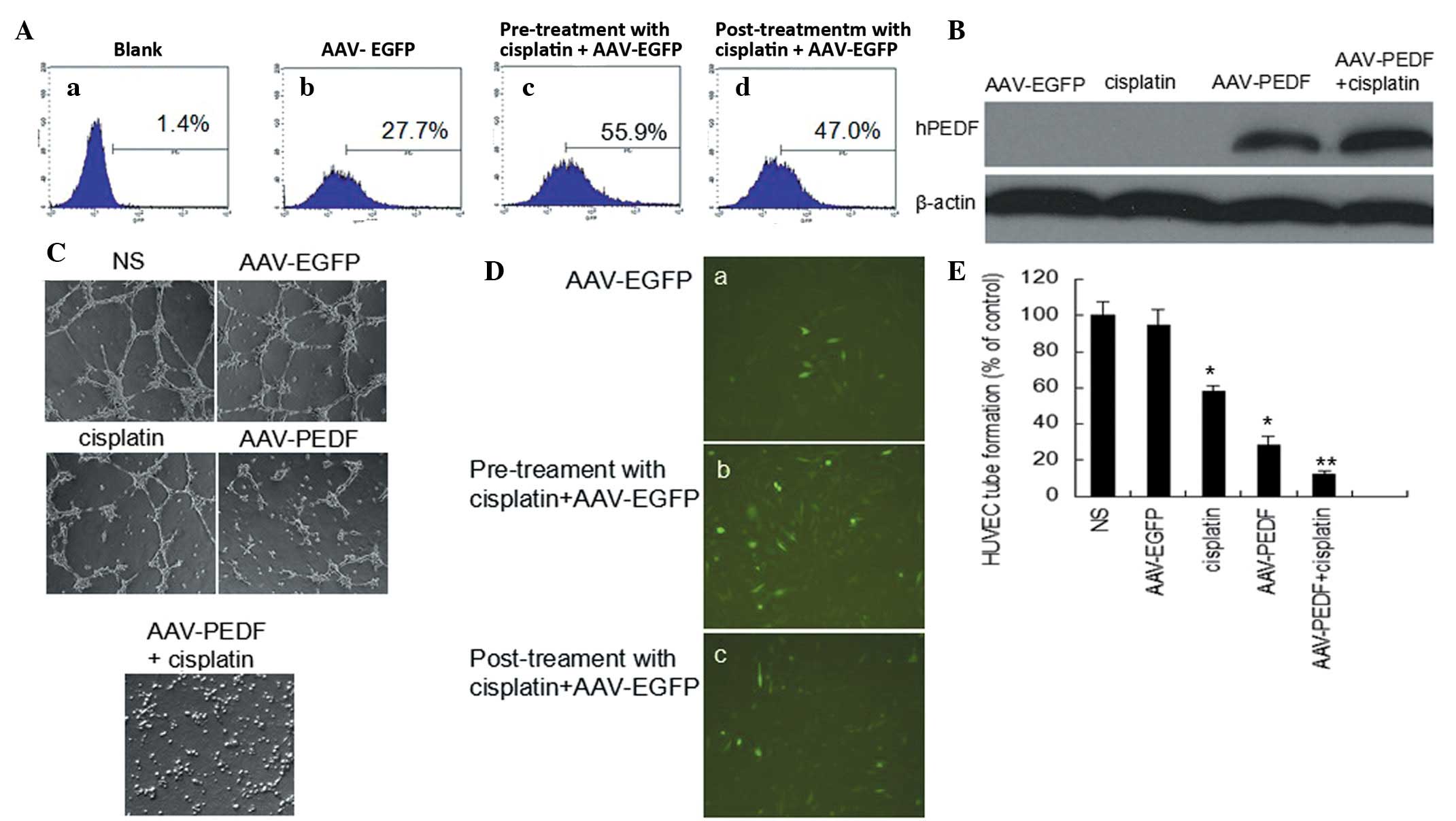
The ability of cisplatin to induce AAV infection was
first investigated using AAV-EGFP (Fig. 1). LLC cells were pre-exposed to 250
ng/ml cisplatin for 2 h prior to AAV-EGFP infection. As revealed in
Fig. 1D(a-c), a marked improvement
in EGFP expression was observed in the pre-exposed cells compared
with cells that received either no treatment, or treatment with
cisplatin following AAV-EGFP infection. EGFP expression levels in
LLC cells were then analyzed using flow cytometry, and the results
demonstrated that pre-treatment with 250 ng/ml cisplatin for 2 h
prior to AAV-EGFP transduction led to an EGFP-positive rate in LLC
cells of 55.9%, whereas no treatment or treatment with cisplatin
following AAV-EGFP infection resulted in EGFP-positive rates of
27.7 and 47%, respectively (Fig.
