Introduction
At present, lung cancer is a malignant tumor with
the globally highest morbidity and fatality rates. ~80–85% of lung
cancers are non-small-cell lung carcinoma (NSCLC). Surgical
resection is considered the main treatment for NSCLC, with surgery
being indicated for ~20–30% of patients and the postoperative
recurrence and the metastasis rate being ≥50% (1). Therefore, for the lung cancer
patients who are unable to undergo surgery and those with
postoperative recurrence or metastasis, combinations of
platinum-containing agents are the primary chemical treatment to
extend the lifetime. However, to date, no effective measures have
been established for patients who are subject to such regimen
control, and who present with new cancer progression. Sorafenib is
a small molecule with activity against neoplasms via multiple
biological targets. Sorafenib has a dual function of inhibiting
tumor cell proliferation and angiogenesis. At present, the Food and
Drug Administration (FDA, Silver Spring, MD, USA) has approved
sorafenib for the use in the clinical treatment of kidney and liver
cancers (2,3). To date, basic research and clinical
trials using sorafenib for the prevention and treatment of lung
cancer have made positive progress globally (4,5). The
present study used the A549/DDP cisplatin-resistant lung
adenocarcinoma cell strain to study the biological effects of
sorafenib. Effects on proliferation, apoptosis and invasion of the
A549/DDP cisplatin-resistant lung adenocarcinoma cell strain
was observed, which provided scientific experimental data and a
theoretical basis for the application of sorafenib for the
subsequent treatment of cisplatin-resistant lung cancer.
Materials and methods
Cell strains
The A549 lung adenocarcinoma cell strain and the
A549/DDP cisplatin-resistant lung adenocarcinoma cell strain
were purchased from the tumor cell bank of the Chinese Academy of
Sciences (Shanghai, China), with a resistance index of 13
times.
Main reagents
Sorafenib was purchased from Bayer (Leverkusen,
Germany), RPMI-1640 culture medium and fetal bovine serum (FBS)
were from HyClone (Logan, UT, USA), cisplatin (DDP) from Haosen
Pharmacy, Ltd. (Ganzhou, China), MTT and dimethylsulfoxide (DMSO)
from Sigma-Aldrich (St. Louis, MO, USA), Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) apoptosis kit
from BioVision (Mountain View, CA, USA) and Matrigel®
from BD Biosciences (Franklin Lakes, NJ, USA).
Cell culture
The A549 and A549/DDP cells were cultured in
RPMI-1640 medium at 37°C with 5% CO2 and 95% saturated
humidity. To maintain the stable drug-resistance of A549/DDP
cells, 2 μmol/l DPP was added into the RPMI-1640 with 10%
FBS for joint culture and the cells were cultured in the medium
without DDP for one week prior to beginning the trial.
Experimental groups
The experiment involved five groups: S0 and
experimental groups S1, S2, S3 and S4. The control group S0 was
incubated with the culture medium RPMI-1640 only; S1–4 were
incubated with 2, 4, 8 and 16 μmol/l sorafenib,
respectively.
MTT assay
Cells were collected at the exponential growth
phase, the cell density was adjusted to 3×104
ml−1 and 100 μl was inoculated into each well on 6
culture plates and 7 repeated wells set for each 96-well plate. One
culture plate was removed each day at the same time, MTT reagent
(20 μl) was added, placed in a CO2 incubator and
incubated for 4 h. Following removal of the medium, DMSO (100 μl)
was added to each well and the plate was placed on an agitator for
10 min to fully dissolve the crystals. The optical density (OD) of
each well was measured at 490 nm using an ELISA reader (R&D
Systems, Minneapolis, MN, USA). The blank controls (containing
medium, MTT and DMSO) and control wells (containing cells, medium,
MTT and DMSO) were measured. The formula adopted for the
calculation of the inhibition rate of cell proliferation is:
Inhibition rate = 100% × [(OD value of the control well − OD value
of the blank control well) − (OD value of the experimental well −
OD value of the blank control well)]/(OD value of control
well − OD value of the blank control well). Each experiment was
repeated three times.
