Introduction
Gliomas and astrocytomas are brain tumors that are
associated with a high mortality rate and current treatments have
not significantly improved patient survival (1). The gap junction, which is required
for maintaining cell growth, has been reported to be absent in
gliomas and astrocytomas. The gap junction is made of connexin
proteins, which have been proposed to act as tumor suppressors
(2,3). Connexin 43 (Cx43) is the major
protein forming gap junction channels in astrocytes and has been
proposed to have growth inhibitory effects. Expression of Cx43 is
inversely correlated with the degree of malignancy (3). It is accepted that connexins not only
act as critical gatekeepers of cell proliferation by controlling
the intercellular exchange of essential growth regulators, but they
may also affect cell cycling by non-gap junctional intercellular
communication (4).
By contrast, the abnormal expression of microRNAs
(miRNAs) has been linked with several types of cancer, including
glioma (5). miRNAs are endogenous
eukaryotic small, non-coding RNAs that negatively regulate gene
expression (6). The study of the
expression profile of miRNA in glioblastoma indicated that mir-451
is significantly decreased in glioblastoma compared with normal
brain tissue (7). Overexpression
of miR-451 in glioblastoma cells led to the inhibition of
glioblastoma cell growth, invasive ability and enhanced apoptosis
(8).
Another line of evidence indicated that higher
grades of human brain tumors are associated with lower adenyl
cyclase activity and/or cellular cAMP concentrations (9). It was stated that the β2-adrenergic
receptor (β2-AR) is expressed in glioblastomas, as well as in the
human-derived 1321N1 astrocytoma cell line (10). cAMP and its related compounds
inhibit the proliferation of tumor cells (11). Furthermore, studies have revealed
an association between cAMP and Cx43 (12), and also between cAMP levels and the
expression of miRNA (13). By
contrast, other studies have demonstrated an association between
miRNAs and Cx43 expression (14).
Since the data in the Oncomine cancer profiling
database (http://www.oncomine.org) suggests a
significant portion of gliomas and astrocytomas express the β2-AR
to a greater extent than in normal brain tissue, this receptor
represents a potential therapeutic target for the treatment of
these tumors (15). In the present
study, we analyzed the possible interaction between the cAMP-Epac
signaling pathway, Cx43 and miR-451 expression in astrocytoma
cells.
Materials and methods
Reagents
All drugs, including a selective β2-AR agonist,
clenbuterol hydrochloride (C5423), adenylyl cyclase inhibitor (SQ
22,536; S153), PKA specific inhibitor (H-89; B1427) and
Epac-specific activator 8-(4-chlorophenylthio)-2
-O-methyladenosine-3,5-cyclic monophosphate (8CPT; C8988) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies
including mouse monoclonal to cx43/GJA1 (ab79010), rabbit
polyclonal to Epac2 (ab21238), mouse monoclonal to β-actin
(ab6276), rabbit polyclonal secondary antibody to mouse IgG
(ab6728) and goat polyclonal secondary antibody to rabbit IgG
(ab6721) were purchased from Abcam (Cambridge, MA, USA).
Cell culture
The human astrocytoma cell line 1321N1 was obtained
from the National Cell bank of Iran, (Tehran, Iran) and maintained
in DMEM containing 10% FBS supplemented with 100 unit/ml penicillin
and 50 mg/ml streptomycin in a humidified atmosphere at 5%
CO2 and 37°C. Cells were plated for RNA extraction and
western blot analysis in six-well plates. Prior to drug exposure in
all experiments, the medium of 70–80% confluent cells was changed
with DMEM containing 1% FBS.
Cell proliferation assay
Cell proliferation was quantified using an MTT assay
(Sigma-Aldrich). Mir-451 transfected and untransfected cells were
seeded in 96-well plates with a density of 0.5×104
cells/well containing DMEM. The attached cells were incubated for
another 24 and 48 h with of 10 μg/ml β2 agonist; 10 μg/ml β2
agonist + 20 μg/ml AC inhibitor; and 20 μg/ml Epac activator, with
DMEM as a control. Next, cells were incubated with 5 mg/ml of MTT
solution at 37°C for 3 h. We dissolved the formazan crystals in
DMSO. The optical density of each well was measured using a
microplate reader (BioTek Instruments, Inc., Winooski, VT, USA)
measuring at a wavelength of 570 nm with background subtraction at
630 nm. The experiments were performed and repeated 3 times. We
determined each treatment for mean values ± SE.
Real-time RT-PCR
Total RNA, isolated with QIAzol (Qiagen, Hilden,
Germany) according to the manufacturer’s instructions, was used for
complementary DNA (cDNA) synthesis by reverse transcriptase
(Vivantis, Oceanside, CA, USA; cat no: RTPL12). Specific primers
for human Cx43 (forward: 5′-GAT GAG GAA GGA AGA GAA G-3′, reverse:
5′-CGC TAG CTT GCT TGT TGT AA-3′) and β-actin (forward: 5′-GCA AGA
GAG GTA TCC TGA CC-3′, reverse: 5′-CCC TCG TAG ATG GGC ACA GT-3′)
were used for this experiment using Takara SYBR Premix Ex Taq
Master (Takara Bio, Inc., Shiga, Japan). Gene expression levels
were quantified by Rotor-Gene 6000 (Corbett, Concorde, NSW,
Australia). The relative expression ratio of Cx43 and β-actin were
calculated using the relative expression software tool (REST).