1A).
LLC cells were treated with 250 ng/ml cisplatin or
transduced with AAV-hPEDF in the presence or absence of cisplatin
at an MOI of 105 particles per cell. hPEDF transgene
expression in LLC cells was analyzed 72 h after infection with
AAV-hPEDF, using western blot analysis. In the presence of
AAV-hPEDF transduction, hPEDF protein was expressed in LLC cells,
and this expression was significantly increased by cisplatin
treatment (Fig. 1B).
These results indicate that cisplatin increased
AAV-mediated transgene expression in LLC cells.
Bioactivity of hPEDF produced by
AAV-hPEDF-transduced LLCs in vitro
When HUVECs are seeded onto a Matrigel matrix, the
cells elongate and form capillary-like cords (21). This assay was used to assess the
ability of cells to form capillary-like structures in conditioned
media, using LLC cells transfected with NS, AAV-EGFP, cisplatin,
AAV-hPEDF or cisplatin plus AAV-hPEDF. Treatment with the
conditioned media from AAV-hPEDF markedly inhibited the tube
formation; however, the combined treatment of cisplatin and
AAV-hPEDF (12±2.3%) resulted in marked inhibition of HUVEC tube
formation compared with treatment with either AAV-hPEDF alone
(28.5±4.8%) or 250 ng/ml cisplatin alone (58±3.0%) (Fig. 1C and E, P<0.05). The data reveal
that the PEDF protein produced by transfected cells was highly
bioactive and that treatment with cisplatin and AAV-hPEDF in
