Spandidos Publications style
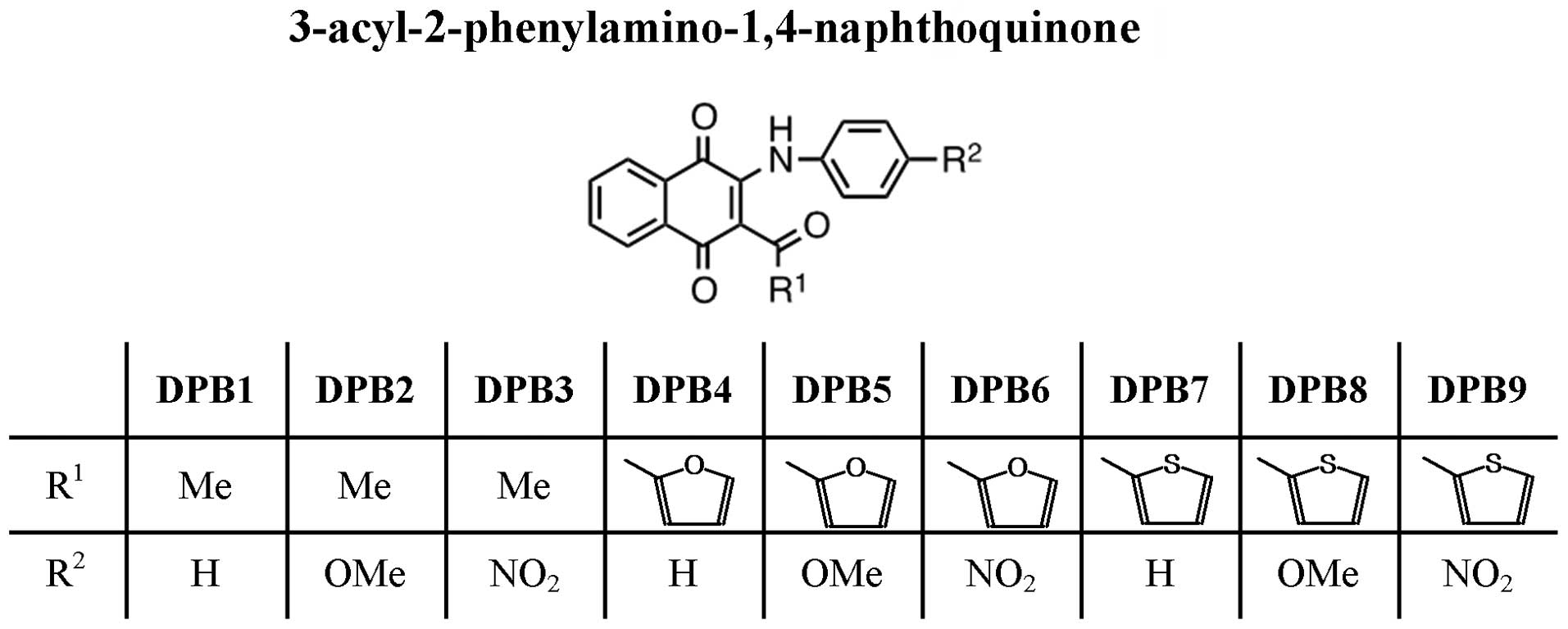
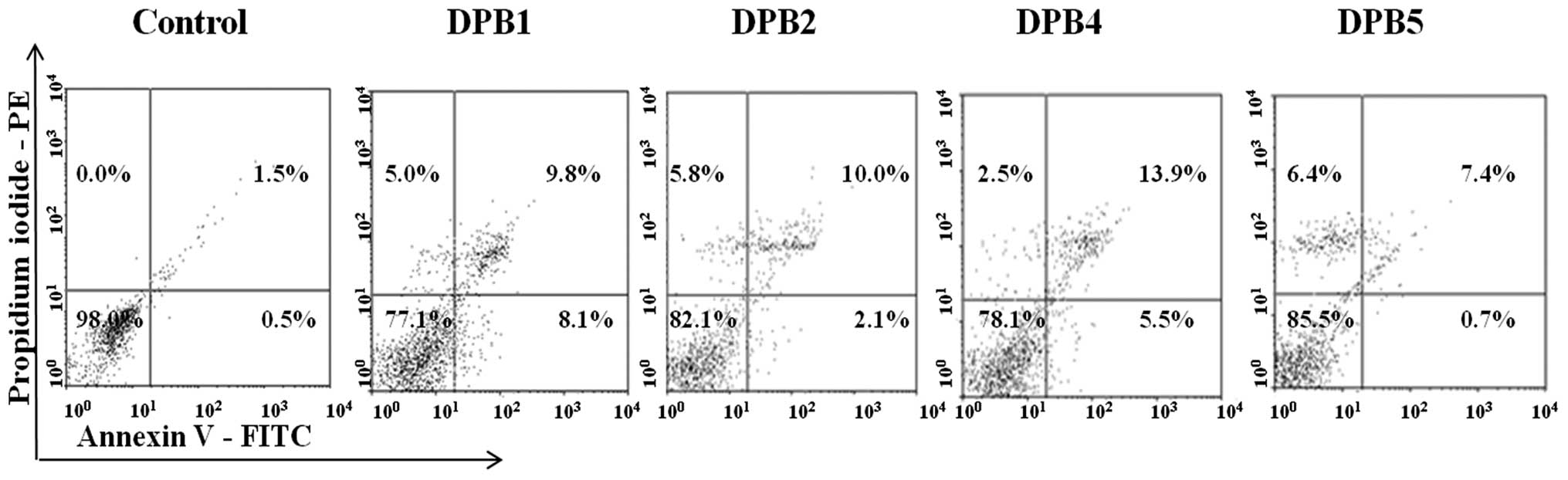
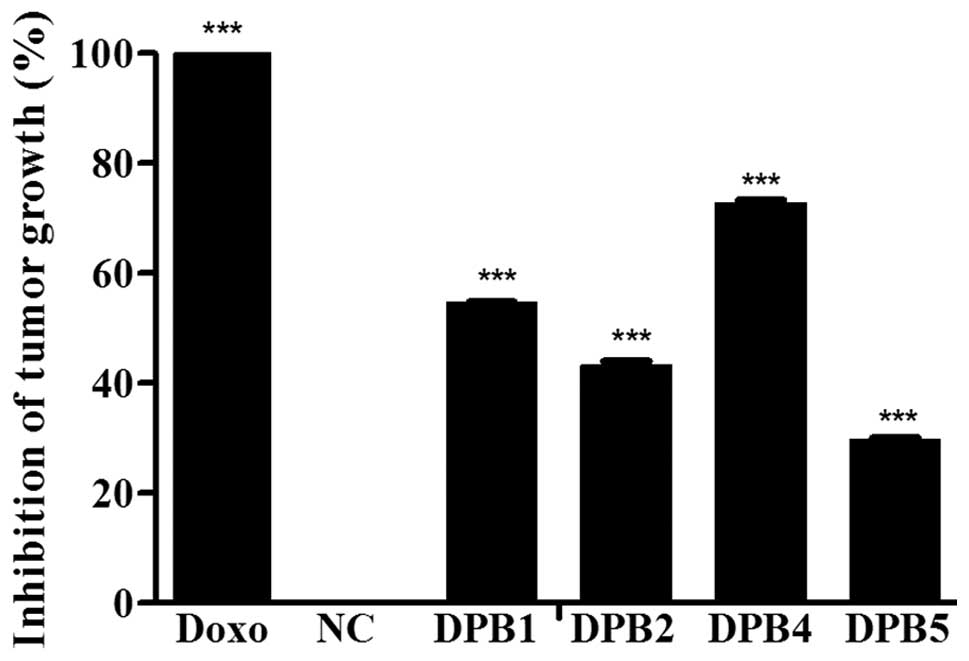
Farias MS, Pich CT, Kviecinski MR, Bucker NC, Felipe KB, Da Silva FO, Günther TM, Correia JF, Ríos D, Benites J, Benites J, et al: Substituted 3‑acyl‑2‑phenylamino‑1,4‑naphthoquinones intercalate into DNA and cause genotoxicity through the increased generation of reactive oxygen species culminating in cell death. Mol Med Rep 10: 405-410, 2014.
APA
Farias, M.S., Pich, C.T., Kviecinski, M.R., Bucker, N.C., Felipe, K.B., Da Silva, F.O. ... Pedrosa, R.C. (2014). Substituted 3‑acyl‑2‑phenylamino‑1,4‑naphthoquinones intercalate into DNA and cause genotoxicity through the increased generation of reactive oxygen species culminating in cell death. Molecular Medicine Reports, 10, 405-410. https://doi.org/10.3892/mmr.2014.2160
MLA
Farias, M. S., Pich, C. T., Kviecinski, M. R., Bucker, N. C., Felipe, K. B., Da Silva, F. O., Günther, T. M., Correia, J. F., Ríos, D., Benites, J., Valderrama, J. A., Calderon, P. B., Pedrosa, R. C."Substituted 3‑acyl‑2‑phenylamino‑1,4‑naphthoquinones intercalate into DNA and cause genotoxicity through the increased generation of reactive oxygen species culminating in cell death". Molecular Medicine Reports 10.1 (2014): 405-410.
Chicago
Farias, M. S., Pich, C. T., Kviecinski, M. R., Bucker, N. C., Felipe, K. B., Da Silva, F. O., Günther, T. M., Correia, J. F., Ríos, D., Benites, J., Valderrama, J. A., Calderon, P. B., Pedrosa, R. C."Substituted 3‑acyl‑2‑phenylamino‑1,4‑naphthoquinones intercalate into DNA and cause genotoxicity through the increased generation of reactive oxygen species culminating in cell death". Molecular Medicine Reports 10, no. 1 (2014): 405-410. https://doi.org/10.3892/mmr.2014.2160