Immunoblotting assay
Total cell lysates were prepared following
harvesting cells in ice-cold PBS and homogenization in cold lysis
buffer (RIPA buffer). Protease inhibitor cocktail (S8820;
Sigma-Aldrich) was added to the lysis buffer. The lysate was
centrifuged at 13,680 × g for 10 min and the supernatant was
transferred to a new tube. Protein content was measured using the
bicinchoninic acid assay (BCA protein kit; Pierce Biotechnology,
Inc., Rockford, IL, USA). An equal amount of protein (25 μg) per
well was loaded to 12.5% standard SDS-PAGE, then by using a wet
system (Bio-Rad, Hercules, CA, USA), the proteins and page ruler
were transferred to PVDF membranes. Membranes were blocked in 2%
skimmed milk in TBST 20 (Tris-buffered saline (TBS)/Tween-20; 0.5%)
for 2 h at room temperature. Membranes were incubated overnight at
4°C with primary antibodies against cx43 and Epac2 diluted in TBST
(1:1000; 0.5%). Membranes were washed three times with TBST 20 and
subsequently incubated for 1 h at room temperature with the
following corresponding HRP-conjugated secondary antibodies: rabbit
polyclonal secondary antibody to mouse IgG and goat polyclonal
secondary antibody to rabbit IgG. Following three washes with TBST
20 (0.5%), detection was performed by applying Pierce ECL Plus
Western Blotting Substrate (#32134), which produced
chemiluminescence from the membrane for manual X-ray film
development. Levels of the analyzed proteins were normalized to
β-actin levels.
Virus packaging, concentration and
transduction of 1321N1 cells
For virus packaging of PB_miR-451 (contained green
fluorescent protein (GFP) and puromycin resistance genes), we used
a human embryonic kidney 293T cell line (HEK 293T). These cells
were maintained in DMEM containing 10% FBS supplemented with 100
unit/ml penicillin and 50 mg/ml streptomycin in a humidified
atmosphere at 5% CO2 and 37°C. Cells were co-transfected
with psPAX2 plasmid containing gag/pol packaging genes, pMDG
plasmid containing VSV-G and PB_miR-451 (as well as an empty
vector) using the calcium-phosphate method. Following 24 h of
transfection, GFP expression was assessed using an inverted
fluorescence microscope (TE2000; Nikon, Tokyo, Japan). The medium
containing the produced viruses were collected 24, 48 and 72 h
following transfection and kept at 4°C followed by concentration
processing, including centrifuging at 21,000 rpm or 40,000 g at 4°C
for 2.5 h. The concentrated supernatants were used to infect 1321N1
human astrocytoma cells.
Puromycin treatment and total RNA
extraction
Following 24 h of transduction, transducted cells
were treated with puromycin (2 μg/ml) and treatment was continued
for 3 days. Cells in the negative control groups were also treated
with puromycin and as expected died after 48 h. The experimental
group (that was transfected with PB_miR-451) was resistant to
puromycin and survived the antibiotic selection. Total RNA
(containing miRNAs) was collected following 72 h puromycin
treatment using QIAzol Lysis Reagent (Qiagen, Valencia, USA). An
RNA pellet was dissolved in 20 μl distilled water and maintained in
−70°C until further use.
miRNA expression verification
To assess the constructs’ functionality,
overexpression of miR-451 was evaluated by RT-PCR in transducted
1321N1 cells. Following 72 h, transducted cells were harvested for
miRNA extraction by Qiazol reagent (Qiagen). The extracted
microRNAs were subjected to cDNA synthesis by Stratagene (La Jolla,
CA, USA) real-time RT-PCR kit according to the manufacturer’s
instructions. Briefly, following poly-adenylation of total RNAs,
the cDNA was synthesized by an adaptor primer provided by
Stratagene. The relative quantification of miRNAs in comparison to
control cells was assayed by real-time RT-PCR with Stratagene SYBR
green master mix (Stratagene), according to the manufacturer’s
instructions in a Rotor-Gene 6000 system (Corbett, Concorde, NSW,
Australia; n=3). The relative expression ratio of miRNA in 1321N1
cells under control conditions and following 24 h of treatment with
our drugs or overexpression was normalized relative to a U6
endogenous control and was then calculated using REST. Specific
primers for human miR-451 and U6 were: miR-451 sense: 5′-GGA AGA
TCT TGA CAA GGA GGA CAG GAG AG-3′, miR-451 reverse: 5′-CCC AAG CTT
GCC TTG TTT GAG CTG GAG TC-3′, U6 sense: 5′-CTC GCT TCG GCA GCA
CA-3′, U6 reverse: 5′-AAC GCT TCA CGA ATT TGC GT-3′.
Statistical analysis
The obtained data were analyzed using the Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference (indicated with an asterisk *).
Each experiment was repeated independently at least three
times.
Results
Epac2 expression in 1321N1 astrocytoma
cells
Previous studies demonstrated the presence of Epac2
in the developing and mature brain (16). To determine the putative presence
of Epac2 in the 1321N1 astrocytoma cell line, we performed a
western blot analysis to detect endogenous Epac2 and to test the
effect of the specific β2-AR agonist and 8CPT on its expression in
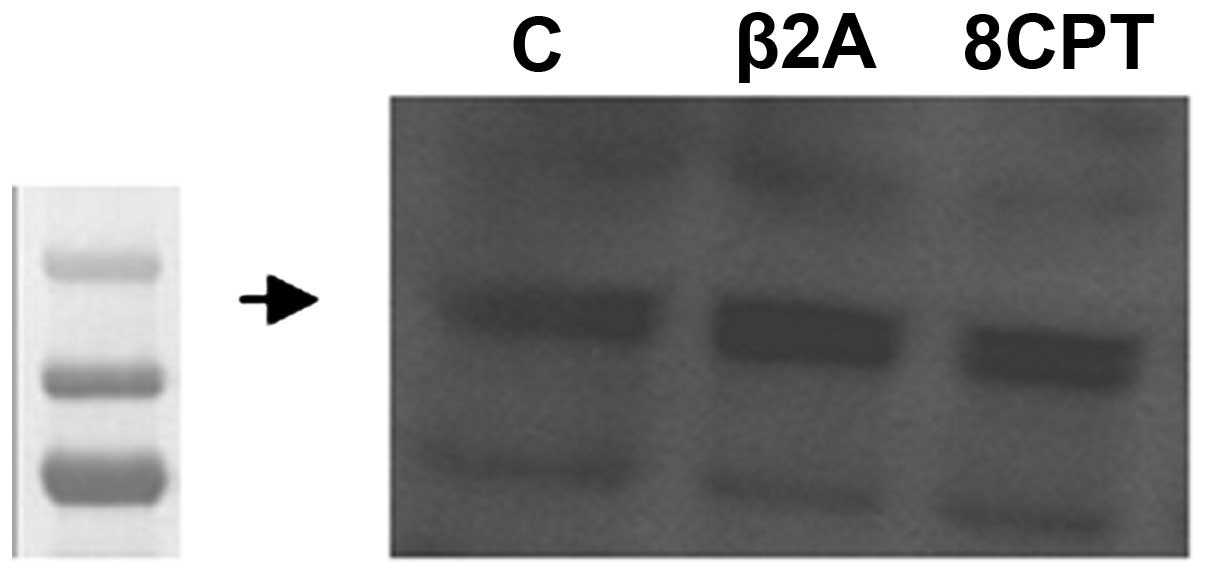
astrocytoma cells. As depicted in Fig.