combination resulted in greater suppression of angiogenesis in
vitro by inhibiting tube formation.
Antitumor efficacy of AAV-hPEDF combined
with cisplatin in mice with established Lewis lung carcinoma
A mouse LLC tumor model was used to examine the
potential inhibitory effect of combination therapy on tumor growth.
Treatment was initiated when the tumor nodule reached an average
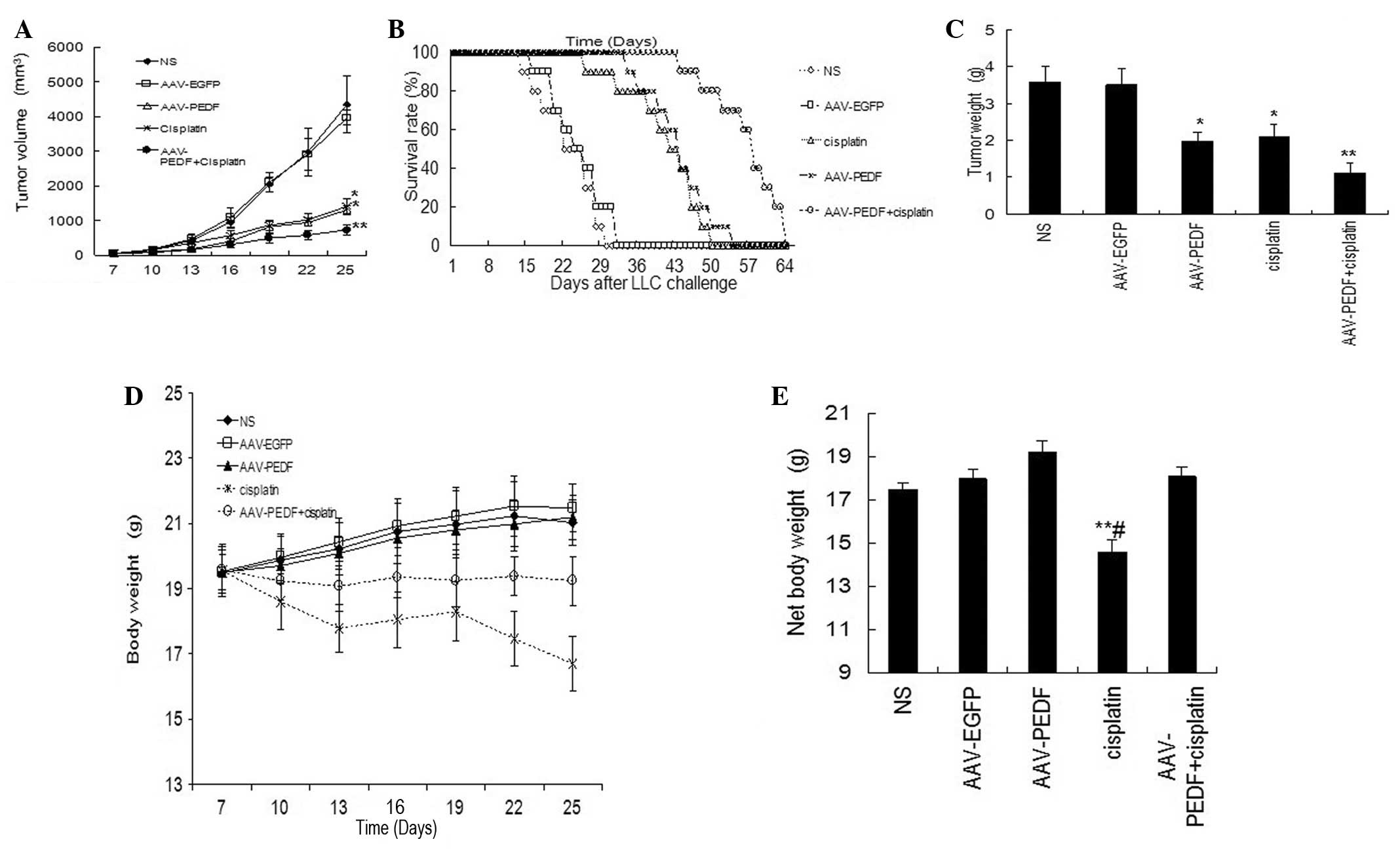
size of 4×5 mm. As shown in Fig.
2A, treatment with either cisplatin or AAV-hPEDF alone resulted
in a marked reduction in tumor volume compared with that of the two
control groups, NS and AAV-EGFP, during the treatment period
(P<0.05). However, treatment with cisplatin plus AAV-hPEDF
demonstrated an enhanced inhibitory effect on tumor growth
(Fig. 2A, P<0.01). On the 25th
day post-inoculation, the mice were sacrificed and solid tumor
tissues were surgically resected and weighed (Fig. 2C). The tumor weights of the
combination-treated group were markedly lower than those in the
groups treated with either cisplatin or AAV-hPEDF alone (Fig. 2C, P<0.05), as well as those in
the NS or AAV-EGFP groups (Fig.
2C, P<0.01). Moreover, the group treated with a combination
of cisplatin and AAV-hPEDF demonstrated an increased survival rate
compared with the control groups (Fig.
2B, P<0.01) or either of the cisplatin- or AAV-hPEDF-treated
groups (Fig. 2B, P<0.05).