Flow cytometric analysis of
apoptosis
The inoculated cells were cultured in a 5%
CO2 incubator for 24 h and the original culture medium
was discarded. Different concentrations of sorafenib (2, 4, 8 and
16 μmol/l) were added to the experimental groups at 2
ml/well in RPMI medium. The control group was added with the same
amount of culture medium without sorafenib. Three complexes were
prepared for each group. The culture plates were incubated for a
further 72 h. The cells of each group were digested with EDTA-free
pancreatin (Gibco, Grand Island, NY, USA) and collected. Binding
buffer (500 μl; KeyGen Biotech, Nanjing, China) was added to
prepare a cell suspension. Under the exclusion of light, 5 μl
Annexin V-FITC and 5 μl PI was added to each group of cell
suspension. The groups were allowed to react for 15 min at room
temperature in the dark. Flow cytometric analysis was performed
within one hour and the percentage of apoptotic cells was
calculated using CellQuest software, version 3.0 (BD
Biosciences).
Transwell assay
A549/DDP cells were collected during the
exponential growth phase, the concentration was adjusted to
5×105 ml−1 and the cells were inoculated into
the upper chamber of a Transwell plate (6.5 mm; 24-well plate,
Costar; Corning, NY, USA) at 200 μl/well and three repeated wells
were set for each group. Sorafenib was added to the upper chamber
with a final concentration of 2, 4, 8 and 16 μmol/l for the
experimental groups, and no reagent was added for the control
group. At the same time, RPMI-1640 (500 μl) culture medium
containing 10% FBS was added into the lower chamber and the plate
was cultured for a further 24 h. The cells were removed from the
Transwell chamber, washed twice with phosphate-buffered saline and
the filter membrane was fixed with 4% paraformaldehyde for 30 min.
The filter membrane was subjected to crystal violet staining
(Beyotime Institute of Biotechnology, Shanghai, China) for 20 min
following air drying and was then rinsed with double distilled
water. Five fields of view at upper, lower, left, right and central
locations were captured under a light microscope (x400). To assess
the number of cells migrated through the membrane to the lower
chamber surface, the average values were analyzed. The number of
migrated cells reflected the level of tumor cell invasiveness.
Accordingly, a reduced number of cells permeating the septum
indicated marked inhibition of invasion.
Statistical analysis
Values are presented as the mean ± standard
deviation and were determined by the statistical software SPSS 11.0
(SPSS Inc., Chicago, IL, USA). Groups were compared using one-way
analysis of variance. Homogeneity of variance was tested by the
least significant difference method, abd heterogeneity of variance
was tested using Dunnett’s T method. P<0.05 was considered to
indicate a statistically significant difference.
