Introduction
Cancer is a heterogeneous group of diseases
resulting from accumulated genetic changes. Data from the World
Health Organization demonstrate that these diseases are the main
cause of death in economically developed countries and the second
leading cause of death in developing countries (1). Although chemotherapy is currently the
most common treatment regimen for the majority of cancers, several
chemotherapies exhibit a low therapeutic index, resulting in
serious side effects (2). For this
reason, studies in the field of cancer therapy have led to the
development of novel drugs that aim to maximize the effectiveness
against cancer, to avoid the side effects and to prevent multidrug
resistance (3).
Among numerous natural and synthetic compounds that
were investigated for their anticancer potential, compounds
containing quinone moieties, including doxorubicin and other
anthracyclines, are widely used as efficient anticancer drugs
(4). The drugs’ main mechanisms of
action are thought to involve: i) A direct interaction with DNA;
ii) topoisomerase inhibition; and iii) generation of reactive
oxygen species (5,6). However, their use is limited by side
effects, such as cardiotoxicity (7). Quinones and particularly
1,4-naphthoquinones are abundant in nature and display diverse
pharmacological properties, including antibacterial, antifungal,
antiviral, anti-inflammatory, antipyretic and anticancer activities
(8,9). The presence of electron-donating or
electron-withdrawing substituents linked to the vinyl double bond
of 1,4-naphthoquinones modulates the generation of quinone radical
anion species. The redox properties of the radical anion species
enable these compounds to undergo a catalytic cycle and in the
presence of molecular oxygen they generate reactive oxygen species
(ROS), including hydrogen peroxide and superoxide anions, which
damage the DNA and certain essential proteins. These agents
represent a promising approach for targeting cancer cells (10–12).
Indeed, as a result of metabolic changes and oncogene activation,
cancer cells generally exhibit high levels of ROS, which can
stimulate cell proliferation and promote genetic instability
(13). Additionally, it has been
revealed that cancer cells exhibit a low antioxidant defense
activity compared with healthy cells (14). The biochemical differences between
normal and cancer cells represent a specific vulnerability that can
be selectively targeted in cancer therapy.
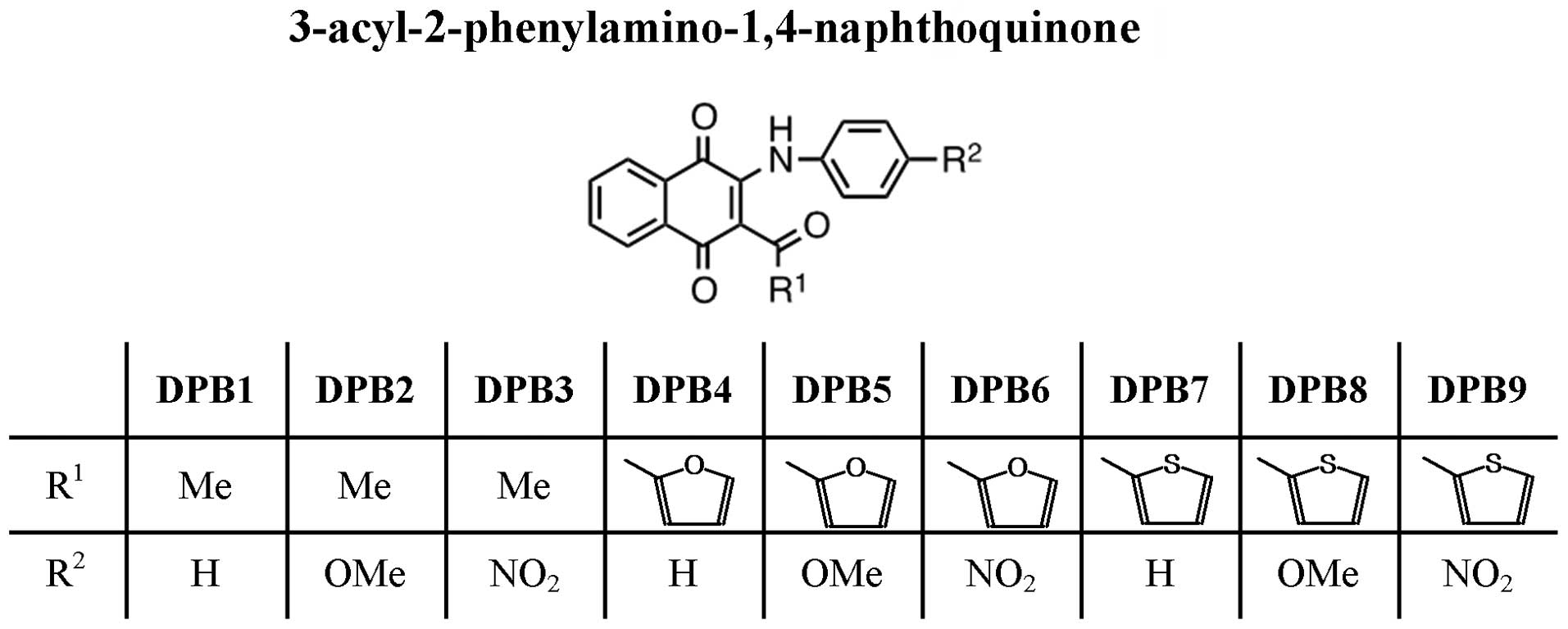
The aim of the present study was to evaluate the
activity of a variety of 3-acyl-2-phenylamino-1,4-naphthoquinones
(Fig. 1) in order to obtain
insights into the influence of the combined electronic
donor/acceptor effects (known as push-pull) of the phenylamine/acyl