1, we found that these cells express the Epac2 protein
endogenously. Protein bands were detected at a molecular weight of
126 kDa for Epac2. The selective β2-AR agonist and Epac activator
did not significantly increase Epac2 expression.
Effect of β2-AR stimulation and the Epac
signaling pathway on Cx43 expression
Next, we tested the specificity of cAMP signaling on
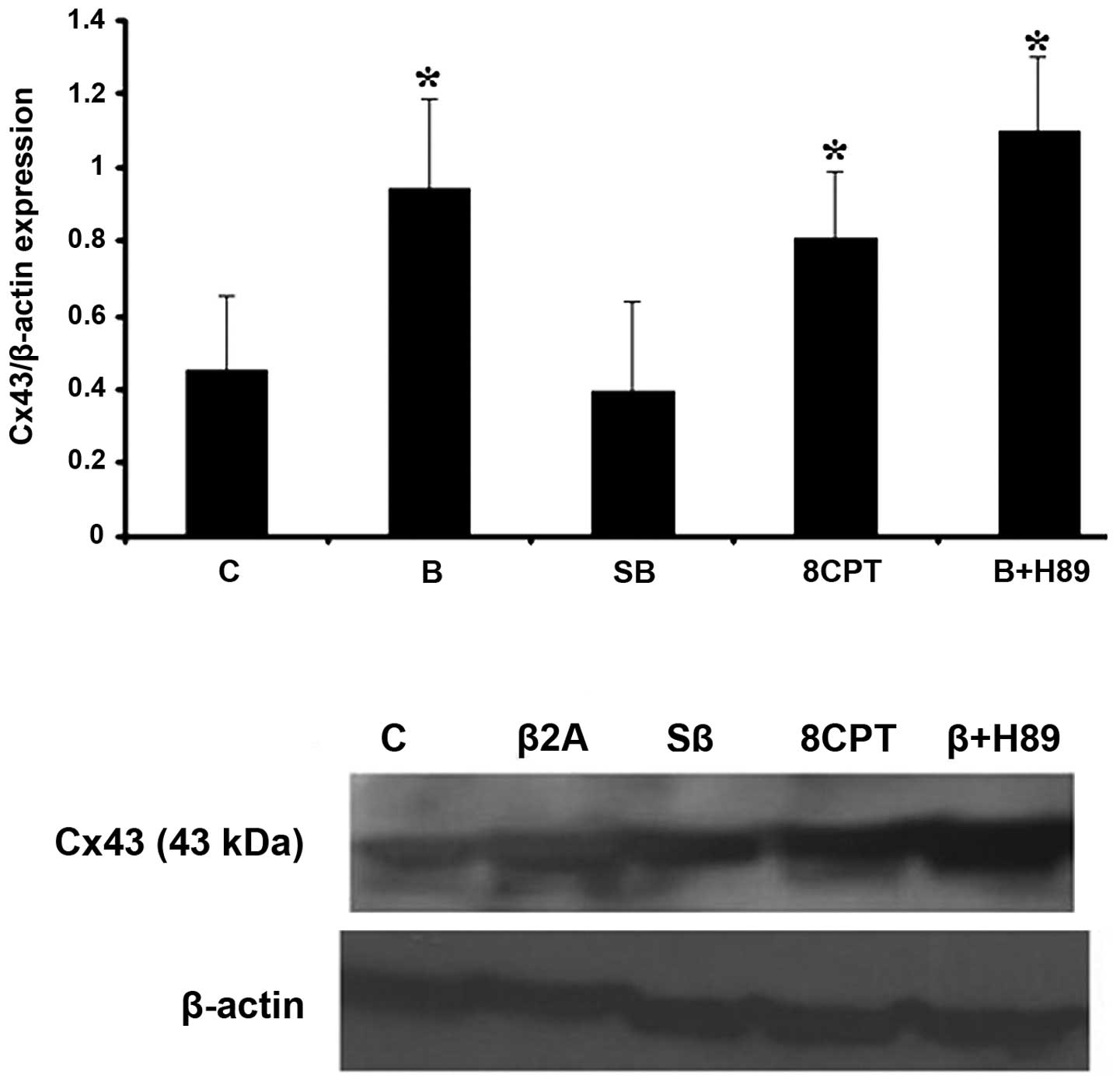
Cx43 expression in astrocytoma cells. Following 24 h treatment of
cells with our drugs, selective β2-AR stimulation with clenbuterol
hydrochloride (10 μg/ml) led to an increase in Cx43 protein level
(Fig. 2). This effect was not dose
dependent (data not shown). 1321N1 cells were pretreated with
adenyl cyclase (AC) inhibitor, SQ 22,536 (20 μg/ml) and H-89
dihydrochloride (10 μg/ml), as a selective PKA inhibitor, for 45
min in the presence of clenbuterol. Pretreatment with SQ prevented
clenbuterol upregulation of Cx43 while cells pretreated with H-89
had no effect on Cx43 upregulation, suggesting that upregulation of
Cx43 observed in the presence of clenbuterol does not occur without
the PKA pathway. To further investigate the possible role of Epac
in the upregulation of Cx43 we used the selective Epac activator
8-pCPT-2′-O-Me-cAMP (20 μg/ml). We observed that the protein level
of Cx43 was increased following 24 h treatment with this drug.