Results
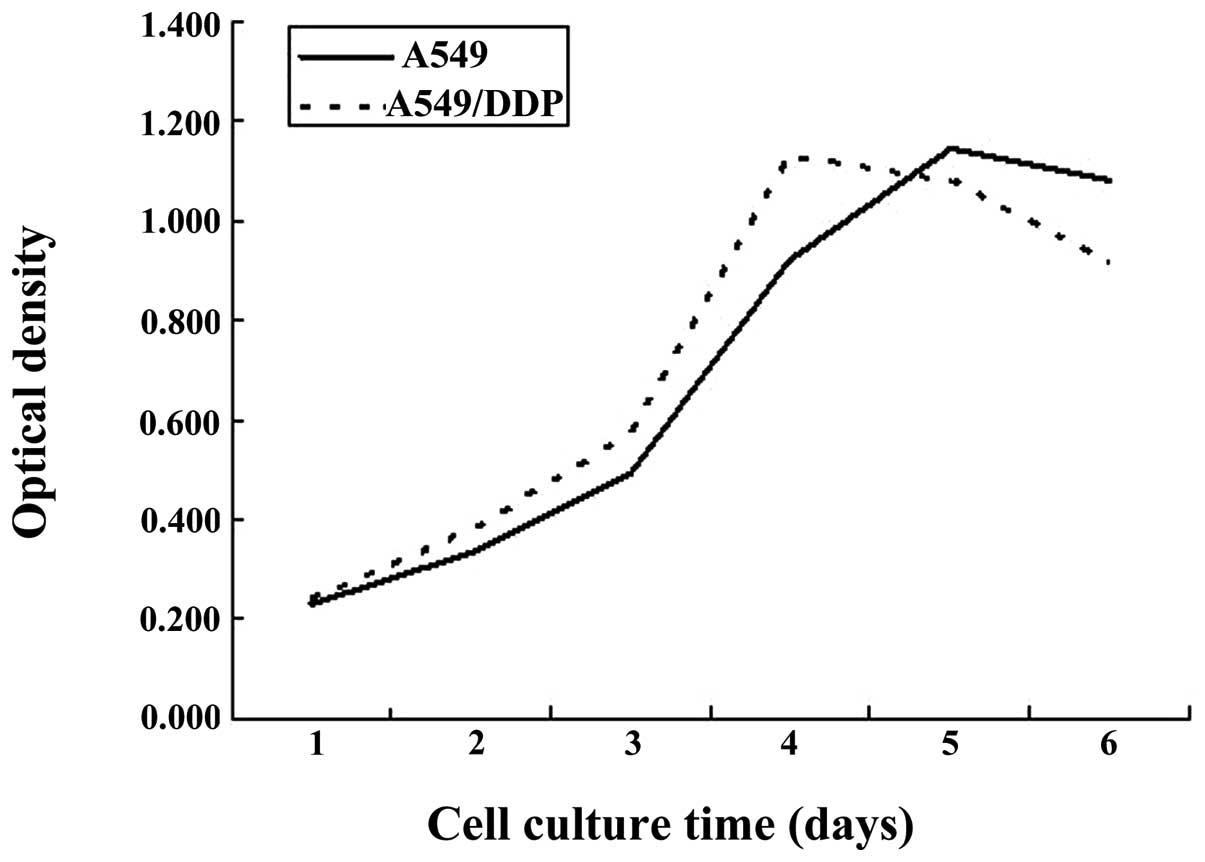
Comparison between the growth curves of
A549/DDP cisplatin-resistant lung adenocarcinoma cells and A549
parental cells
Microscopic observation indicated that the adherent
growth of cisplatin-resistant lung adenocarcinoma cells
A549/DDP was marked and vigorous, indicating a satisfying
light refraction of cytoplasm. Compared with A549 cells, the
A549/DDP cells were slightly larger in volume, growing in
fusiform or polygonal structures, with a large cell nucleus.
Compared with parental A549 cells, the A549/DDP
cisplatin-resistant cells exhibited a higher cell proliferation
rate and reached the exponential growth phase 2–4 days following
passage, while the A549 cells required 3–5 days to reach the
exponential growth phase (Fig.
1).