substituents, located at the vinyl double bond of the
1,4-naphthoquinone core, on the survival (including the cell death
mechanism) of MCF7 breast cancer cells and Ehrlich ascites tumor
growth in a mouse model. Additional assays were performed to assess
the physical interaction of these compounds with plasmid DNA and
their DNA intercalation properties.
Materials and methods
Chemicals
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS) and antibiotics were purchased from Cultilab
(Campinas, SP, Brazil). Dimethylsulfoxide (DMSO),
dichlorofluorescein diacetate (DCFH-DA), agarose, distamycin,
catalase, calf thymus DNA (CT-DNA), N-acetylcysteine and
bovine serum albumin were purchased from Sigma-Aldrich
(Sigma-Aldrich, St. Louis, MO, USA). The annexin V-fluorescein
isothiocyanate (FITC) apoptosis detection kit was purchased from BD
Pharmingen (San Diego, CA, USA). The Perfectprep Plasmid Mini
extraction kit was purchased from Eppendorf (Hamburg, Germany). All
the other chemicals ACS grade reagents.
General procedure for the preparation of
3-acyl-2-phenylamino-1,4-naphthoquinones
The preparation of the core
3-acyl-2-phenylamino-1,4-naphthoquinones was initiated with the
synthesis of 2-acylnaphthohydroquinones via solar-induced
Photo-Friedel-Crafts acylation of 1,4-naphthoquinone with aldehydes
according to a previously reported procedure (12). The resulting compounds were then
oxidized with silver (I) oxide in dichloromethane, and the
corresponding 2-acyl-1,4-naphthoquinones were reacted in
situ with selected phenylamines in a one-step procedure to
produce the corresponding 3-acyl-2-phenylamino-1,4-naphthoquinones
DPB1–DPB9 (Fig. 1) (15). The quinone structures were
confirmed by comparing their infrared and proton/carbon nuclear
magnetic resonance spectral properties to those reported in the
literature (15).
In vitro assays
Cytotoxicity assays
The MCF7 human breast carcinoma cells were obtained
from the Rio de Janeiro Cell Bank (Rio de Janeiro, Brazil). The
cells were cultured at 37°C in the presence of 5% CO2
and 95% humidity. DMEM supplemented with 10% FBS, penicillin (100
U/ml) and streptomycin (100 μg/ml) was used as the culture medium.
The cytotoxic effect of 3-acyl-2-phenylamino-1,4-naphthoquinones on
breast cancer cells was tested using the MTT assay as described
previously (16). Briefly,
104 cells/well were plated in 96-well plates. After
reaching confluence, the cells were exposed to the respective
3-acyl-2-phenylamino-1,4-naphtoquinones (0–80 μM) for up to 24 h.
The cells were then washed twice with phosphate-buffered saline
(PBS) and incubated for 2 h with MTT (0.5 mg/ml). The formazan
crystals were solubilized by adding DMSO (100 μl/well) and the
absorbances of the colored solutions were read at 550 nm.