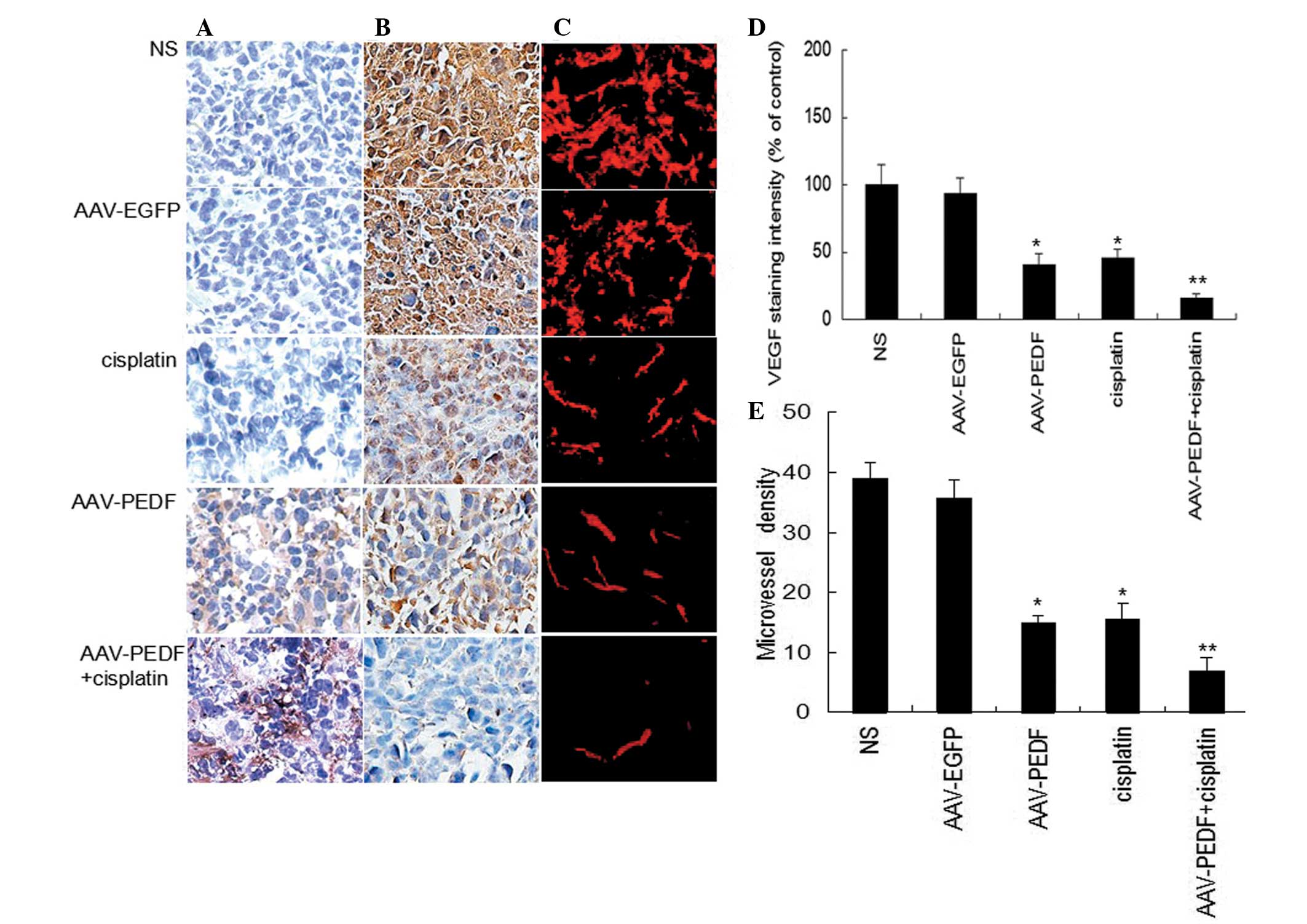
hPEDF expression was investigated in tumor sections
from each group by immunohistochemistry. hPEDF staining was
strongly positive in the tumor tissue in the group treated with
AAV-hPEDF alone and the group treated with a combination of
AAV-hPEDF plus cisplatin. Compared with the AAV-hPEDF group, there
was stronger hPEDF staining in the group treated with a combination
of AAV-hPEDF and cisplatin (Fig.
3A).
These results demonstrate that the combined use of
cisplatin and AAV-hPEDF markedly inhibited tumor growth and
prolonged the survival time in C57BL/6 mice.
Enhanced effect of AAV-hPEDF and
cisplatin combination treatment on suppression of tumor
angiogenesis and induction of tumor apoptosis in vivo
To analyze whether the inhibitory effect of the
combination therapy on tumor growth was associated with the
suppression of tumor angiogenesis, tumor tissues from each group
were immunostained for CD31 and MVD. MVD expression was markedly
lower in the combination treatment group compared with the groups
treated with cisplatin or AAV-hPEDF alone (Fig. 3C and E, P<0.05) as well as with
the two control groups (Fig. 3C and
E, P<0.01). VEGF expression in the cisplatin, AAV-hPEDF, and
combination groups was lower than that in the control groups, and
VEGF expression in the cisplatin plus AAV-hPEDF group was lower
than in either of the groups treated with cisplatin or AAV-hPEDF
alone (Fig. 3B and D). These data
demonstrate that combination treatment significantly inhibits tumor
angiogenesis in vivo.
