Article
Therapeutic effects of sorafenib on the A549/DDP human lung adenocarcinoma cell line in vitro
- Authors:
- Xiang‑Qi Chen
- Yu‑Lan Wang
- Zhi‑Ying Li
- Ting‑Yan Lin
-
View Affiliations / Copyright
Affiliations:
Teaching and Research Department of Respiratory Medicine, Union Clinical Medical College of Fujian Medical University, Fuzhou, Fujian 350001, P.R. China
-
Pages:
347-352
|
Published online on:
April 24, 2014
https://doi.org/10.3892/mmr.2014.2163
- Expand metrics +
Metrics:
Total
Views: 0
(Spandidos Publications: | PMC Statistics:
)
Metrics:
Total PDF Downloads: 0
(Spandidos Publications: | PMC Statistics:
)
This article is mentioned in:
Abstract
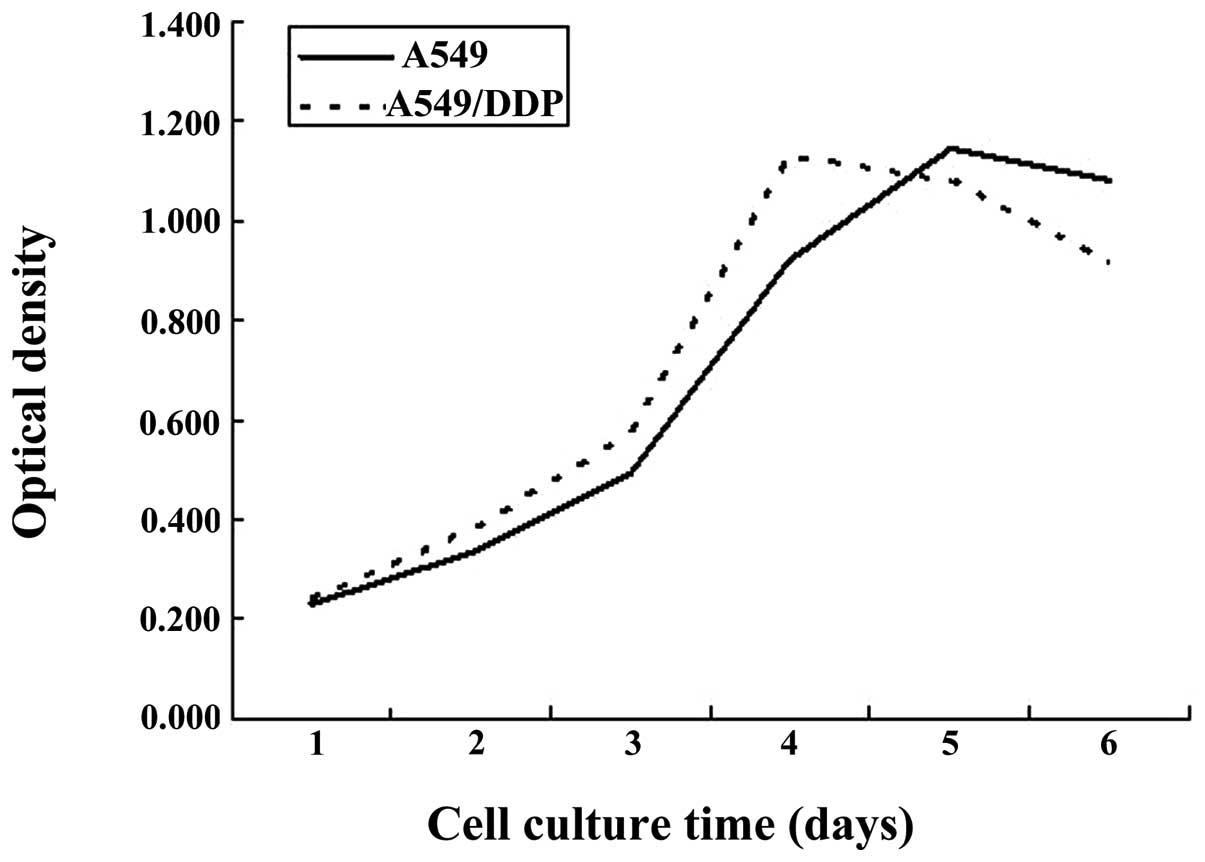
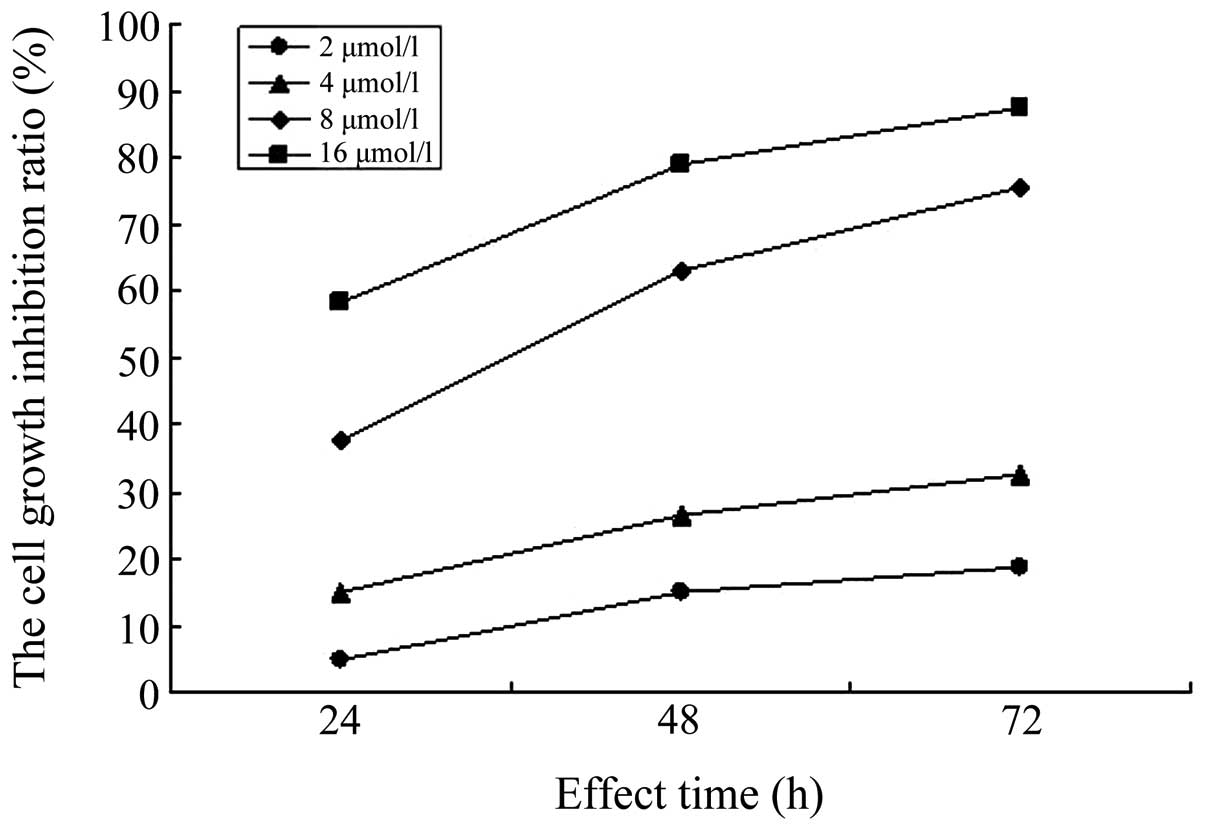
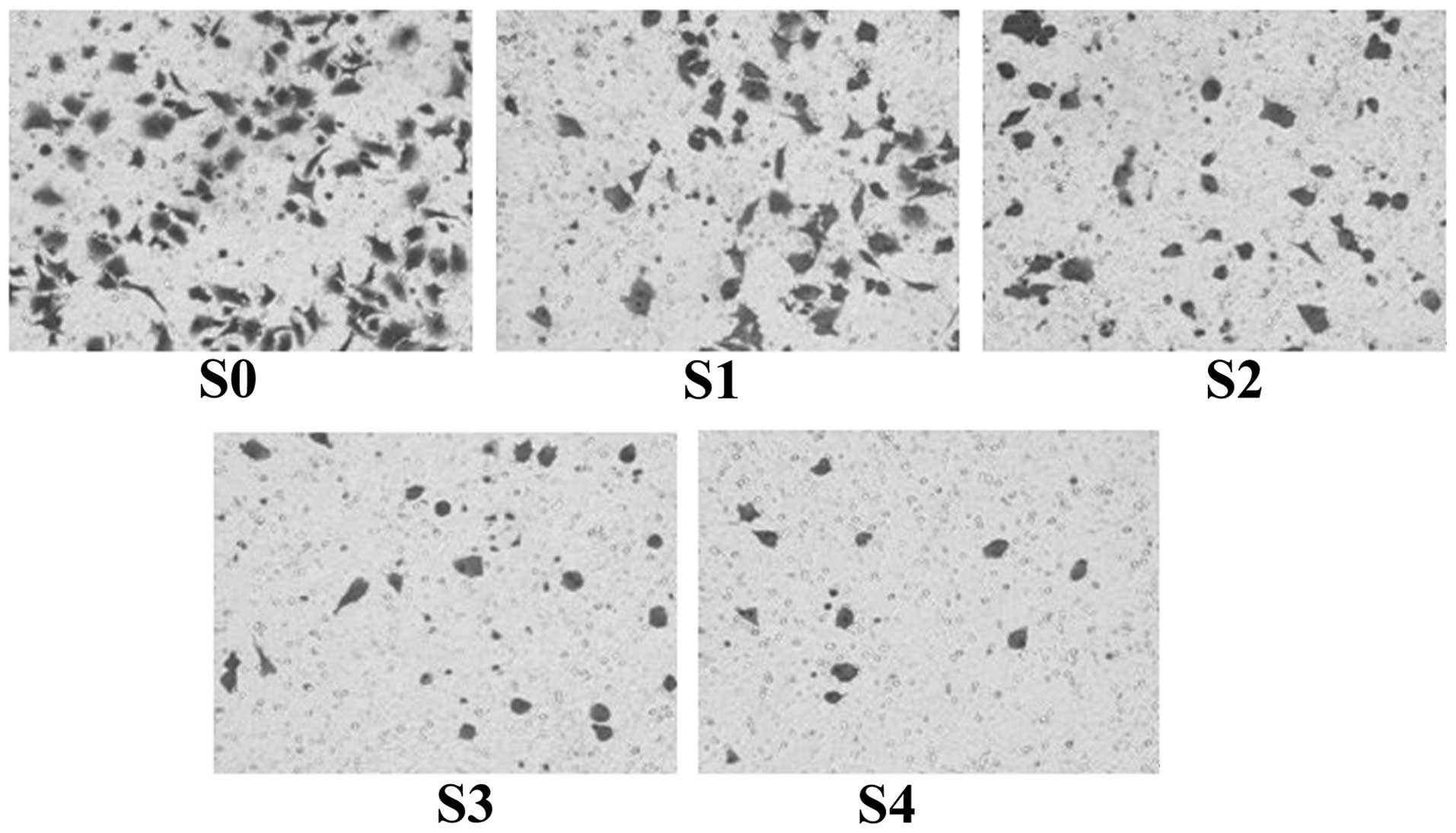
The aim of the present study was to observe the effects of sorafenib on the proliferation, apoptosis and invasion of A549/DDP cisplatin‑resistant lung adenocarcinoma cells cultured in vitro. The A549/DDP cisplatin‑resistant lung adenocarcinoma cell strain was cultured in vitro, the cell culture group incubated in culture medium only was set as the control group (Group S0) and the four concentration gradients of sorafenib were