Plasmid DNA cleavage
The plasmid DNA cleavage assay was performed as
previously described by Günther et al (17) with minor adaptations. For this
assay, the supercoiled PUC 9.1 plasmid DNA was isolated from the
DH5SαFIQ Escherichia coli strain using a Maxi Prep kit
(Qiagen, Hilden, Germany) and according to the manufacturer’s
instructions. The plasmid DNA (600 ng) was treated with the test
compounds (0.05 μM) in hydroxyethyl-piperazineethanesulfonic acid
(HEPES) buffer at pH 7.4 for 16 h in the dark at 37°C. The samples
were electrophoresed for 40 min at 90 V on a 0.8% agarose gel in
Tris-borate-ethylenediaminetetraacetic acid buffer. The gel was
stained with 0.5 μg/ml ethidium bromide and then visualized and
photographed under ultraviolet light. The images were analyzed
using ImageJ 1.46 bioinformatics software (National Institute of
Health, Bethesda, MD, USA) in order to compare the band intensity
and the pixel number presented by the supercoiled intact form (FI),
open circular (single strand break - FII) and linear plasmid form
(double strand break - FIII). Furthermore, the DNA intensity of the
FI form was corrected by a factor of 1.47 due to its reduced
ability to be stained by ethidium bromide (18).
Intercalation assay
In order to determine whether the
3-acyl-2-phenylamino-1,4-naphthoquinones interact with CT-DNA by
intercalation, the DNA-intercalating agent ethidium bromide was
used (18). The CT-DNA was diluted
with 50 mM phosphate buffer containing 0.1 M NaCl (pH 7.4). NaCl
was included to avoid second binding of ethidium bromide to the
DNA. The concentration of CT-DNA used was 20 μM. The emission
spectrum of ethidium bromide in the presence and absence of DNA was
recorded at concentrations in the range of 10–110 μM in order to
identify the saturation point of the DNA sites corresponding to the
binding of this compound. Once the saturating concentration of
ethidium bromide was established (30 μM), the fluorescence
absorbance was measured using increasing concentrations of
3-acyl-2-phenylamino-1,4 naphthoquinones (DPB1, DPB2, DPB4 and
DPB5). The absorbance was recorded at room temperature 10 min after
the addition of the compounds. A TECAN Infinite M200 fluorescence
reader (Tecan Austria GmbH, Grödje, Austria) was used for the
measurements using a protocol adapted from Silveira et al
(19). The excitation wavelength
used was 492 nm and the emitted fluorescence was measured at ~620
nm.
Intracellular ROS determination
Intracellular ROS were evaluated according to the
procedure described by Glorieux et al (20). The cells (15,000) were loaded with
10 μM DCFH-DA in Hank’s balanced salt solution (HBSS) at 37°C and
incubated for 30 min. Excess DCFH-DA was removed by washing with
fresh Hank’s buffer solution HBSS. The cells were then incubated
for 2 h with the test compounds, washed twice more with HBSS and
then 100 μl of HBSS/well was added. The fluorescence intensity of
dichlorofluorescein was measured at an excitation wavelength of 485
nm and an emission wavelength of 520 nm. Changes in the ROS levels
were determined by calculating ΔF = (Ft - Fc) / Fc, where Ft
represents the fluorescence read at each time-point and Fc the
control fluorescence.
Assessment of the mechanism of
apoptosis
Apoptosis was quantified using an annexin V-FITC
apoptosis detection kit from BD Pharmingen (San Diego, CA, USA),
which detects phosphatidylserine that is externalized during the
early phases of apoptosis. Briefly, MCF7 cells were plated at a
density of 106 cells/well in 12-well plates. Subsequent
to an overnight incubation, the cells were treated with
3-acyl-2-phenylamino-1,4-naphthoquinones (15 μM) for 24 h. The
negative control was treated with 0.25% DMSO in fresh culture
medium. The cells were then washed with PBS (three times) at 37°C,
trypsinized, washed again with PBS (three times) at 37°C and then
resuspended in 200 μl binding buffer [0.1 M HEPES/NaOH (pH 7.4),
1.4 M NaCl and 25 mM CaCl2] diluted 1:10 in distilled
water. Next, 5 μl annexin V-FITC was added and the samples were
incubated for 15 min in the dark. Finally, 5 μl propidium iodide
was added and flow cytometric analysis was performed. The
fluorescence intensities of annexin V-FITC and propidium iodide
were measured using a 488 nm excitation filter, and the emission
was detected in the range of 515–545 nm and 564–606 nm,
respectively. The data were analyzed using the WinMDI software (BD
Pharmingen).
