Introduction
Gliomas are the most common and devastating primary
central nervous system neoplasms and account for 77% of all brain
tumors (1). Gliomas are subdivided
into oligodendrogliomas, ependymomas, astrocytomas and
oligoastrocytomas based on the resemblance of tumor cells to the
original parental cells. According to World Health Organization
(WHO) classifications, astrocytomas are further classified into
four clinical grades: Pilocytic astrocytomas (WHO grade I), diffuse
astrocytomas (WHO grade II), anaplastic astrocytomas (WHO grade
III) and glioblastoma multiforme (WHO grade IV) (2). In excess of 51,000 individuals are
diagnosed with primary brain tumors in the United States each year,
and ~75% of those diagnosed with astrocytoma are likely to succumb
within five years of diagnosis (3). Although the length of survival has
been enhanced by surgery, radiation and chemotherapy, astrocytoma
mortality remains high. The median survival of patients with
glioblastoma is between 12 and 15 months and between two and five
years for patients with anaplastic astrocytoma (1,2).
Therefore, novel strategies for the treatment of astrocytoma,
particularly glioblastoma, are required. However, the mechanism
underlying the malignant progression of astrocytomas has not yet
been fully elucidated.
Sperm-associated antigen 9 (SPAG9) is one of a
family of scaffolding proteins that selectively mediates c-Jun
N-terminal kinase (JNK) signaling by aggregating specific
components of the mitogen-activated protein kinase cascade to form
a functional JNK signaling module (4). Previous studies have shown that SPAG9
is overexpressed in a number of human cancers, including renal,
breast, thyroid, cervical and colon carcinomas (5–9).
Furthermore, SPAG9-small interfering RNA treatment has been
shown to inhibit tumor cell proliferation and invasion (5–9). A
recent study demonstrated that SPAG9 was overexpressed in human
astrocytomas and that SPAG9 depletion in astrocytoma cells
inhibited cell invasion through downregulation of matrix
metalloproteinase-9 (MMP-9) (10),
suggesting that SPAG9 has an important role in astrocytoma
invasion.
Podocalyxin (PODXL) is a transmembrane protein that
is expressed by several types of human cells, including
hematopoietic progenitors and vascular endothelial cells, as well
as platelets (11). Increased
PODXL expression has been associated with a subset of aggressive
types of cancer, including acute myeloid and lymphoid leukemia,
myeloid sarcomas and certain breast, liver, pancreatic and kidney
tumors (11,12). PODXL has been shown to lead to
increased cell invasion and MMP expression in breast and prostate
cancer cells (13). Furthermore,
PODXL expression has been detected in 42.9% of anaplastic
astrocytoma samples and 54.8% of glioblastoma samples, suggesting
that PODXL is associated with the malignant progression of
astrocytoma (14). A recent study
showed that PODXL promotes cell invasion through the upregulation
of MMP-9 expression in human astrocytoma cells (15), indicating that PODXL also has an
important role in astrocytoma invasion.
In the present study, the association between SPAG9
and PODXL in human astrocytoma invasion and the underlying
mechanisms involved were investigated for the first time, to the
best of our knowledge.
Materials and methods
Cells lines, plasmids and reagents
SW1783 and U87 human astrocytoma cell lines were
purchased from the American Type Culture Collection (Rockville, MD,
USA). Human full-length SPAG9 and PODXL cDNAs were
subcloned into pcDNA 3.1 expression vectors, respectively. Human
PODXL promoter-luciferase reporter construct was generated
as previously described (16).