added to the culture groups as the experimental groups: S1, 2 µmol/l; S2, 4 µmol/l; S3, 8 µmol/l; and S4, 16 µmol/l. The MTT assay was used to determine the growth inhibition rate of the cells, which were respectively subjected to sorafenib treatment for 24, 48 and 72 h. Flow cytometry was used to determine the rate of apoptosis of cells in each group following sorafenib treatment for 72 h. Furthermore, the Transwell invasion experiment was used to determine the effect on A549/DDP cell invasion following sorafenib treatment for 24 h. Based on the MTT assay, it was found that the inhibition rates of A549/DDP cisplatin‑resistant lung adenocarcinoma cells in groups S1‑4 following sorafenib treatment for 24 h were 4.58±2.82, 14.93±2.62, 37.58±7.13 and 58.39±8.15%, respectively. For 48 h, inhibition rates in S1‑4 were 14.98±2.93, 26.28±7.31, 63.00±3.05 and 78.84±3.96%, respectively, and for 72 h, inhibition rates were 18.80±2.82, 32.71±2.55, 75.51±4.73 and 87.50±3.36%, respectively. The difference in the inhibition rates of cells among the experimental groups for the same incubation time showed statistical significance (P<0.05). Flow cytometric analysis indicated that the rate of apoptosis in the control group was 8.88±0.81% following sorafenib treatment for 72 h, and the rates of apoptosis in groups S1‑4 were, 12.84±0.24, 17.27±0.78, 21.98±0.75 and 49.67±1.38%, respectively. The rate of apoptosis in each experimental group was higher compared with that in the control group (P<0.05). The difference in the rate of apoptosis among the experimental groups was statistically significant (P<0.05). The Transwell assay showed that the number of cells permeating the septum in the control group was 82.7±2.3/high power lens (HP), while the average number of cells permeating septum in groups S1‑4 following treatment with sorafenib for 24 h was 58.2±2.5, 41.3±1.3, 22.6±2.1 and 14.7±1.1/HP, which was significantly lower compared with the control group. The number of cells permeating the septum in each experimental group decreased with the enhancement of the concentration gradient. The differences were statistically significant (P<0.05). In conclusion, sorafenib inhibits the proliferation of A549/DDP cisplatin‑resistant lung adenocarcinoma cells in a time‑ and concentration‑dependent manner. In addition, sorafenib induces apoptosis in A549/DDP cisplatin‑resistant lung adenocarcinoma cells, thus reducing their invasiveness.