In vivo antitumor activity
Animals and treatments
Male isogenic Balb/c mice weighing 20±2 g were
housed under controlled conditions (12-h light/dark cycle, 21±2°C,
60% humidity) and had free access to standard food and water. All
the animals were allowed to acclimatize for at least five days
prior to the first treatment. The animals fasted for 12 h prior to
the treatment with water provided ad libitum. All the animal
procedures were conducted in accordance with legal requirements
appropriate to the species (NIH publication no. 80–23, revised in
1978) and with approval from the local ethics committee
(CEUA-PP00744) of the University of Santa Catarina (Florianópolis,
Brazil).
The Ehrlich ascites carcinoma (5×106
cells) were inoculated in the peritoneum of mice under aseptic
conditions; this time-point was regarded as day zero. The animals
were divided into six groups (n=6): A negative control treated with
0.9% NaCl (50 μl); a positive control treated with doxorubicin (1.2
mg/kg); plus four test-groups treated with DPB1, DPB2, DPB4 and
DPB5 (0.16 mg/kg), respectively. The intraperitoneal treatments
began 24 h after tumor inoculation and they were repeated every 24
h for nine days. On day ten, all the animals were anesthetized and
sacrificed by cervical dislocation.
Inhibition of tumor growth
The inhibition of tumor growth was determined by
measuring the change in the abdominal circumference of mice between
day zero and day ten based on previously reported methods (21). The following formula was used:
Inhibition of tumor growth (%) = [(variation in waist circumference
of the treated group × 100) / variation in waist circumference of
the control group] - 100.
Data analysis
The assays were performed in triplicate and in
vitro assays were repeated at least three times. Unless
otherwise specified, values are expressed as the mean ± standard
deviation. Data were analyzed for statistical differences using the
one-way analysis of variance test followed by the Bonferroni test.
The EC50 values were determined using the GraphPad Prism
software (San Diego, CA, USA). Differences between values with
P<0.05 were considered statistically significant.
Results and Discussion
Cytotoxic effect of
3-acyl-2-phenylamino-1,4-naphthoquinones on MCF7 cells
The EC50-values obtained following
incubation of MCF7 cells for 24 h with
3-acyl-2-phenylamino-1,4-naphthoquinones at different
concentrations (5–80 μM) are shown in Table I. The quinones exerted significant
cytotoxic effects (EC50<60 μM). DPB4 was the most
cytotoxic (EC50 15.0 μM) and DPB6 (EC50 56.0
μM) the least cytotoxic compound. The differences in the
EC50-values indicated that the nature of the substituent
group influences the cytotoxicity of these quinones. A previous
study showed that changes in the quinoid core alter the
cytotoxicity of quinones in a panel of cancer cells (MCF7, DU145,
T24, J82, AGS, and BEM-SK-1) and impair the proliferative capacity
of T24 cells (12). Additionally,
the electronic properties of the substituent bound to the quinone
core may interfere with the redox cycling process and thus affect
the production of ROS (11).
 | Table IEC50 values obtained by the
MTT reduction assay. MCF7 cells were treated for 24 h with
3-acyl-2-phenylamino-1,4-naphthoquinones (5–80 μM). |
Table I
EC50 values obtained by the
MTT reduction assay. MCF7 cells were treated for 24 h with
3-acyl-2-phenylamino-1,4-naphthoquinones (5–80 μM).
| Compounds | EC50,
μM |
|---|
| DPB1 | 20.3±1.8 |
| DPB2 | 22.6±8.0 |
| DPB3 | 48.6±6.6 |
| DPB4 | 15.0±8.5 |
| DPB5 | 23.9±8.1 |
| DPB6 | 56.0±8.7 |
| DPB7 | 34.8±8.2 |
| DPB8 | 28.6±7.5 |
| DPB9 | 30.1±5.5 |
Cleavage of plasmid DNA by
3-acyl-2-phenylamino-1,4- naphthoquinone
It was demonstrated that the
3-acyl-2-phenylamino-1,4-naphthoquinones at 0.05 μM were able to
cause mild plasmid DNA fragmentation (Fig. 2). In parallel with the cytotoxicity
results, DPB1 and DPB4 were the most genotoxic compounds. They
caused single-strand breaks in the DNA as indicated by the
appearance of bands of the FII DNA form at ratios of ~22 and 30%,
respectively. EcoRI was used as the positive control,
causing 71% of DNA single-strand cleavage resulting in the open
circular form (FII) and 5% double-strand cleavage resulting in the
linear FIII form.