Sorafenib inhibits the proliferation of
A549/DDP cisplatin-resistant lung adenocarcinoma cells
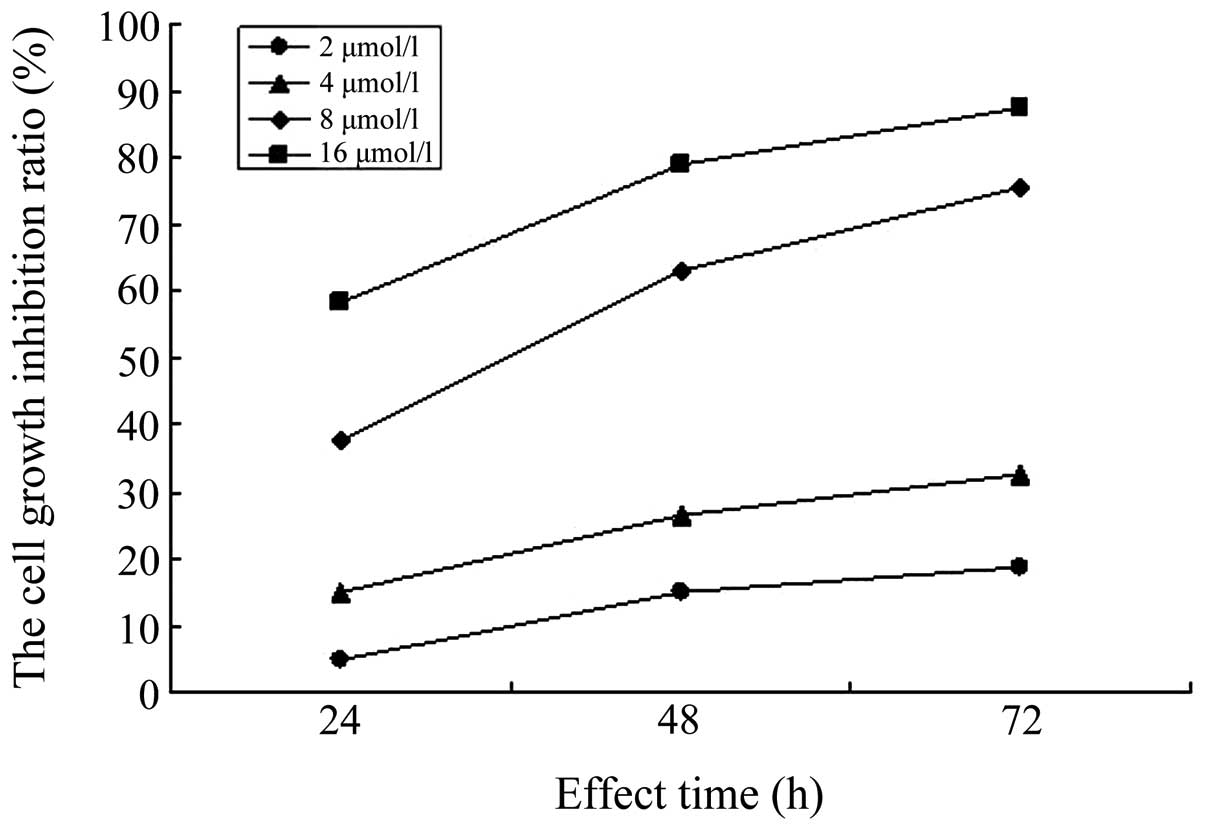
The MTT assay was used to determine the inhibition
rate of sorafenib on the proliferation of A549/DDP
cisplatin-resistant lung adenocarcinoma cells at different
concentrations and time-points. The results showed that, compared
with the control group, sorafenib inhibited the proliferation of
A549/DDP cisplatin-resistant lung adenocarcinoma cells
within the concentration range of 2, 4, 8 and 16 μmol/l.
With the extension of the action time and increase of the
concentration, the inhibition rate of sorafenib on A549/DDP
cisplatin-resistant lung adenocarcinoma cells gradually increased
(P<0.05). In addition, the differences in the inhibition rates
of sorafenib on A549/DDP cisplatin-resistant lung
adenocarcinoma cells at different times and concentrations were
statistically significant (P<0.05), as shown in Table I and Figs. 2 and 3.
 | Table IThe inhibition rate of sorafenib on
A549/DDP cisplatin-resistant lung adenocarcinoma cells at
different incubation times (mean ± standard deviation). |
Table I
The inhibition rate of sorafenib on
A549/DDP cisplatin-resistant lung adenocarcinoma cells at
different incubation times (mean ± standard deviation).