Effect of β2-AR stimulation and the Epac
signaling pathway on miR-451 expression
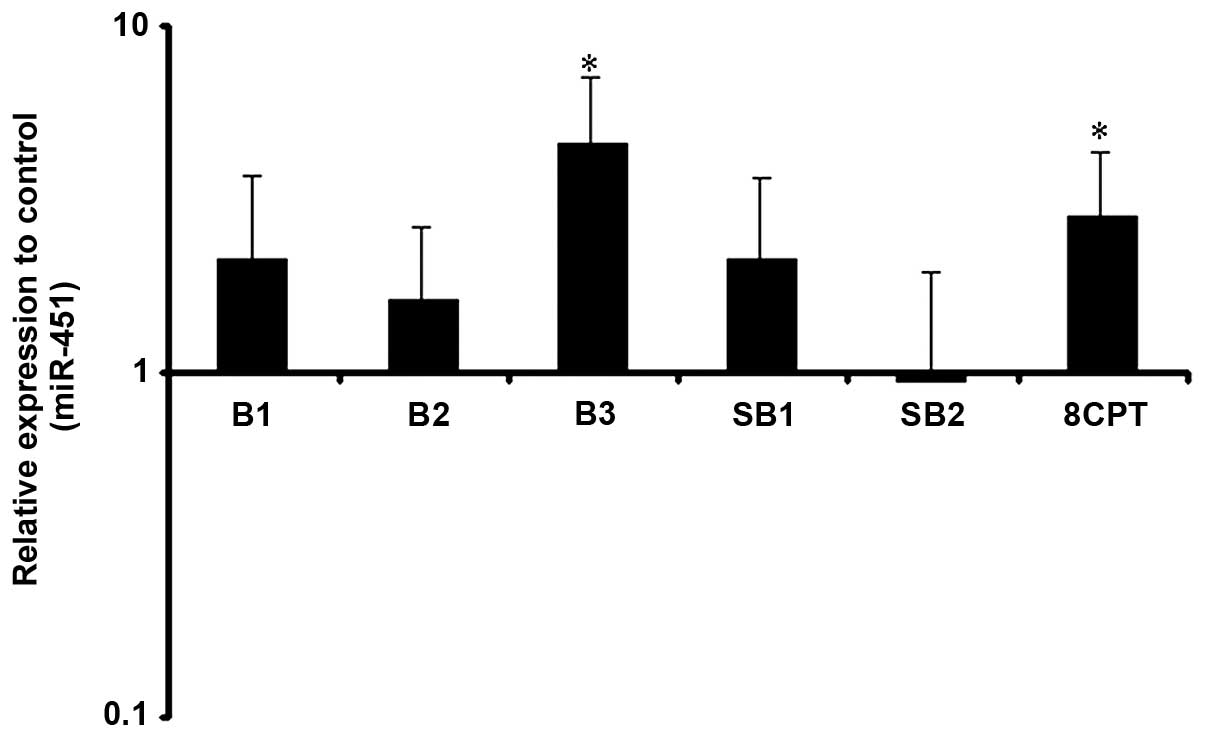
Putative regulation of miR-451 by our drugs was
verified by real time-PCR. Following 24 h treatment, a higher dose
of β2-AR selective stimulation significantly increased miR-451
levels, as 10 and 20 μg/ml of clenbuterol had no significant effect
on miR-451 expression. The minimum dose of clenbuterol for the
modulation of miR-451 was 40 μg/ml. Adenyl cyclase inhibition
prevented these changes suggesting adenyl cyclase activation is
able to increase the miR-451 expression level. To examine whether
Epac activation is also capable of affecting miR-451 expression, we
treated cells with 8CPT. Selective activation of Epac had the same
effect of β2-AR stimulation with higher doses, as miR-451 increased
significantly upon treatment with 8CPT (Fig. 3).
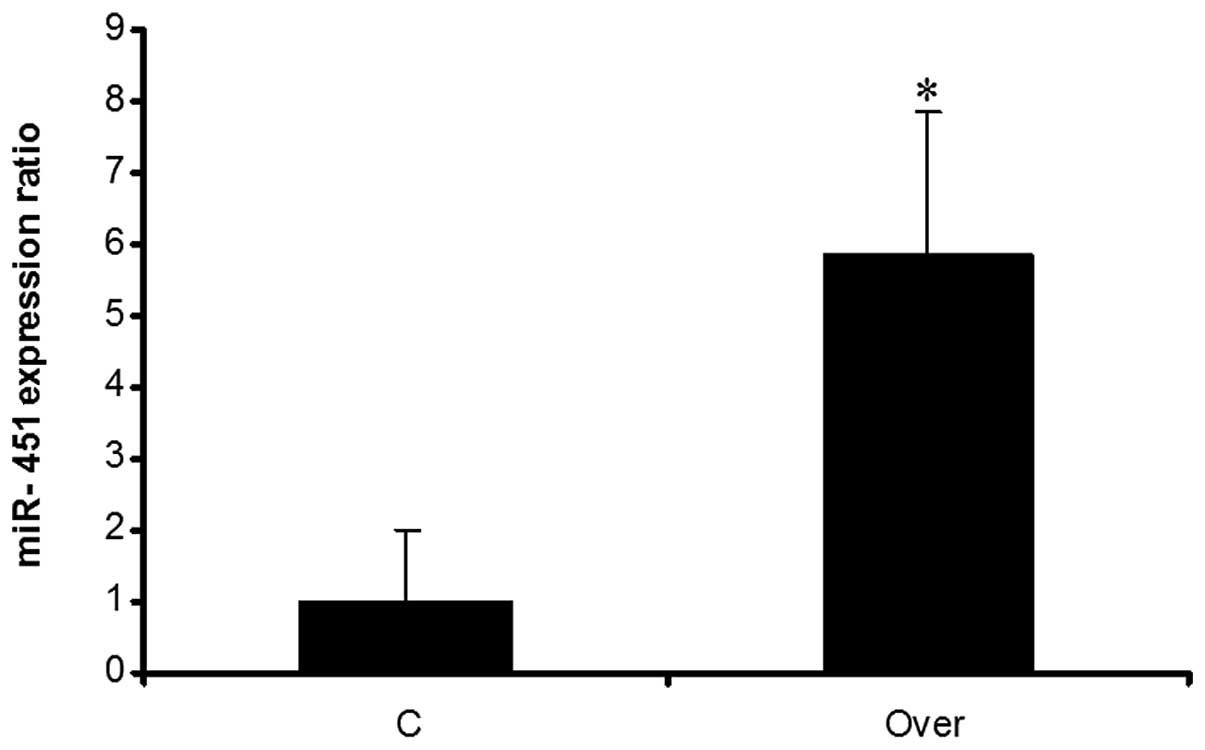
Evaluation of miR-451 overexpression in
1321N1 cells