Briefly, a 1,528-bp DNA fragment of the 5′ regulatory region of the
human PODXL gene, comprising 1,297 bp upstream from the
transcription start site plus 231 bp of 5′-untranslated region, was
amplified and inserted upstream of the luciferase gene. Human
PODXL short hairpin RNA (shRNA) plasmid (RHS3979-98487921)
and pLKO.1 empty plasmid (RHS4080) were purchased from Open
Biosystems (Huntsville, AL, USA). Anti-PODXL (3D3) (39-3800)
antibody was purchased from Invitrogen Life Technologies (Carlsbad,
CA, USA). Human JIP-4/SPAG9-shRNA lentiviral particles
(sc-62513-V), control shRNA lentiviral particles-A (sc-108080) and
anti-MMP-9 (sc-13520) and anti-JIP-4/SPAG9 (sc-67649) antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). SuperFect™ transfection reagent was purchased from Qiagen
(Valencia, CA, USA). A dual-luciferase reporter assay system was
obtained from Promega Corporation (Madison, WI, USA). The selective
JNK inhibitor SP600125, the JNK agonist anisomycin, puromycin, G418
and all chemicals of reagent grade were purchased from Sigma (St.
Louis, MO, USA).
Transfection and lentiviral
transduction
The SPAG9 and PODXL expression constructs were
transfected into cells using SuperFect™ transfection reagent
(Qiagen) in accordance with the manufacturer’s instructions. Pools
of stable transductants were generated via selection with G418 (800
μg/ml). Lentiviral transduction was performed and pools of stable
transductants were generated via selection with puromycin (5
μg/ml).
Western blot analysis
Immunoblotting was performed using the respective
antibodies. Briefly, cells were dissolved in 250 μl 2× SDS loading
buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 25% glycerol, 0.01%
bromophenol blue and 5% 2-mercaptoethanol) and incubated at 95°C
for 10 min. Equal quantities of proteins for each sample were
separated using 10% SDS-PAGE and blotted onto a polyvinylidene
difluoride microporous membrane (Millipore, Billerica, MA, USA).
Membranes were incubated for 1 h with a 1/1,000 dilution of primary
antibody (1/10,000 for 3D3 PODXL blotting) and then washed and
revealed using secondary antibodies with horseradish peroxidase
conjugate (1/5,000; 1 h; Santa Cruz Biotechnology, Inc.).
Peroxidase was revealed using the GE Healthcare enhanced
chemiluminescence kit (Piscataway, NJ, USA). Proteins were
quantified prior to being loaded onto the gel. Expression of TIMP-1
and TIMP-2 was also examined with western blot analysis using
antibodies (sc-365905 for TIMP-1 and sc-6835 for TIMP-2) from Santa
Cruz Biotechnology, Inc.
Quantitative transcription polymerase
chain reaction (qPCR)
RNA was prepared from cells using TRIzol®
reagent followed by purification with TURBO DNA-free system
(Ambion, Austin, TX, USA). The cDNAs were synthesized using
SuperScript II reverse transcriptase (Invitrogen Life
Technologies). qPCR was performed on the LightCycler thermal cycler
system (Roche Diagnostics, Indianapolis, IN, USA) using a
SYBR-Green I kit (Roche Diagnostics) in accordance with the
manufacturer’s instructions. The results were normalized against
the housekeeping gene GAPDH in the same sample. The primers
used were as follows: PODXL, 5′-AATTCCTTTCCCAGTTGT-3′
(forward) and 5′-TTCTCA GTAAATTCCAGTGTA-3′ (reverse); GAPDH,
5′-GACTCA TGACCACAGTCCATGC-3′ (forward) and 5′-AGAGGC
AGGGATGATGTTCTG-3′ (reverse). Each experiment was repeated two
times and performed in triplicate.
Luciferase assay
SW1783 and U87 cells were transfected with human
PODXL promoter-luciferase reporter constructs using
SuperFect™ transfection reagent (Qiagen). The plasmid pRL-CMV
encoding Renilla reniformis luciferase (at 1/5 molar ratio
to test plasmids) was co-transfected with test plasmids in each
transfection as an internal control for data normalization.
Luciferase assays were performed using a dual-luciferase reporter
assay system (Promega Corporation) in accordance with the
manufacturer’s instructions. Each experiment was repeated three
times and performed in duplicate.