View References
|
1
|
Lee MW, Kim DS, Min NY and Kim HT: Akt1
inhibition by RNA interference sensitizes human non-small cell lung
cancer cells to cisplatin. Int J Cancer. 122:2380–2384. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuen JS, Sim MY, Siml HG, et al:
Inhibition of angiogenic and non-angiogenic targets by sorafenib in
renal cell carcinoma (RCC) in a RCC xenograft model. Br J Cancer.
104:941–947. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huynh H, Ngo VC, Koong HN, et al:
Sorafenib and rapamycin induce growth suppression in mouse models
of hepatocellular carcinoma. J Cell Mol Med. 13:2673–2683. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smit EF, Dingemans AM, Thunnissen FB, et
al: Sorafenib in patients with advanced non-small cell lung cancer
that harbor K-ras mutations: a brief report. J Thorac Oncol.
5:719–720. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okamoto I, Miyazaki M, Morinaga R, et al:
Phase I clinical and pharmacokinetic study of sorafenib in
combination with carboplatin and paclitaxel in patients with
advanced non-small cell lung cancer. Invest New Drugs. 28:844–853.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim S, Yazici YD, Calzada G, et al:
Sorafenib inhibits the angiogenesis and growth of orthotopic
anaplastic thyroid carcinoma xenografts in nude mice. Mol Cancer
Ther. 6:1785–1792. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Escudier B, Eisen T, Stadler WM, et al:
Sorafenib for treatment of renal cell carcinoma: final efficacy and
safety results of the phase III treatment approaches in renal
cancer global evaluation trial. J Clin Oncol. 27:3312–3318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dy GK, Hillman SL, Rowland KM Jr, et al: A
front-line window of opportunity phase 2 study of sorafenib in
patients with advanced nonsmall cell lung cancer: North Central
Cancer Treatment Group Study N0326. Cancer. 116:5686–5693. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spigel DR, Burris HA 3rd, Greco FA, et al:
Randomized, double-blind, placebo-controlled, phase II trial of
sorafenib and erlotinib or erlotinib alone in previously treated
advanced non-small-cell lung cancer. J Clin Oncol. 29:2582–2589.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giard DJ, Aaronson SA, Todaro GJ, et al:
In vitro cultivation of human tumors: establishment of cell lines
derived from a series of solid tumors. Natl Cancer Inst.
51:1417–1423. 1973.PubMed/NCBI
|
|
11
|
Yu C, Friday BB, Lai JP, et al: Cytotoxic
synergy between the multikinase inhibitor sorafenib and the
proteasome inhibitor bortezomib in vitro: induction of apoptosis
through Akt and c-Jun NH2-terminal kinase pathways. Mol Cancer
Ther. 5:2378–2387. 2006. View Article : Google Scholar
|
|
12
|
Mao WF, Shao MH, Gao PT, et al: The
important roles of RET, VEGFR2 and the RAF/MEK/ERK
pathway in cancer treatment with sorafenib. Acta Pharmacol Sin.
33:1311–1318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carter CA, Chen C, Brink C, et al:
Sorafenib is efficacious and tolerated in combination with
cytotoxic or cytostatic agents in preclinical models of human
non-small cell lung carcinoma. Cancer Chemother Pharmacol.
59:183–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Pan YY and Zhang Y: Sorafenib
combined with gemcitabine in EGFR-TKI-resistant human lung cancer
cells. Oncol Lett. 5:68–72. 2013.PubMed/NCBI
|
|
15
|
Giovannetti E, Labots M, Dekker H, et al:
Molecular mechanisms and modulation of key pathways underlying the
synergistic interaction of sorafenib with erlotinib in
non-small-cell-lung cancer (NSCLC) cells. Curr Pharm Des.
19:927–939. 2013. View Article : Google Scholar
|