 | Figure 2DNA plasmid damage induced by
3-acyl-2-phenylamino-1,4-naphthoquinones (DPB1–DPB9). Experiments
were performed in triplicate, and the results are expressed as the
mean ± standard deviation of the percentage of the plasmid DNA form
observed. Left-hand lane, NC of DNA treated with vehicle alone
(dimethylsulfoxide); right-hand lane, EcoRI, positive
control DNA treated with vehicle and the restriction enzyme
EcoRI. Significant differences between the treated and the
NC groups are denoted by * and ***,
indicating P<0.1 and P<0.001, respectively. NC, negative
control; FI, supercoiled intact form; FII, open circular form
resulting from a single-strand break; FIII, linear plasmid form
resulting from a double-strand break. |
DNA strand breaks compromise the integrity of the
genetic information and therefore decrease the cell viability. It
is likely that 3-acyl-2-phenylamino-1,4-naphthoquinones exert
cytotoxic effects resulting in apoptosis of tumor cells since they
can induce DNA fragmentation.
DNA intercalation by certain
3-acyl-2-phenylamino-1,4-naphthoquinones
The fluorescence spectra of CT-DNA/ethidium bromide
associated with 3-acyl-2-phenylamino-1,4 naphthoquinones (1–40 μM)
were evaluated to check whether these compounds have an
intercalating activity. Substances able to intercalate into DNA
cause a reduction in the amount of ethidium bromide bound to DNA
with respective decreases in their fluorescence intensity (18). It was demonstrated that
3-acyl-2-phenylamino-1,4 napthoquinones decreased the fluorescence
intensity in a concentration-dependent manner (Fig. 3). Thus, they are capable of
intercalating into DNA. DPB4 caused the strongest reduction in
ethidium bromide fluorescence; thus it is expected to have the
strongest intercalating activity as compared to DPB1, DPB2 and DPB5
(Fig. 3). It is known that
doxorubicin, besides acting as a nuclease by an oxidative
mechanism, also acts through a non-specific DNA-intercalating
action (22,23), similar to that observed for
3-acyl-2-phenylamino-1,4 naphthoquinones. Intercalating agents may
be useful in treating cancer since they exhibit antiproliferative
effects. Once these agents are present between the DNA strands,
they can block DNA replication and cell proliferation. Several
cellular mechanisms may be triggered under such conditions, leading
either to cell death or senescence. p53 is activated to induce DNA
repair and if this is not effective, apoptosis occurs.
Intracellular ROS levels
MCF7 cells exposed to
3-acyl-2-phenylamino-1,4-naphthoquinones revealed increased
intracellular levels of ROS compared with the negative control
(Fig. 4). In cells exposed to DPB4
and DPB1, the content of intracellular ROS was found increased up
to 10 and 8-fold, respectively (Fig.
4).
A study by Verrax et al (24) indicated that the potential of the
majority of quinone derivatives to induce the generation of
cellular ROS should depend on their redox potential.