In vitro cell invasion assay
Transwell® cell-culture chambers with
8-μm pore size (BD Biosciences, Bedford, MA, USA) for 24-well
plates were coated with 50 μl Matrigel™ (10 mg/ml; BD Biosciences),
which was diluted 1:3 in RPMI-1640. SW1783 and U87 cells were
seeded in the upper chamber at 5×105 cells/well in
RPMI-1640 serum-free medium. Complete medium (600 ml) was added to
the lower chamber. Cells were allowed to migrate for 24 h followed
by fixation and staining with crystal violet. Invasion cells were
counted in 10 random fields/chamber under the microscope. Each
experiment was repeated three times and performed in
triplicate.
Statistical analysis
Statistical analysis was performed using SPSS for
Windows 10.0 (SPSS, Inc., Chicago, IL, USA). Data values are
expressed as the mean ± standard deviation. Comparisons of the
means among multiple groups were performed using a one-way analysis
of variance followed by post hoc pairwise comparisons using Tukey’s
tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of the overexpression and
knockdown of SPAG9 on PODXL expression in human astrocytoma
cells
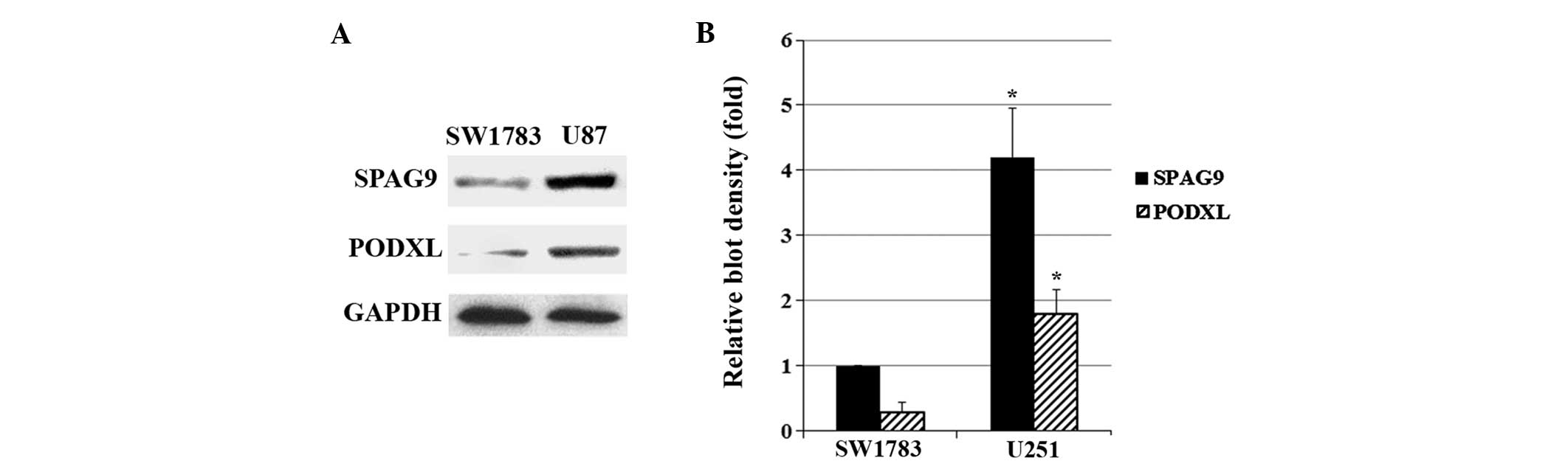
As shown in Fig. 1,
SW1783 (grade III astrocytoma) cells exhibited a relatively low
constitutive expression of SPAG9 and PODXL compared with U87 (grade
IV astrocytoma; glioblastoma) cells. Western blot analysis showed
that stable transfection of SPAG9 led to SPAG9
overexpression in SW1783 cells, which was not affected by the JNK
inhibitor SP600125 (5 μM). However, knockdown of SPAG9 by
shRNA resulted in >75% decrease in endogenous SPAG9 protein
expression in U87 cells, which was not affected by the JNK agonist
anisomycin (25 ng/ml) (Fig. 2).