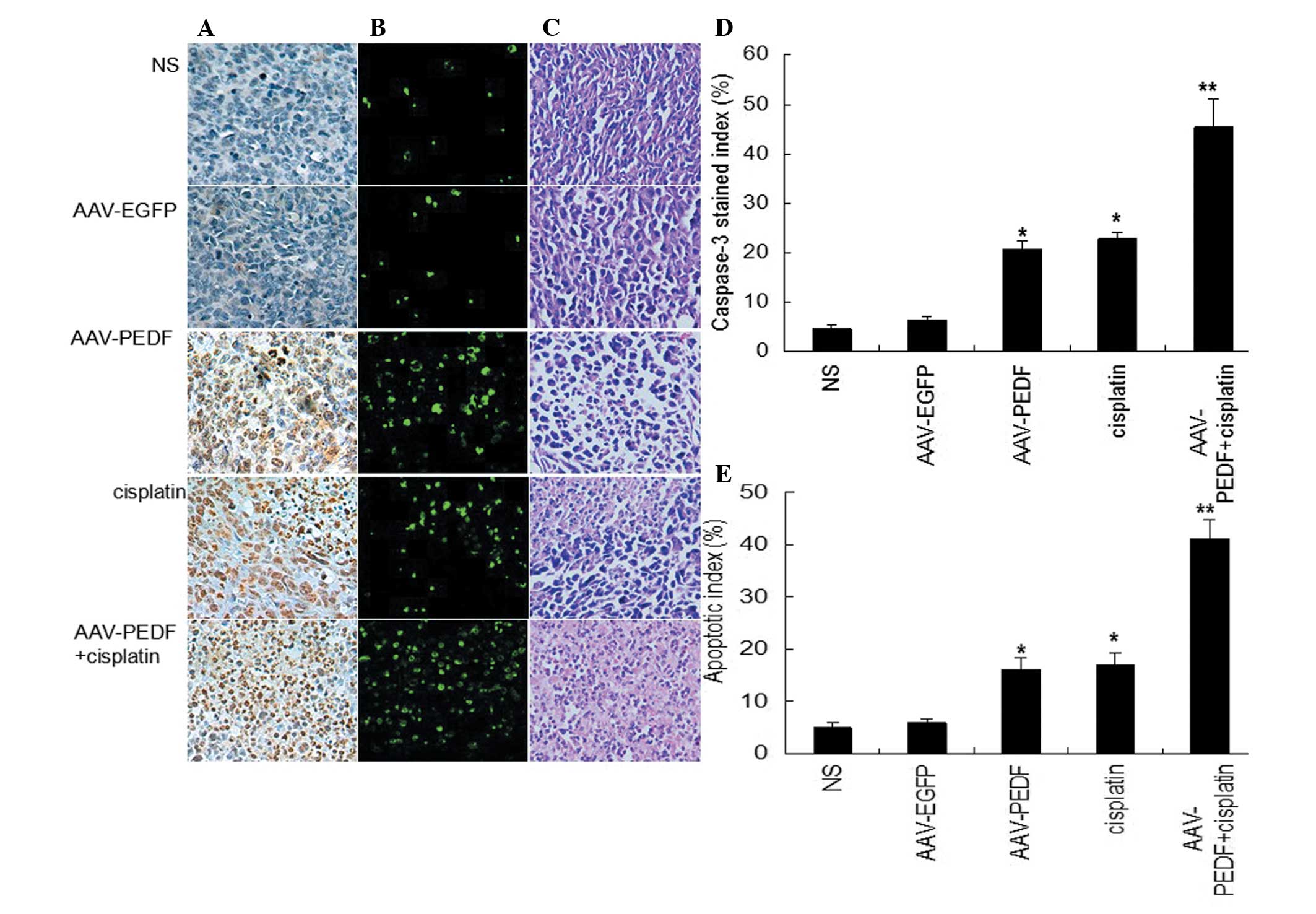
Treatment-induced tumor apoptosis was detected by a
TUNEL assay. Apoptotic cells were present at low levels in tumors
from the NS-treated and AAV-EGFP-treated groups, whereas cisplatin
or AAV-hPEDF treatment markedly induced apoptosis in tumor cells.