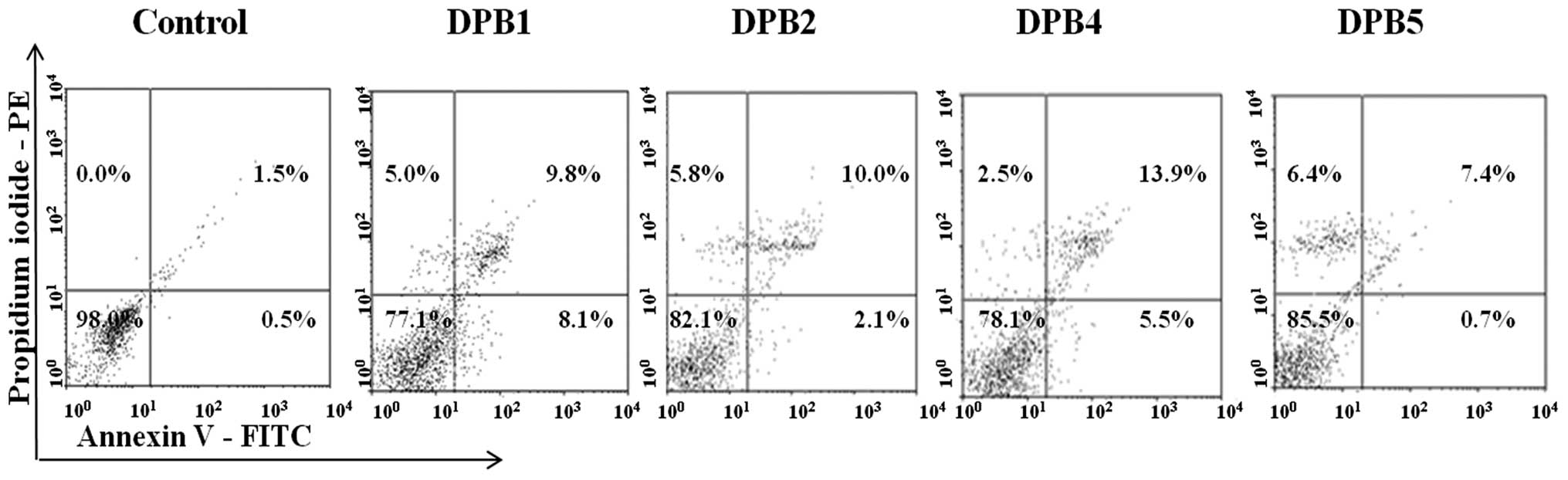
Mechanisms of cell death
The treatment of MCF-7 cells with
3-acyl-2-phenylamino-1,4-naphthoquinones increased the frequency of
both apoptotic and necrotic cells in comparison to the negative
control (Fig. 5 and Table II). Apoptosis was identified to be
the main mechanism underlying induced cell death. Particularly,
cells were found mostly in the stage of late apoptosis.
 | Table IITypes of MCF7 cell death induced by
3-acyl-2-phenylamino-1,4-naphthoquinones (15 μM) administered for
24 h. |
Table II
Types of MCF7 cell death induced by
3-acyl-2-phenylamino-1,4-naphthoquinones (15 μM) administered for
24 h.
| | | Apoptotic cells
(%) |
|---|
| | |
|
|---|
| Treatments | Viable cells,
% | Necrotic cells,
% | Initial
apoptosis | Late apoptosis | Total apoptotic
cells |
|---|
| Negative
control | 98.1 | 0.0 | 0.5 | 1.5 | 1.9 |
| DPB1 | 77.1 | 5.0 | 8.1 | 9.8 | 18.0 |
| DPB2 | 82.1 | 5.8 | 2.1 | 10.0 | 12.1 |
| DPB4 | 78.1 | 2.5 | 5.5 | 13.9 | 19.4 |
| DPB5 | 85.5 | 6.4 | 0.7 | 7.4 | 8.1 |
Although apoptosis induced by
3-acyl-2-phenylamino-1,4-naphthoquinones could have been triggered
by the intercalation into DNA, it is also well-known that increased
levels of ROS can culminate in both apoptosis and necrosis
(25,26). For instance, a previous study
demonstrated that juglone (5-hydroxy-1,4-naphthoquinone) induced
apoptotic death in HL60 leukemia cells, and this effect was avoided
in the presence of the antioxidants N-acetylcysteine and
catalase (27). A study indicated
that the oxidative stress induced by certain quinones can lead to
cell death either through necrosis or apoptosis, depending on the
concentration and exposure time to the oxidizing agent, as well as
the cell type (28). DPB4 exerts
strong antitumor effects due to its ability to kill cancer cells
via intercalating into DNA and by increasing the generation of ROS
that cleave DNA. All these events culminate in increased levels of
apoptosis and necrosis (Fig. 5 and
Table II) and they are involved
in the mechanism underlying the cytotoxicity of the series of
3-acyl-2-phenylamino-1,4-naphthoquinones investigated in the
present study.