PODXL protein expression in SW1783 cells increased in parallel with
SPAG9 overexpression, and this was inhibited by SP600125. In
U87 cells, PODXL protein expression decreased in parallel with
SPAG9-knockdown, and this was rescued by anisomycin
(Fig. 3). Similar data trends were
observed with PODXL mRNA expression in SW1783 and U87 cells
(Fig. 3).
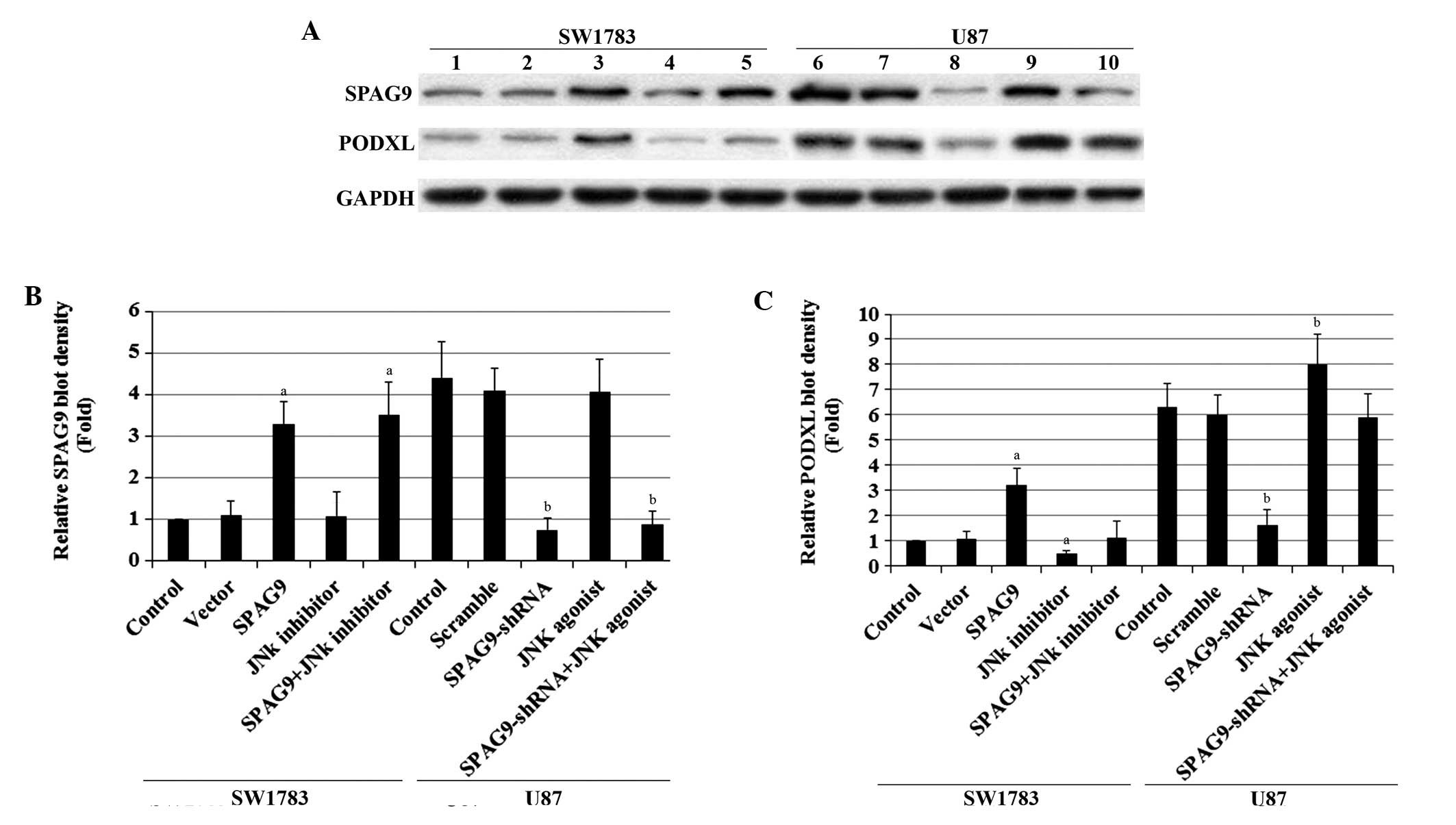 | Figure 2SPAG9 and podocalyxin PODXL expression
in astrocytoma cells with overexpression and knockdown of
SPAG9. (A) In SW1783 cells, expression of SPAG9 in SW1783
control cells, cells stably transfected with empty pcDNA3 vector
(Vector) and cells stably transfected with SPAG9 with or
without JNK inhibitor SP600125 treatment (5 μM, 24 h) was analyzed
using western blotting (lane 1, Control; lane 2, Vector; lane 3,
SPAG9; lane 4, JNK inhibitor; lane 5, SPAG9+JNK inhibitor). In U87
cells, the PODXL protein level in the U87 control cells, cells
stably transduced with scramble control shRNA (Scramble) and cells
stably transduced with SPAG9-shRNA with or without JNK
agonist anisomycin treatment (25 ng/ml, 24 h) was analyzed with
western blot analysis (lane 6, Control; lane 7, Scramble; lane 8,
SPAG9-shRNA; lane 9, JNK agonist; lane 10,
SPAG9-shRNA+JNK agonist). GAPDH blotting was used as a
loading control. Density of the (B) SPAG9 and (C) PODXL blots was
normalized against GAPDH to obtain the relative blot density,
expressed as fold-change to the relative (B) SPAG9 or (C) PODXL
blot density of SW1783 control cells (designated as 1). In SW1783
cells, aP<0.05 compared with Control and Vector. In
U87 cells, bP<0.05 compared with Control and
Scramble. SPAG9, sperm-associated antigen 9; PODXL, podocalyxin;