For overexpression of miR-451, HEK 293T cells were
used for virus packaging and these cells were transfected by the
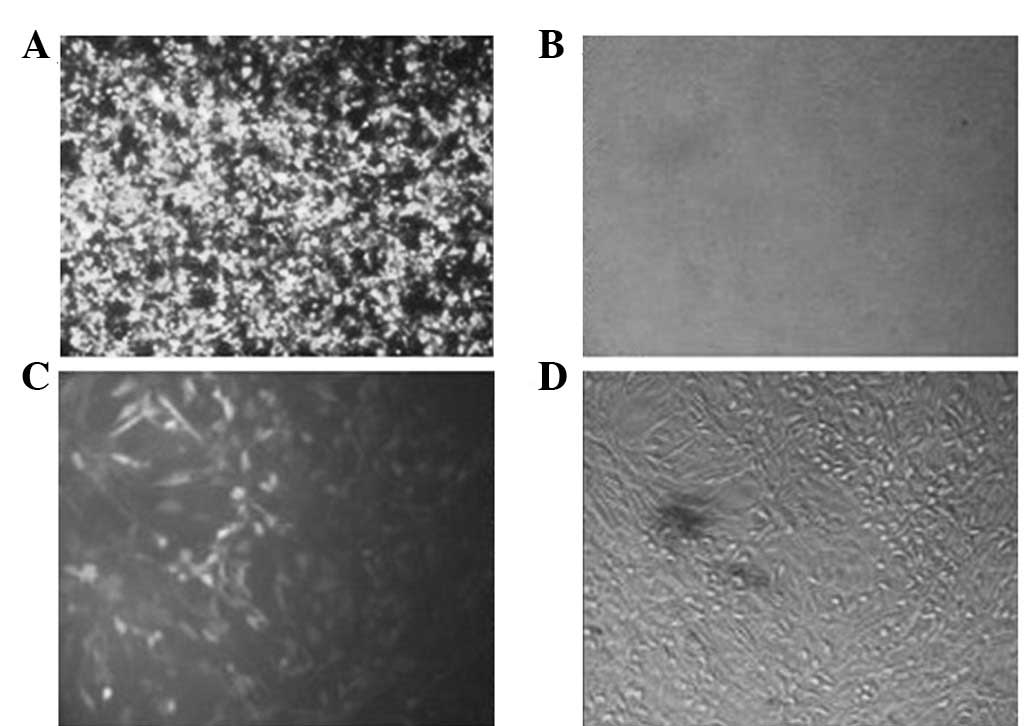
recombinant vector PB_miR-451. The fluorescent image of HEK 293T
cells following transfection indicated that the majority of the
cells were green due to the existence of GFP in the vector
(Fig. 4A and B). Produced viruses
were used for transduction. The fluorescent image of 1321N1 cells
following transduction indicated that the majority of the cells
were green (Fig. 4C and D). Total
RNA was extracted from 1321N1 cells and real-time PCR was employed
for evaluating the expression of miRNA. Results showed that miR-451
was overexpressed in comparison with the negative control (Fig. 5).
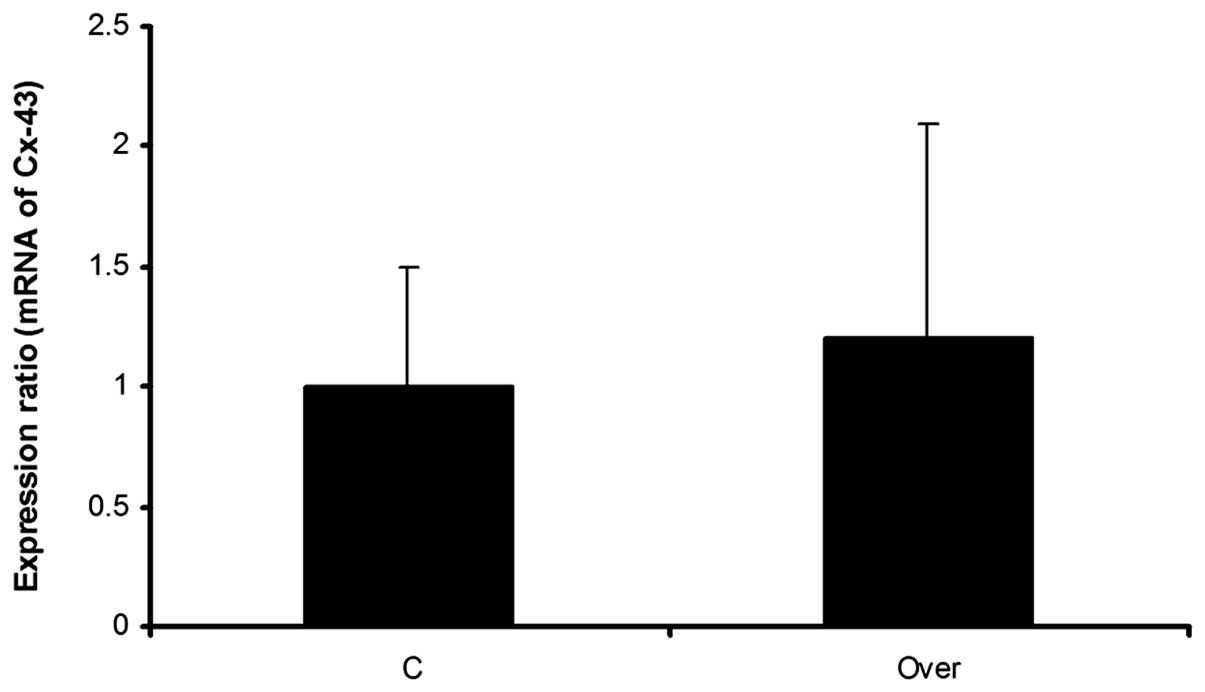
Effect of miR-451 overexpression on Cx43
and mRNA expression
Three days following puromycin treatment, the
expression level of Cx43 mRNA, was evaluated using real-time PCR.