However, the apoptosis rate in the combination treatment group was
markedly higher than that in the cisplatin- or AAV-hPEDF-treated
groups (Fig. 4B). Furthermore, the
activated cell death form of caspase-3 was assessed by
immunohistochemistry analysis. The average caspase-3 stained index
in the cisplatin- or AAV-hPEDF-treated groups were similar
(Fig. 4A and D). The combined
therapy resulted in a marked increase in the caspase-3 index,
exceeding those of the single treatments (Fig. 4A and D, P<0.05). Additionally,
in hematoxylin and eosin staining of tumors, larger areas of tumor
necrosis were observed in the cisplatin plus AAV-hPEDF group
(Fig. 4C).
Reduction of body weight loss caused by
cisplatin after AAV-hPEDF treatment in mice
Notably, the body weight of mice treated with
cisplatin alone (2 mg/kg intraperitoneally every three days) was
found to be reduced by 20.6, 22.3 and 21.2% during the experimental
period, compared with the NS-, AAV-EGFP-, and AAV-hPEDF-treated
mice, respectively (Fig. 2D),
whereas that of mice in the combined therapy group did not change
(Fig. 2D). In addition, the
average net body weight of the animals was calculated to remove the
impact of tumor weight on body weight. The net body weight of
cisplatin monotherapy-treated animals demonstrated a marked
reduction compared with the other four groups (Fig. 2E, P<0.05). These findings
indicate that AAV-hPEDF may markedly decrease cisplatin-induced
side effects in mice.
Discussion
Cisplatin is an attractive anticancer agent,
frequently used for treatment of multiple human cancers, including
ovarian, head and neck, liver and lung cancer, and other solid
tumors. The usefulness of cisplatin is limited by its toxicity to
nonmalignant tissues or organs, and by intrinsic and acquired
resistance to the drug (1,2). Therefore, novel therapeutic
approaches are required to decrease the drug dosage, diminish side
effects and enhance the therapeutic efficacy to achieve successful
use of cisplatin in cancer treatment. Certain studies have reported
that supplementing conventional treatment with gene therapy may
have synergistic or enhanced efficacy on inhibiting tumor growth
(3,5), as gene therapy and chemotherapy act
by different mechanisms. Recombinant vectors based on AAV are safe
vehicles with potential for gene transfer and gene therapy
(6). In the present study,
cisplatin was combined with AAV-mediated hPEDF gene to investigate
the effect of this combined treatment on tumor growth.
Although initially labeled as a neurotrophic factor,
PEDF was later identified to have potent antivascular activity
(13), with a demonstrated ability
to suppress the growth of various malignancies in vivo
(22–24). As a potent endogenous inhibitor of
neovascularization, PEDF appears to inhibit pathological vessel
formation without altering native vasculature (25), and is nontoxic and stable when
applied by virus-mediated gene transfer (23,24).
The virus (adenovirus and AAV)-meditated PEDF gene has been
intensively investigated in the treatment of various types of
cancers (22–24). However, it is difficult to
eradicate cancer cells using AAV-PEDF alone. Thus, a combined
approach of AAV-PEDF and traditional cytotoxic drugs may be
beneficial in eradicating lung cancer cells. In the present study,
the results suggest that AAV-PEDF conjugated to cisplatin
efficiently expressed the PEDF gene in the target cells; this
expression was significantly increased by cisplatin treatment and
the combination treatment resulted in decreased angiogenesis in
vitro by inhibiting tube formation (Fig. 1). The in vivo experiments in
the present study indicated that the combination therapy of
AAV-PEDF and cisplatin inhibited tumor growth more efficiently,
prolonged survival time, resulted in greater suppression of tumor
angiogenesis and exhibited more marked induction of tumor apoptosis
in vivo, than either treatment alone. In addition, the
combination therapy protected tumor-inoculated mice from
cisplatin-induced body weight loss (Fig. 2). Therefore, the combinational
strategy of AAV-PEDF and cisplatin has potential for use in
clinical lung cancer therapy.