JNK, c-Jun N-terminal kinase; shRNA, short hairpin RNA. |
 | Figure 3PODXL mRNA levels in
astrocytoma cells with overexpression and knockdown of
SPAG9. In SW1783 cells, the PODXL mRNA levels in
SW1783 control cells, cells stably transfected with empty pcDNA3
vector (Vector) and cells stably transfected with SPAG9 with
or without JNK inhibitor SP600125 treatment (5 μM, 24 h) were
analyzed using qPCR. In U87 cells, the PODXL mRNA levels in
the U87 control cells, cells stably transduced with scramble
control shRNA (Scramble) and cells stably transduced with
SPAG9-shRNA with or without JNK agonist anisomycin treatment
(25 ng/ml, 24 h) were analyzed using qPCR. PODXL mRNA levels
are shown as fold-changes compared with SW1783 control cells
(designated as 1). In SW1783 cells, aP<0.05 compared
with Control and Vector. In U87 cells, bP<0.05
compared with Control and Scramble. SPAG9, sperm-associated antigen
9; PODXL, podocalyxin; JNK, c-Jun N-terminal kinase; shRNA, short
hairpin RNA; qPCR, quantitative polymerase chain reaction. |
Effect of the overexpression and
knockdown of SPAG9 on PODXL promoter activity in human astrocytoma
cells
To determine whether SPAG9 regulates PODXL
expression in human astrocytoma cells by altering the PODXL
gene promoter activity, SW1783 and U87 cells were transfected with
human PODXL promoter-luciferase reporter plasmids.
Luciferase assays showed that the PODXL gene promoter
activity in SW1783 cells was increased by SPAG9
overexpression, and this effect was inhibited by SP600125 (5 μM).
In U87 cells, the PODXL gene promoter activity was decreased
by SPAG9-knockdown, while activity was completely restored
by anisomycin (25 ng/ml) (Fig.