Real-time PCR results demonstrated non significant alterations in
the expression level of the Cx43 gene. Although the expression
ratio of Cx43 mRNA was 1:2 in the overexpressed group compared with
the negative control group, this difference was not significant
(Fig. 6).
Synergic inhibitory effects of cAMP-Epac
and miR-451 on cell proliferation
The cell proliferation assay revealed that growth
was inhibited in transfected and treated cells in comparison with
control cells. Maximum inhibition was observed in transfected cells
that were treated with cAMP-Epac related drugs. These results
demonstrate that miR-451 and the cAMP-Epac pathway may have a
synergic effect on the inhibition of glioma cell growth (Fig. 7).
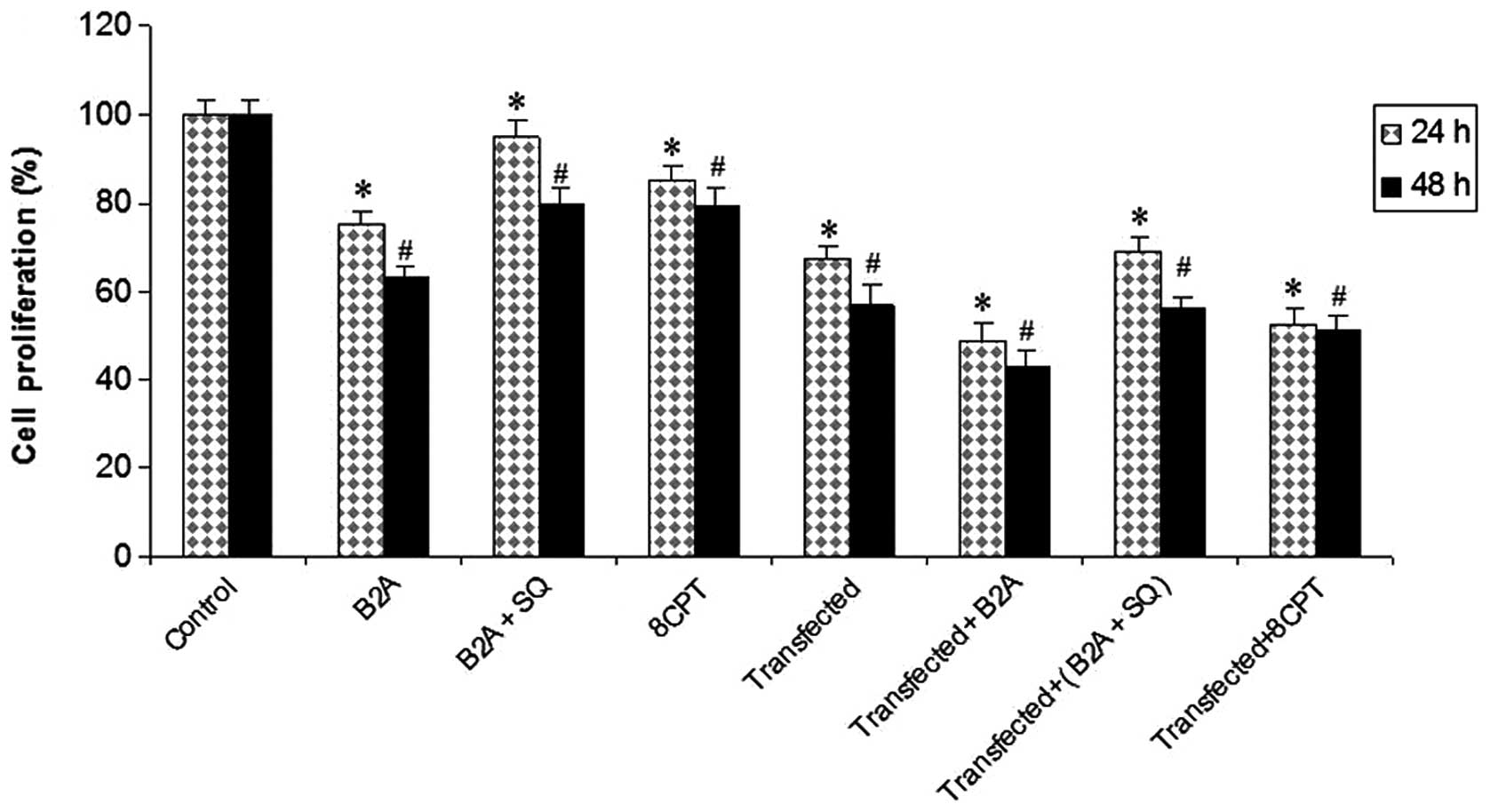 | Figure 7Synergic effects of cAMP-Epac
signaling stimulation and overexpression of miR-451 on cell
proliferation. MTT assay in C, 24 and 48 h treated cells with B2A,
B2A+SQ, 8CPT, miR-451 transfected, transfected cells treated with
B2A, B2A+SQ and 8CPT. Error bars show the S.D., n=3.
*P<0.05, significantly different from control 24 h;
**P<0.05, significantly different from control 48 h.
C, control or untreated; B2A, 10 μg/ml β2 agonist; B2A+SQ, 20 μg/ml
AC inhibitor + 10 μg/ml β2 agonist; 8CPT, 20 μg/ml Epac
activator. |
Discussion
There is widespread agreement that gap junction
channels formed by the Cx43 protein are likely to play important
roles (17). Cx43 has a high
turnover rate with a half-life of ~1–2 h, this condition permits
rapid adjustments of cell-cell communication with transcriptional
regulation and degradation (18).