| Group | 24 h | 48 h | 72 h |
|---|
| S1 | 4.58±2.81 | 14.98±2.93a | 18.80±2.82b |
| S2 | 14.93±2.62 | 26.28±7.31a | 32.71±2.55b |
| S3 | 37.58±7.13 | 63.00±3.05a | 75.51±4.73c |
| S4 | 58.39±8.15 | 78.84±3.96a | 87.50±3.36c |
Sorafenib induces apoptosis of A549/DDP
cisplatin-resistant lung adenocarcinoma cells
The A549/DDP cisplatin-resistant lung
adenocarcinoma cells in the experimental groups S1–4 were
respectively treated with sorafenib at the concentrations of 2, 4,
8 and 16 μmol/l for 72 h, and the S0 control group was
cultured with RPMI-1640 medium only for 72 h. The rate of apoptosis
was determined using Annexin V-FITC/PI staining and flow
cytometric analysis. It was observed that the rate of apoptosis of
the control group S0 was 8.89±0.81% and the rates of apoptosis in
the experimental groups S1–4 were 12.84±0.24, 17.27±0.78,
21.98±0.75 and 49.67±1.38%, respectively. Compared with the control
group, the differences were statistically significant (P<0.01);
the differences of further pairwise comparison in the same group
were also statistically significant (P<0.01), as shown in
Fig. 4.
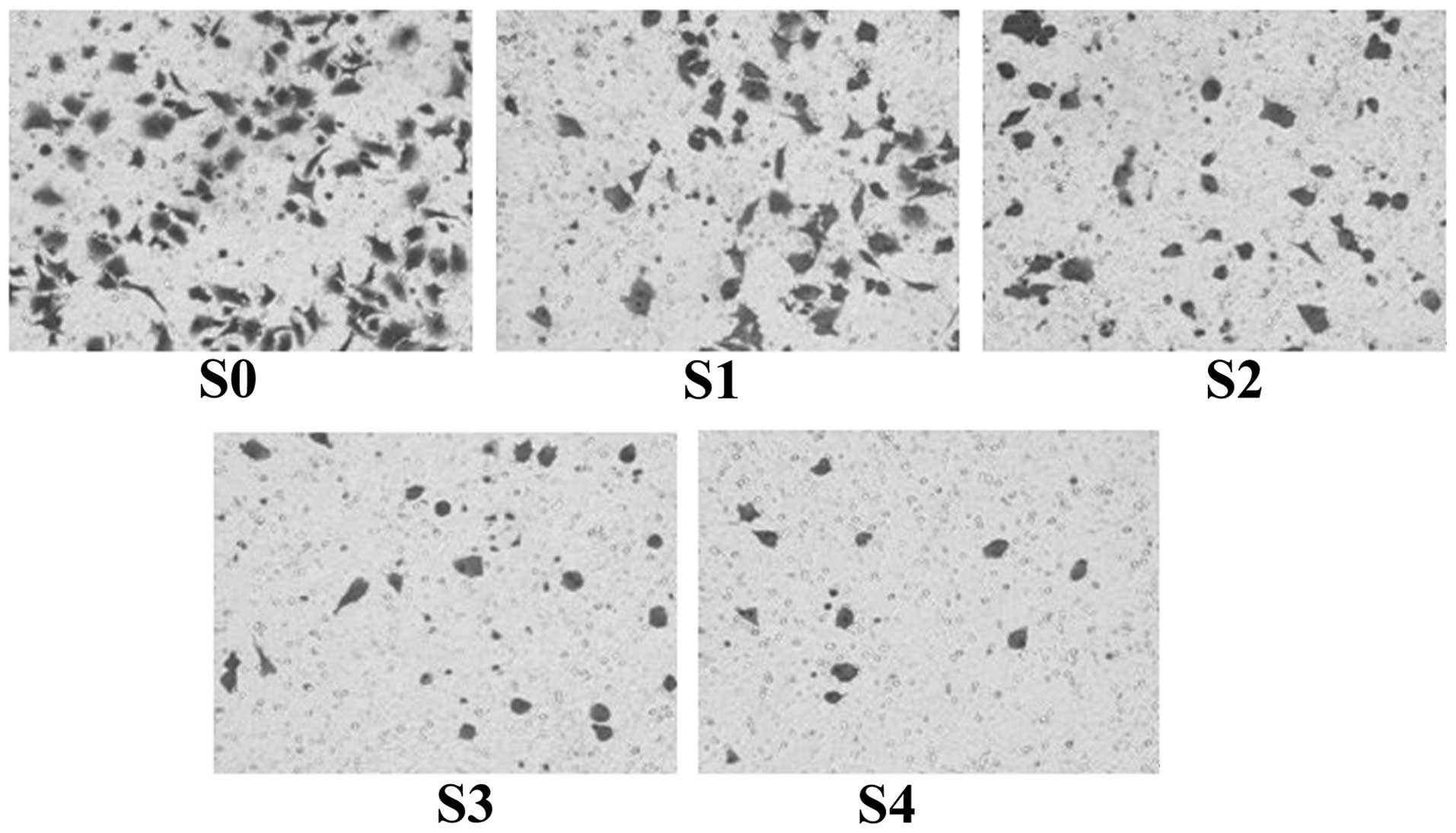
Sorafenib inhibits the invasion of
A549/DDP cisplatin-resistant lung adenocarcinoma cells
The Transwell assay was used to determine the number
of cells permeating the Transwell membrane in each group. The
results showed that, following 24 h of incubation, the average
number of cells permeating the septum in the normal control group
S0 reached 82.7±2.3/high power lens (HP), while the
average number of cells permeating the septum in S1–4 in the
presence of sorafenib at concentrations of 2, 4, 8 and 16
μmol/l, were decreased to 58.2±2.5, 41.3±1.3, 22.6±2.1 and
14.7±1.1/HP, respectively. Compared with the control group,
the difference in the number of cells permeating the
Matrigel® of each group was statistically significant
(P<0.01). When the concentration increased and the cell
permeation of the septum gradually decreased, the differences
obtained by pairwise comparisons among the groups also became
statistically significant (P<0.01). In addition, observation by