The results indicate that AAV-PEDF and low-dose
cisplatin may each potentiate the anticancer properties of the
other; however the molecular mechanism responsible for the
interaction between AAV-PEDF and low-dose chemotherapy remains
unclear. AAV-PEDF exerts its antitumor effects through
overexpression of PEDF mediated by the AAV vector. PEDF
overexpression not only results in a decrease of tumor microvessel
density and downregulation of VEGF expression, but also an increase
of tumor cell apoptosis, which has both an indirect and direct
effect on tumors (14).
Conversely, as a cytotoxic agent, cisplatin interferes with DNA
synthesis and causes DNA cross-linking that modulates cell cycle
progression, thus ultimately inducing tumor cell apoptosis
(26). Additionally, previous
studies have shown that AAV vector-mediated infection, together
with irradiation, UV light and various chemotherapeutic agents,
enhances transgene expression (19,20).
In the present study, it was demonstrated that low-dose cisplatin
was capable of increasing AAV infection in murine LLC cells using
AAV-EGFP viral particles. The infection efficiency of the AAV
vector harboring hPEDF was similar to that of AAV-EGFP, as
predicted. In animal experiments, cisplatin was also revealed to
enhance AAV vector-mediated PEDF expression. These effects may
contribute to the synergistic suppression of tumor growth. However,
the precise antitumor mechanism of the combination treatment
requires further investigation.
Although cisplatin is effective against certain
tumors, its severe side effects in normal tissues and organs limits
its application in cancer therapy. In the present study,
intraperitoneal injection of cisplatin resulted in an apparent
decrease in body weight; however, AAV-hPEDF was observed to protect
tumor-inoculated mice from cisplatin-induced body weight loss
(Fig. 2D). Furthermore, the net
body weight of the combined therapy group was markedly higher than
that of the cisplatin only-treated group (Fig. 2E). However, whether AAV-hPEDF
protects against the nephrotoxicity caused by cisplatin requires
further investigation.
In conclusion, to the best of our knowledge, the
present study is the first to combine AAV-mediated PEDF and low
concentrations of cisplatin for cancer therapy. The combined
treatment of AAV-hPEDF and cisplatin markedly prolonged the
survival time of the mice and suppressed tumor growth, and also
protected against cisplatin-related toxicity. These findings
suggest that combination of AAV-hPEDF and cisplatin has potential
as a novel therapeutic strategy for human lung cancer and other
solid tumors.
Acknowledgements
This study was funded by the National Key Basic
Research Program (973 Program) of China (grant no.
2010CB529900).
Abbreviations:
|
PEDF
|
pigment epithelium-derived factor
|
|
AAV
|
adeno-associated virus
|
|
MVD
|
microvessel density
|
|
LLC
|
lewis lung carcinoma
|
|
VEGF
|
vascular endothelial growth factor
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
NS
|
normal saline
|
References
|
1
|
Adam M, Bayer C, Henke J, et al:
Tirapazamine plus cisplatin and irradiation in a mouse model:
improved tumor control at the cost of increased toxicity. J Cancer
Res Clin Oncol. 134:137–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verweij J, de Wit R and de Mulder PH:
Optimal control of acute cisplatin-induced emesis. Oncology.
53(Suppl 1): 56–64. 1996. View Article : Google Scholar
|
|
3
|
Jiang M, Liu Z, Xiang Y, et al:
Synergistic antitumor effect of AAV-mediated TRAIL expression
combined with cisplatin on head and neck squamous cell carcinoma.
BMC Cancer. 11:542011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanazawa T, Mizukami H, Okada T, et al:
Suicide gene therapy using AAV-HSVtk/ganciclovir in combination
with irradiation results in regression of human head and neck
cancer xenografts in nude mice. Gene Ther. 10:51–58. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Huang F, Cai H, et al: The
efficacy of combination therapy using adeno-associated virus-TRAIL
targeting to telomerase activity and cisplatin in a mice model of
hepatocellular carcinoma. J Cancer Res Clin Oncol. 136:1827–1837.