Modulation of cAMP and its relevant signaling pathway Epac are
reported to affect the level of Cx43 and its phosphorylation in the
heart (19,20) and endothelial cells (21). However, the molecular nature of
β2-AR stimulation and cAMP derivative induced Cx43 expression in
astrocytoma cells remains unclear. We demonstrated that β2-AR
stimulation through activation of the cAMP cascade upregulates Cx43
expression by increasing its mRNA and protein expression in 1321N1
astrocytoma cell lines. Investigation of the signaling pathway
demonstrated that cAMP and its newly described signaling pathway,
Epac2, mediates β2-AR-enhanced Cx43 expression. Previous studies
which treated myocardial cells with a non selective beta adrenergic
agonist stated that β adrenergic receptor and cAMP signaling
pathway activation led to upregulation of Cx43 via activation of
PKA and CREB (12,22). One study in 2009 stated that
corticotropin-releasing hormone upregulates Cx43 expression partly
via the PKA-CREB signaling pathway in cultured astrocytes (23). Since the connexin content of tumors
decreases (2), the upregulation of
connexins may be of therapeutic value (3). We hypothesized that selective
stimulation of β2-AR and the cAMP-Epac signaling pathway results in
Cx43 upregulation in astrocytoma cells. To assess this hypothesis,
we evaluated the protein level of Cx43 in 1321N1 cells following
our pharmacological interventions on cultured cells. Our results
demonstrated that 24 h treatment with a β2-AR selective agonist
increased the protein level of Cx43 and this effect was blocked by
adenyl cyclase inhibitor, SQ. To investigate whether upregulation
of Cx43 was working through the Epac signaling pathway, we
inhibited PKA by H89 in the presence of β2-AR agonist. Selective
β2-AR stimulation with simultaneous inhibition of the PKA pathway
and thereby indirect stimulation of the Epac pathway had the same
effect as selective β2-AR stimulation. Also, selective Epac
stimulation in our cells increased the total protein level of Cx43.
Toll et al (2011) demonstrated that selective β2-AR
simulation in 1321N1 cells led to the inhibition of cell
proliferation (15). By contrast,
other studies indicated that connexins may affect the growth of
normal or tumor cells, however the precise molecular mechanism by
which connexins are capable of inducing this effect remains to be
elucidated. Recent studies have demonstrated that connexins,
including Cx43 exert important and extensive effects on gene
expression, particularly those genes linked to growth regulation
(17). Therefore, increasing Cx43
expression in astrocytoma cells may have a beneficial effect in
controlling their growth and responsiveness to current
treatments.
Effect of the cAMP-Epac signaling pathway on the
expression of microRNAs. The profiling of miRNAs in various human
disorders and their role in the etiology or treatment of them
caught the attention of researchers (24). miR-451 was identified as a tumor
suppressor in glioma and it was demonstrated that overexpression of
miR-451 in glioma cells changed their biological behavior (8). Thus, we hypothesized that stimulation
of the cAMP-Epac signaling pathway may alter miR-451 expression
level in the 1321N1 astrocytoma cell line. Our results revealed a
dose-dependent association between selective β2-AR stimulation and
miR-451 expression level. While we have not observed any changes
with 10 and 20 μg/ml of clenbuterol, increasing clenbuterol doses
to 40 μg/ml, upregulates miR-451 expression level. Selective Epac
signaling pathway stimulation by 8CPT upregulated miR-451 as well.
Our findings are in line with other studies which demonstrated that
the cAMP signaling pathway has a regulatory effect on miRNA
expression (13). Our study
focused mainly on the cAMP-Epac pathway and we demonstrated that
Epac signaling is also involved in the alteration of miRNA
biogenesis. We used astrocytoma cell lines in our study and it
appears that other glioma cell lines must be studied before an
accurate conclusion may be acquired. Although studying the
regulation of miRNA transcription is an emerging field and further
study is needed, the evidence we have presented suggests a new
effect for the cAMP-Epac pathway as a regulator of miR-451 at the
transcriptional level in astrocytoma cells. However, higher doses
of the β2-AR agonist are needed in order to alter miR-451
expression. Administration of the β2-AR agonist and Epac selective
stimulation is expected to upregulate miR-451 in vivo.
Functional analyses of the first identified miRNAs
determined their roles in cell growth and differentiation (25). MiR-451 is significantly
downregulated in glioma compared with normal brain tissue. The
overexpression of miR-451 in glioma cell lines led to decreased
proliferation and invasion (8).
Based on our previous results, we demonstrated that cAMP has a
regulatory effect on Cx43 and miR-451, therefore we speculate that
these two components have an association between them. Next, we
overexpressed miR-451 in the 1321N1 astrocytoma cell line and,
following that, Cx43 expression level was evaluated. Our results
demonstrated that overexpression of miR-451 had no effect on Cx43
mRNA expression and protein level. It seems that the effects of
miR-451 and Cx43 in the progression and/or controlling of tumors
were applied independently. However, further investigation is
required in order to elucidate the combinational effect of these
biological components in patho-physiological processes. For
example, simultaneous overexpression of miR-451 and Cx43 in
astrocytoma cells may uncover their cooperative effect.
This study yielded several new findings. To the best
of our knowledge, we demonstrated, for the first time, that
selective β2-AR and its related cAMP-Epac signaling pathway
stimulation led to the increase of Cx43 expression in astrocytoma
cells. Secondly, the β2-adrenoceptor-cAMP-Epac signaling pathway
positively regulates miR-451 expression. Thirdly, overexpression of
miR-451 had no effect on Cx43 expression. These findings not only
increase our knowledge of certain aspects of the molecular biology
of astrocytoma cells, but also may provide new potential
therapeutic and drug targets. There are certain fundamental
implications of our findings. In astrocytoma and glioma, Cx43 and
miR-451 were downregulated. Upregulation of them may aid the
treatment of astrocytoma and glioma, due to their specific effects
since the effects of Cx43, including decreasing cell proliferation
(4) and the bystander effect
(26) have been proven in previous
studies. MiR-451 overexpression by gene delivery or pharmacological
intervention may be used with chemotherapy due to its effect in the
enhancement of tumor cell sensitivity to chemotherapy agents.