optical microscopy following crystal violet staining indicated that
the cells in the normal control group which had not been treated
with sorafenib were of fusiform, polygonal or oval shaped, with a
larger cell volume a dense distribution at the lower surface of the
Transwell chamber, while the cells of the experimental groups,
which had been treated with sorafenib, gradually became smaller,
pseudopodia decreased and the shape of the cells became round and
sparsely scattered at the lower surface of the Transwell chamber
(Fig. 5).
Discussion
Sorafenib is an antineoplastic agent with multiple
biological targets, which inhibits tumor growth, invasion and
metastasis by preventing the proliferation of tumor cells and
angiogenesis in tumor tissue. In 2005 and 2007, the FDA approved
the use of sorafenib for the clinical treatment of advanced kidney
and liver cancer (6,7). At present, basic research and
clinical trials using sorafenib for the prevention and treatment of
lung cancer are progressing with positive preliminary results
(4,5). Dy et al (8) have tested sorafenib in a Phase II
clinical trial as a first-line treatment of advanced NSCLC and the
results showed that among the 25 patients selected for the study,
three went into partial remission and six achieved a stable
condition, with progression-free survival (PFS) for 2.8 months, and
a certain clinical efficacy was obtained. Spigel et al
(9) performed a clinical trial of
combining sorafenib with erlotinib, or using only erlotinib as the
second-line treatment of patients with advanced NSCLC. A total of
168 patients were involved. The results showed that the disease
control rates of the combination group and the group with only
erlotinib were 54 and 38%, respectively, and PFSs were 3.38 and
1.94, respectively, indicating that sorafenib has potential as a
second-line treatment. In the present study, in order to provide a
novel method for the second-line treatment of NSCLC, the
A549/DDP cisplatin-resistant lung adenocarcinoma cell strain
was chosen to assess the effect of sorafenib on the proliferation,
apoptosis and invasion in vitro.