4).
 | Figure 4Effect of SPAG9 on human
PODXL promoter activity. SW1783 and U87 cells were
transfected with human PODXL promoter-luciferase reporter
plasmids. After 24 h, luciferase assays were performed. In SW1783
cells, luciferase activities in the SW1783 control cells, cells
stably transfected with empty pcDNA3 vector (Vector) and cells
stably transfected with SPAG9 with or without JNK inhibitor
SP600125 treatment (5 μM, 24 h) were analyzed. In U87 cells,
luciferase activities in the U87 control cells, cells stably
transduced with scramble control shRNA (Scramble) and cells stably
transduced with SPAG9-shRNA with or without JNK agonist
anisomycin treatment (25 ng/ml, 24 h) were analyzed. Luciferase
activities are expressed as fold-changes compared with SW1783
control cells (designated as 1). In SW1783 cells,
aP<0.05, compared with Control and Vector. In U87
cells, bP<0.05, compared with Control and Scramble.
SPAG9, sperm-associated antigen 9; PODXL, podocalyxin; JNK, c-Jun
N-terminal kinase; shRNA, short hairpin RNA. |
Functional role of PODXL in
SPAG9-enhanced cell invasion and MMP-9 expression in human
astrocytoma cells
SPAG9 and PODXL have been suggested to promote cell
invasion through the upregulation of MMP-9 expression in human
astrocytoma cells (10,15). Since the above findings showed that
SPAG9 expression may affect PODXL expression in human astrocytoma
cells, the functional role of PODXL in SPAG9-enhanced cell invasion
and MMP-9 expression in astrocytoma cells was then examined. In
vitro cell invasion assays showed that SPAG9
overexpression significantly increased cell invasion in SW1783