Acknowledgements
This study was supported by a grant (no. 12290) from
the Deputy of Research, Tehran University of Medical Sciences
(Tehran, Iran). In addition, we want to thank Stem Cell Technology
Research Center (Tehran, Iran) for their support.
References
|
1
|
Furnari FB, Stegh A, Chin L, Fenton T,
Bachoo RM, Hahn WC, et al: Malignant astrocytic glioma: genetics,
biology, and paths to treatment. Genes Dev. 21:2683–2710. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pu P, Xia Z, Yu S and Huang Q: Altered
expression of Cx43 in astrocytic tumors. Clin Neurol Neurosurg.
107:49–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sánchez-Alvarez R, Paíno T,
Herrero-González S, Medina JM and Tabernero A: Tolbutamide reduces
glioma cell proliferation by increasing connexin43, which promotes
the up-regulation of p21 and p27 and subsequent changes in
retinoblastoma phosphorylation. Glia. 54:125–134. 2006.
|
|
4
|
Vinken M, Decrock E, De Vuyst E, Ponsaerts
R, D’hondt C, Bultynck G, et al: Connexins: sensors and regulators
of cell cycling. Biochim Biophys Acta. 1815:13–25. 2011.PubMed/NCBI
|
|
5
|
Novakova J, Slaby O, Vyzula R and Michalek
J: MicroRNA involvement in glioblastoma pathogenesis. Biochem
Biophys Res Commun. 386:1–5. 2009. View Article : Google Scholar
|
|
6
|
Liu NK and Xu XM: MicroRNA in central
nervous system trauma and degenerative disorders. Physiol Genomics.
43:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou X, Ren Y, Moore L, Mei M, You Y, Xu
P, et al: Downregulation of miR-21 inhibits EGFR pathway and
suppresses the growth of human glioblastoma cells independent of
PTEN status. Lab Invest. 90:144–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nan Y, Han L, Zhang A, Wang G, Jia Z, Yang
Y, et al: MiRNA-451 plays a role as tumor suppressor in human
glioma cells. Brain Res. 1359:14–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Racagni G, Pezzotta S, Giordana M, Iuliano
E, Mocchetti I, Spanu G, et al: Cyclic nucleotides in experimental
and human brain tumors. J Neurooncol. 1:61–67. 1983.PubMed/NCBI
|
|
10
|
Wakshull E, Hertel C, O’Keefe EJ and
Perkins JP: Cellular redistribution of β-adrenergic receptors in a
human astrocytoma cell line: a comparison with the epidermal growth
factor receptor in murine fibroblasts. J Cell Biochem. 29:127–141.
1985.
|
|
11
|
Schmitt JM and Stork PJ: Cyclic
AMP-mediated inhibition of cell growth requires the small G protein
Rap1. Mol Cell Biol. 21:3671–3683. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xia Y, Gong KZ, Xu M, Zhang YY, Guo JH,
Song Y, et al: Regulation of gap-junction protein connexin 43 by
β-adrenergic receptor stimulation in rat cardiomyocytes. Acta
Pharmacol Sin. 30:928–934. 2009.
|
|
13
|
Keller DM, Clark EA and Goodman RH:
Regulation of microRNA-375 by cAMP in pancreatic β-Cells. Mol
Endocrinol. 26:989–999. 2012.PubMed/NCBI
|
|
14
|
Lu Y, Zhang Y, Shan H, Pan Z, Li X, Li B,
et al: MicroRNA-1 downregulation by propranolol in a rat model of
myocardial infarction: a new mechanism for ischaemic
cardioprotection. Cardiovasc Res. 84:434–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Toll L, Jimenez L, Waleh N, Jozwiak K, Woo
AY, Xiao RP, et al: Beta2-adrenergic receptor agonists inhibit the
proliferation of 1321N1 astrocytoma cells. J Pharmacol Exp Ther.
336:524–532. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bos JL: Epac proteins: multi-purpose cAMP
targets. Trends Biochem Sci. 31:680–686. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dbouk HA, Mroue RM, El-Sabban ME and
Talhouk RS: Connexins: a myriad of functions extending beyond
assembly of gap junction channels. Cell Commun Signal. 7:42009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Laird D, Puranam K and Revel JP: Turnover
and phosphorylation dynamics of connexin43 gap junction protein in
cultured cardiac myocytes. Biochem J. 273:67–72. 1991.PubMed/NCBI
|
|
19
|
Salameh A and Dhein S: Pharmacology of gap
junctions. New pharmacological targets for treatment of arrhythmia,
seizure and cancer? Biochim Biophys Acta. 1719:36–58. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duquesnes N, Derangeon M, Métrich M, Lucas
A, Mateo P, Li L, et al: Epac stimulation induces rapid increases
in connexin 43 phosphorylation and function without preconditioning
effect. Pflugers Arch. 460:731–741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beese M, Wyss K, Haubitz M and Kirsch T:
Effect of cAMP derivates on assembly and maintenance of tight
junctions in human umbilical vein endothelial cells. BMC Cell Biol.
7:11–68. 2010.PubMed/NCBI
|
|
22
|
Somekawa S, Fukuhara S, Nakaoka Y, Fujita
H, Saito Y and Mochizuki N: Enhanced functional gap junction
neoformation by protein kinase A-dependent and Epac-dependent
signals downstream of cAMP in cardiac myocytes. Circ Res.
97:655–662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanstein R, Trotter J, Behl C and Clement
AB: Increased connexin 43 expression as a potential mediator of the
neuroprotective activity of the corticotropin-releasing hormone.
Mol Endocrinol. 23:1479–1493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Rooij E: The art of microRNA research.
Circ Res. 108:219–234. 2011.
|
|
25
|
Wightman B, Ha I and Ruvkun G:
Posttranscriptional regulation of the heterochronic gene lin-14 by
lin-4 mediates temporal pattern formation in C. elegans. Cell.
75:855–862. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nicholas TW, Read SB, Burrows FJ and Kruse
CA: Suicide gene therapy with Herpes simplex virus thymidine kinase
and ganciclovir is enhanced with connexins to improve gap junctions
and bystander effects. Histol Histopathol. 18:495–507.
2003.PubMed/NCBI
|