2010. View Article : Google Scholar
|
|
6
|
Monahan PE, Jooss K and Sands MS: Safety
of adeno-associated virus gene therapy vectors: a current
evaluation. Expert Opin Drug Saf. 1:79–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wagner JA, Reynolds T, Moran ML, et al:
Efficient and persistent gene transfer of AAV-CFTR in maxillary
sinus. Lancet. 351:1702–1703. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muramatsu S, Fujimoto K, Ikeguchi K, et
al: Behavioral recovery in a primate model of Parkinson’s disease
by triple transduction of striatal cells with adeno-associated
viral vectors expressing dopamine synthesizing enzymes. Hum Gene
Ther. 13:345–354. 2002.PubMed/NCBI
|
|
9
|
Kay MA, Manno CS, Ragni MV, et al:
Evidence for gene transfer and expression of factor IX in
haemophilia B patients treated with an AAV vector. Nat Genet.
24:257–261. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Becerra SP, Sagasti A, Spinella P and
Notario V: Pigment epithelium-derived factor behaves like a
noninhibitory serpin. Neurotrophic activity does not require the
serpin reactive loop. J Biol Chem. 270:25992–25999. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Broadhead ML, Dass CR and Choong PF: In
vitro and in vivo biological activity of PEDF against a range of
tumours. Expert Opin Ther Targets. 13:1429–1438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tombran-Tink J and Barnstable CJ: PEDF: a
multifaceted neurotrophic factor. Nat Rev Neurosci. 4:628–636.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dawson D, Volpert OV, Gillis P, et al:
Pigment epithelium-derived factor: a potent inhibitor of
angiogenesis. Science. 285:245–248. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fernandez-Garcia NI, Volpert OV and
Jimenez B: Pigment epithelium-derived factor as a multifunctional
antitumor factor. J Mol Med (Berl). 85:15–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jaffe EA, Nachman RL, Becker CG and Minick
CR: Culture of human endothelial cells derived from umbilical
veins. Identification by morphologic and immunologic criteria. J
Clin Invest. 52:2745–2756. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He SS, Shi HS, Yin T, et al: AAV-mediated
gene transfer of human pigment epithelium-derived factor inhibits
Lewis lung carcinoma growth in mice. Oncol Rep. 27:1142–1148.
2012.PubMed/NCBI
|
|
17
|
Wu X, Dong X, Wu Z, Cao H, Niu D, et al: A
novel method for purification of recombinant adenoassociated virus
vectors on a large scale. Chinese Sci Bull. 46:485–488. 2001.
View Article : Google Scholar
|
|
18
|
Pang X, Yi Z, Zhang X, et al:
Acetyl-11-ketob-boswellic acid inhibits prostate tumor growth by
suppressing vascular endothelial growth factor receptor 2-mediated
angiogenesis. Cancer Res. 69:5893–5900. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alexander IE, Russell DW and Miller AD:
DNA-damaging agents greatly increase the transduction of
nondividing cells by adeno-associated virus vectors. J Virol.
68:8282–8287. 1994.PubMed/NCBI
|
|
20
|
Kanazawa T, Urabe M, Mizukami H, et al:
Gamma-rays enhance rAAV-mediated transgene expression and cytocidal
effect of AAV-HSVtk/ganciclovir on cancer cells. Cancer Gene Ther.
8:99–106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taraboletti G and Giavazzi R: Modelling
approaches for angiogenesis. Eur J Cancer. 40:881–889. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hase R, Miyamoto M, Uehara H, et al:
Pigment epithelium-derived factor gene therapy inhibits human
pancreatic cancer in mice. Clin Cancer Res. 11:8737–8744. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang LP, Cheng P, Peng XC, et al:
Anti-tumor effect of adenovirus-mediated gene transfer of pigment
epithelium-derived factor on mouse B16-F10 melanoma. J Exp Clin
Cancer Res. 28:752009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu QJ, Gong CY, Luo ST, et al:
AAV-mediated human PEDF inhibits tumor growth and metastasis in
murine colorectal peritoneal carcinomatosis model. BMC Cancer.
12:1292012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stellmach V, Crawford SE, Zhou W and Bouck
N: Prevention of ischemia-induced retinopathy by the natural ocular
antiangiogenic agent pigment epithelium-derived factor. Proc Natl
Acad Sci USA. 98:2593–2597. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siddik ZH: Cisplatin: mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|