cells, and that this effect was reversed by PODXL-knockdown
(Fig. 5). In U87 cells, knockdown
of SPAG9 markedly decreased cell invasion, and this decrease
was completely restored by overexpression of PODXL. Similar
data trends were observed with MMP-9 protein expression in SW1783
and U87 cells (Fig. 6). By
contrast, the expression of tissue inhibitor of metalloproteinase 1
(TIMP1) and TIMP2 was not changed by overexpression or knockdown of
either SPAG9 or PODXL (data not shown).
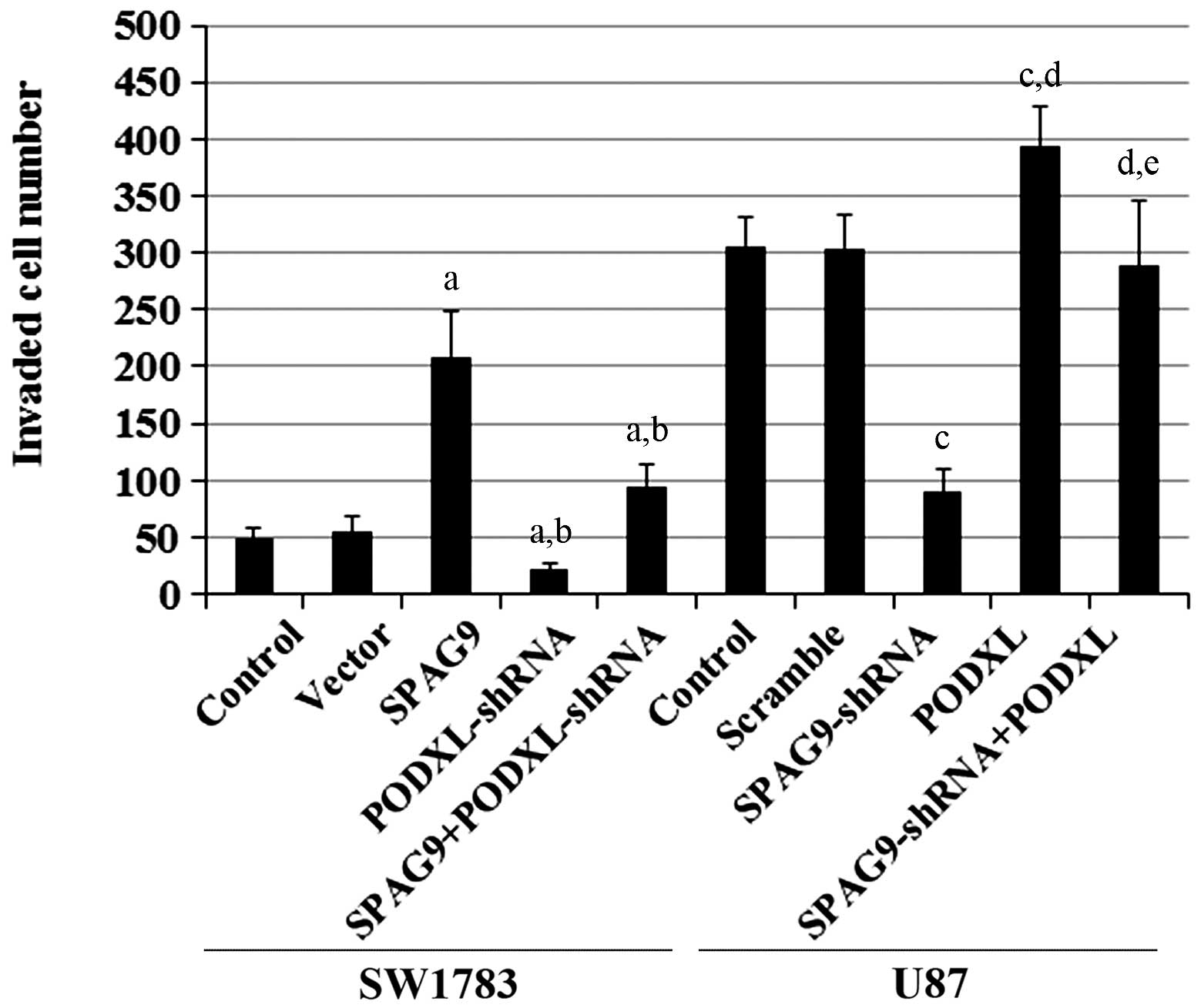 | Figure 5In vitro cell invasion in
astrocytoma cells with overexpression and knockdown of SPAG9
and/or PODXL. In SW1783 cells, in vitro cell invasion
assays were performed in SW1783 control cells, cells stably
transfected with empty pcDNA3 vector (Vector), cells stably
transfected with SPAG9, cells stably transduced with
PODXL-shRNA and cells stably expressing SPAG9 and
PODXL-shRNA. In U87 cells, in vitro cell invasion
assays were performed in U87 control cells, cells stably transduced
with scramble control shRNA (Scramble), cells stably transduced
with SPAG-shRNA, cells stably transfected with PODXL
and cells stably expressing SPAG9-shRNA and PODXL.
Invaded cell numbers were counted. In SW1783 cells,
aP<0.05 compared with Control and Vector;
bP<0.05 compared with SPAG9. In U87 cells,
cP<0.05 compared with Control and Scramble;
dP<0.05 compared with SPAG9-shRNA;
eP<0.05 compared with PODXL. SPAG9, sperm-associated
antigen 9; PODXL, podocalyxin; shRNA, short hairpin RNA. |
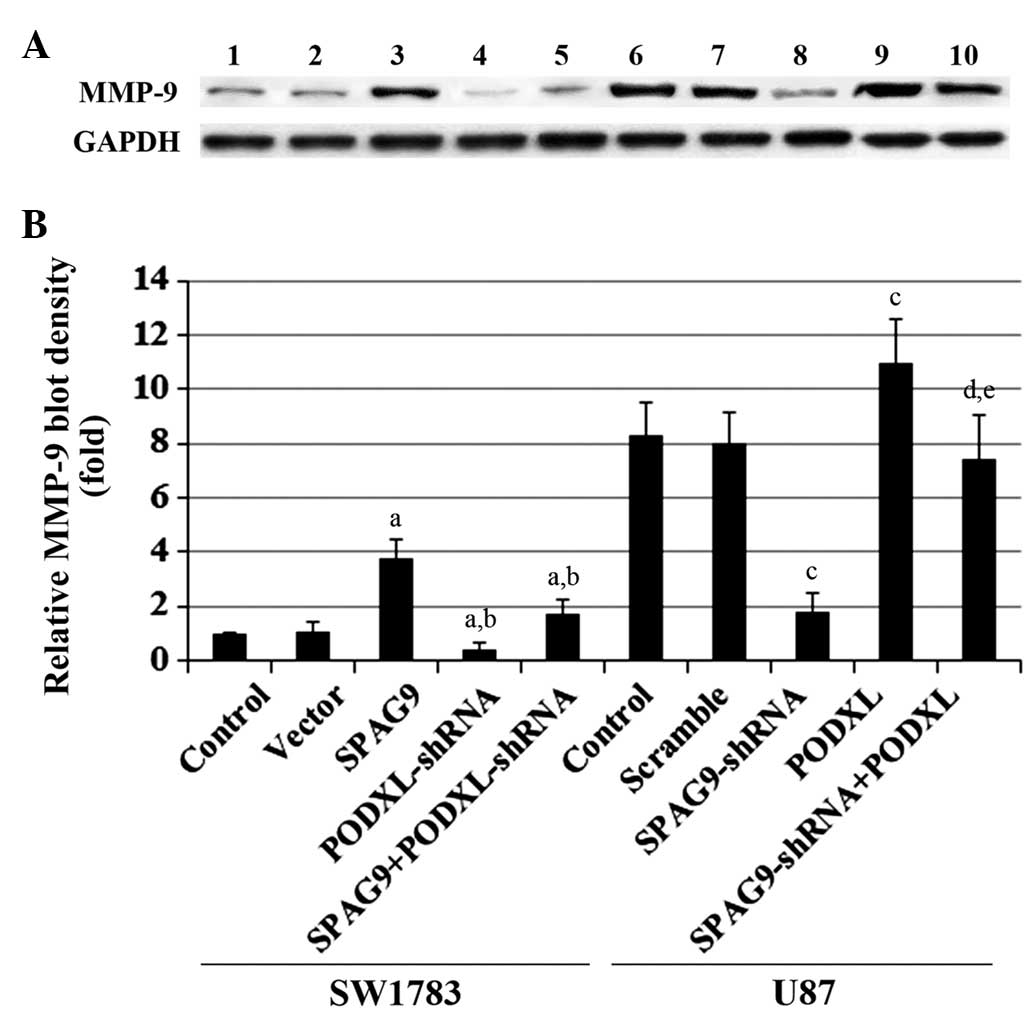 | Figure 6MMP-9 expression in astrocytoma cells