In the present study, the MTT assay was used to
determine the activity of sorafenib on the proliferation of
A549/DDP cisplatin-resistant lung adenocarcinoma cells and
to observe its inhibition effects. The results showed that compared
with the parental A549 cells, the A549/DDP
cisplatin-resistant lung adenocarcinoma cells were slightly larger
in volume, growing in fusiform or polygonal structures, with a
large intracellular nucleus. The cell proliferation was faster,
reaching the exponential phase within 2–4 days following passage,
while A549 cells reached the exponential phase within 3–5 days
following passage, indicating that the metabolic activity of
A549/DDP cisplatin-resistant lung adenocarcinoma cells was
significantly enhanced and marked mitosis was observed following
acquisition of drug-resistance characteristics; thus, they were
able to enter the logarithmic phase faster than the parental A549
cells. This is consistent with the observations on the growth
characteristics of A549/DDP cisplatin-resistant lung
adenocarcinoma cells reported by Giard et al (10). A further analysis of the inhibitory
effect of sorafenib on the proliferation of A549/DDP
cisplatin-resistant lung adenocarcinoma cells was conducted,
showing that sorafenib is capable of inhibiting the proliferation
of the A549/DDP cisplatin-resistant lung adenocarcinoma
cells within the concentration range of 2–16 μmol/l.
Furthermore, following treatment of A549/DDP cells with
varying concentrations of sorafenib, the inhibition rate of cells
was gradually increased with increasing time, while at the same
time, the inhibition rate was gradually increased as well with
increasing drug concentration. The results of this trial showed
that sorafenib was capable of inhibiting the proliferation of
A549/DDP cisplatin-resistant lung adenocarcinoma cells and
this inhibitory effect was time- and concentration-dependent. The
Annexin V-FITC/PI assay was used to determine the rate of
apoptosis of A549/DDP cisplatin-resistant lung
adenocarcinoma cells and to observe the induction effect of
sorafenib on the apoptosis of A549/DDP cells. The results
showed that marked apoptosis appeared in the cells in each
experimental group following treatment with sorafenib at different
concentrations for 72 h. In addition, with the increase of the drug
concentration, the rate of apoptosis also increased. The difference
from the control group was statistically significant (P<0.05),
indicating that sorafenib is capable of promoting the apoptosis of
A549/DDP cisplatin-resistant lung adenocarcinoma cells, and
this effect was dependent on the concentration. The difference
obtained by pairwise comparison among experimental groups was also
statistically significant (P<0.05). The results of the present
study were similar to those investigating the effects of sorafenib
on the proliferation and apoptosis of parental A549 cells reported
by Yu et al (11) and Mao
et al (12). In the present
study, to further determine the influence of sorafenib on the
invasiveness of A549/DDP cisplatin-resistant lung
adenocarcinoma cells, the Transwell assay was used for each
experimental group in the presence of sorafenib for 24 h. The
results showed that with increasing drug concentration, the number
of cells permeating the basement membrane in each experimental
group significantly decreased. The difference between each
experimental and the control group was statistically significant
(P<0.05), indicating that sorafenib had an in vitro
inhibitory effect on the invasion of A549/DDP
cisplatin-resistant lung adenocarcinoma cells. At the same time, it
was noted that the cells permeating the septum in each experimental
group markedly changed their shape, the occurrence of pseudopodia
decreased and the cell volume decreased.
In conclusion, sorafenib inhibited the proliferation
of A549/DDP cisplatin-resistant lung adenocarcinoma cells in
a time- and concentration-dependent manner. Sorafenib also induces
apoptosis and reduces the invasiveness of A549/DDP cells,
which provided scientific experimental evidence for the theoretical
basis of the application of sorafenib in the subsequent treatment
of cisplatin-resistant lung cancer and offers a novel and effective
strategy for further treatment of advanced lung cancer (13–15).