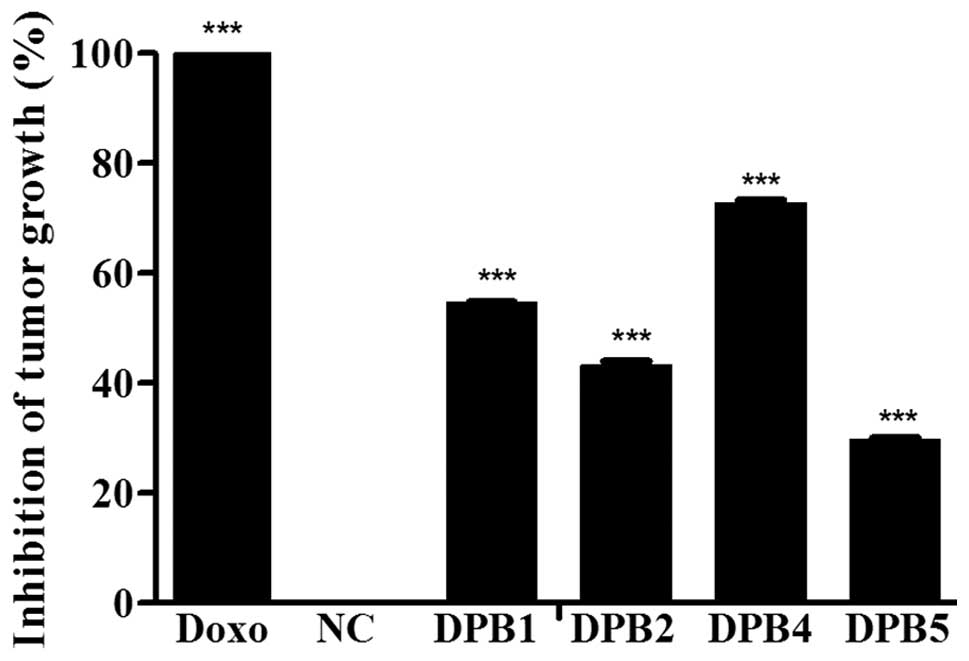
Antitumor effect in vivo
The effects of the
3-acyl-2-phenylamino-1,4-naphthoquinones against the Ehrlich
ascites carcinoma in mice are shown in Fig. 6. All the
3-acyl-2-phenylamino-1,4-naphthoquinones that were assayed
presented a certain effect as compared with the negative control.
DPB4 was initially indicated by the in vitro assays to be
the most potent compound, and was also confirmed to be the most
promising candidate in vivo, as it was capable of reducing
tumor growth by up to 70%. Once the results are confirmed, it will
be possible to predict whether DNA intercalation and the increased
ROS generation are not constrained in vivo by antioxidant
defenses, by the immune system or by drug biotransformation. Due to
its potency in the present study, DPB4 was selected as a potential
prototype for further studies in the development of a novel
antitumor drug.
Acknowledgements
This study was supported by grants from
CNPq/MCT-Brazil. R.C. Pedrosa (Proc. 302404/2011-2) is a recipient
of a research grant from the National Research Council of Brazil.
M.S. Farias, K.B. Felipe, F.O. da Silva and N.F. Bücker are fellows
from CAPES, Brazil. The authors would also like to thank the
National Fund of Science and Technology (FONDECYT grant no.
1100376) in Chile for financial support that was provided for the
synthetic experiments performed in this investigation.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J and
Forman D: Global cancer statistics. CA Cancer J Clin. 61:69–90.
2011. View Article : Google Scholar
|
|
2
|
Adams VR: Adverse events associated with
chemotherapy for common cancers. Pharmacotherapy. 20:S96–S103.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwartz J: Current combination
chemotherapy regimens for metastatic breast cancer. Am J Health
Syst Pharm. 66(Suppl 6): S3–S8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nadas J and Sun D: Anthracyclines as
effective anticancer drugs. Expert Opin Drug Discov. 1:549–568.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carvajal D, Kennedy S, Boustani A, et al:
Induction of cell death by a novel naphthoquinone containing a
modified anthracycline ring system. Chem Biol Drug Des. 78:764–777.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gholivand MB, Kashanian S and Peyman H:
DNA-binding, DNA cleavage and cytotoxicity studies of two
anthraquinone derivatives. Spectrochim Acta A Mol Biomol Spectrosc.
87:232–240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zucchi R and Danesi R: Cardiac toxicity of
antineoplastic anthracyclines. Curr Med Chem Anticancer Agents.