References
|
1
|
Lee MW, Kim DS, Min NY and Kim HT: Akt1
inhibition by RNA interference sensitizes human non-small cell lung
cancer cells to cisplatin. Int J Cancer. 122:2380–2384. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuen JS, Sim MY, Siml HG, et al:
Inhibition of angiogenic and non-angiogenic targets by sorafenib in
renal cell carcinoma (RCC) in a RCC xenograft model. Br J Cancer.
104:941–947. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huynh H, Ngo VC, Koong HN, et al:
Sorafenib and rapamycin induce growth suppression in mouse models
of hepatocellular carcinoma. J Cell Mol Med. 13:2673–2683. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smit EF, Dingemans AM, Thunnissen FB, et
al: Sorafenib in patients with advanced non-small cell lung cancer
that harbor K-ras mutations: a brief report. J Thorac Oncol.
5:719–720. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okamoto I, Miyazaki M, Morinaga R, et al:
Phase I clinical and pharmacokinetic study of sorafenib in
combination with carboplatin and paclitaxel in patients with
advanced non-small cell lung cancer. Invest New Drugs. 28:844–853.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim S, Yazici YD, Calzada G, et al:
Sorafenib inhibits the angiogenesis and growth of orthotopic
anaplastic thyroid carcinoma xenografts in nude mice. Mol Cancer
Ther. 6:1785–1792. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Escudier B, Eisen T, Stadler WM, et al:
Sorafenib for treatment of renal cell carcinoma: final efficacy and
safety results of the phase III treatment approaches in renal
cancer global evaluation trial. J Clin Oncol. 27:3312–3318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dy GK, Hillman SL, Rowland KM Jr, et al: A
front-line window of opportunity phase 2 study of sorafenib in
patients with advanced nonsmall cell lung cancer: North Central
Cancer Treatment Group Study N0326. Cancer. 116:5686–5693. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spigel DR, Burris HA 3rd, Greco FA, et al:
Randomized, double-blind, placebo-controlled, phase II trial of
sorafenib and erlotinib or erlotinib alone in previously treated
advanced non-small-cell lung cancer. J Clin Oncol. 29:2582–2589.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giard DJ, Aaronson SA, Todaro GJ, et al:
In vitro cultivation of human tumors: establishment of cell lines
derived from a series of solid tumors. Natl Cancer Inst.
51:1417–1423. 1973.PubMed/NCBI
|
|
11
|
Yu C, Friday BB, Lai JP, et al: Cytotoxic
synergy between the multikinase inhibitor sorafenib and the
proteasome inhibitor bortezomib in vitro: induction of apoptosis
through Akt and c-Jun NH2-terminal kinase pathways. Mol Cancer
Ther. 5:2378–2387. 2006. View Article : Google Scholar
|
|
12
|
Mao WF, Shao MH, Gao PT, et al: The
important roles of RET, VEGFR2 and the RAF/MEK/ERK
pathway in cancer treatment with sorafenib. Acta Pharmacol Sin.
33:1311–1318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carter CA, Chen C, Brink C, et al:
Sorafenib is efficacious and tolerated in combination with
cytotoxic or cytostatic agents in preclinical models of human
non-small cell lung carcinoma. Cancer Chemother Pharmacol.
59:183–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Pan YY and Zhang Y: Sorafenib
combined with gemcitabine in EGFR-TKI-resistant human lung cancer
cells. Oncol Lett. 5:68–72. 2013.PubMed/NCBI
|
|
15
|
Giovannetti E, Labots M, Dekker H, et al:
Molecular mechanisms and modulation of key pathways underlying the
synergistic interaction of sorafenib with erlotinib in
non-small-cell-lung cancer (NSCLC) cells. Curr Pharm Des.
19:927–939. 2013. View Article : Google Scholar
|