3:151–71. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Babula P, Adam V, Havel L and Kizek R:
Naphthoquinones and their pharmacological properties. Ceska Slov
Farm. 56:114–120. 2007.(Article in Czech).
|
|
9
|
Kviecinski MR, Pedrosa RC, Felipe KB, et
al: Inhibition of cell proliferation and migration by oxidative
stress from ascorbate-driven juglone redox cycling in human
bladder-derived T24 cells. Biochem Biophys Res Commun. 421:268–273.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith MT: Quinones as mutagens,
carcinogens, and anticancer agents: introduction and overview. J
Toxicol Environ Health. 16:665–672. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benites J, Valderrama JA, Taper H and Buc
Calderon P: Part 2: influence of 2-euryfuryl-1,4-naphthoquinone and
its peri-hydroxy derivatives on both cell death and metabolism of
TLT cells, a murine hepatoma cell line. Modulation of cytotoxicity
by vitamin C. Chem Pharm Bull. 57:615–619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Benites J, Ríos D, Díaz P and Valderrama
J: The solar-chemical-Friedel-Crafts heteroacylation of
1,4-quinones. Tetrahedron Lett. 52:609–611. 2011. View Article : Google Scholar
|
|
13
|
Trachootham D, Zhou Y, Zhang H, et al:
Selective killing of oncogenically transformed cells through a
ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer
Cell. 10:241–252. 2006. View Article : Google Scholar
|
|
14
|
Verrax J, Beck R, Dejeans N, et al:
Redox-active quinones and ascorbate: an innovative cancer therapy
that exploits the vulnerability of cancer cells to oxidative
stress. Anticancer Agents Med Chem. 11:213–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ríos D, Benites J, Valderrama JA, et al:
Biological evaluation of 3-acyl-2-arylamino-1,4-naphthoquinones as
inhibitors of Hsp90 chaperoning function. Curr Top Med Chem.
12:2094–2102. 2012.PubMed/NCBI
|
|
16
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 16:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Günther TMF, Kviecinski MR, Baron CC, et
al: Sodium orthovanadate associated with pharmacological doses of
ascorbate causes an increased generation of ROS in tumor cells that
inhibits proliferation and triggers apoptosis. Biochem Biophys Res
Commun. 430:883–888. 2013.
|
|
18
|
Sreedhara A and Cowan JA: Catalytic
hydrolysis of DNA by metal ions and complexes. J Biol Inorg Chem.
4:337–347. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
da Silveira VC, Benezra H, Luz JS, et al:
Binding of oxindole-Schiff base copper (II) complexes to DNA and
its modulation by the ligand. J Inorg Biochem. 105:1692–1703.
2011.PubMed/NCBI
|
|
20
|
Glorieux C, Dejeans N, Sid B, et al:
Catalase overexpression in mammary cancer cells leads to a less
aggressive phenotype and an altered response to chemotherapy.
Biochem Pharmacol. 15:1384–1390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kviecinski MR, Benelli P, Felipe KB, et
al: SFE from Bidens pilosa Linné to obtain extracts rich in
cytotoxic polyacetilenes with antitumor activity. J Supercrit
Fluid. 56:243–248. 2010.
|
|
22
|
Neidle S: The molecular basis for the
action of some DNA-binding drugs. Prog Med Chem. 16:151–221. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kennedy S, DiCesare JC and Sheaff RJ:
Topoisomerase I/II inhibition by a novel naphthoquinone containing
a modified anthracycline ring system. Biochem Biophys Res Commun.
408:94–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Verrax J, Delvaux M, Beghein N, et al:
Enhancement of quinone redox cycling by ascorbate induces a
caspase-3 independent cell death in human leukaemia cells. An in
vitro comparative study. Free Radic Res. 39:649–657. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deeb D, Gao X, Jiang H, et al: Oleanane
triterpenoid CDDO-Me inhibits growth and induces apoptosis in
prostate cancer cells through a ROS-dependent mechanism. Biochem
Pharmacol. 79:350–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hyun MS, Hur JM, Mun YJ, Kim D and Woo WH:
BBR induces apoptosis in HepG2 cell through an
Akt-ASK1-ROS-p38MAPKs-linked cascade. J Cell Biochem. 109:329–338.
2010.PubMed/NCBI
|
|
27
|
Xu HL, Yu JF, Qu SC, Jiang YF and Sui da
Y: Juglone, from Juglans mandshruica Maxim, inhibits growth and
induces apoptosis in human leukemia cell HL-60 through a reactive
oxygen species-dependent mechanism. Food Chem Toxicol. 50:590–596.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Verrax J, Cadrobbi J, Marques C, et al:
Ascorbate potentiates the cytotoxity of menadione leading to an
oxidative stress that kills cancer cells by a non-apoptotic
caspase-3 independent form of cell death. Apoptosis. 9:223–333.
2004. View Article : Google Scholar
|