with overexpression and knockdown of SPAG9 and/or
PODXL. (A) In SW1783 cells, MMP-9 expression was determined
in SW1783 control cells, cells stably transfected with empty pcDNA3
vector (Vector), cells stably transfected with SPAG9, cells
stably transduced with PODXL-shRNA and cells stably
expressing SPAG9 and PODXL-shRNA (lanes 1–5,
respectively). In U87 cells, MMP-9 expression was determined in U87
control cells, cells stably transduced with scramble control shRNA
(Scramble), cells stably transduced with SPAG-shRNA, cells
stably transfected with PODXL and cells stably expressing
both SPAG9-shRNA and PODXL (lanes 6–10,
respectively). GAPDH was used as a loading control. (B) Density of
the MMP-9 blot was normalized against GAPDH to obtain the relative
MMP-9 expression, which is expressed as fold-change to the relative
MMP-9 blot density of SW1783 control cells (designated as 1). In
SW1783 cells, aP<0.05 compared with Control and
Vector; bP<0.05 compared with SPAG9. In U87 cells,
cP<0.05 compared with Control and Scramble;
dP<0.05 compared with SPAG9-shRNA;
eP<0.05 compared with PODXL. MMP-9, matrix
metalloproteinase-9; SPAG9, sperm-associated antigen 9; PODXL,
podocalyxin; JNK, c-Jun N-terminal kinase; shRNA, short hairpin
RNA. |
Discussion
PODXL has been found to increase the aggressive
phenotype of a number of cancers, including astrocytoma (15). In addition, the progression of
astrocytoma, as well as that of a number of other human
malignancies, has been associated with SPAG9, a recently
characterized oncoprotein (10).
The present study provides the first evidence, to the best of our
knowledge, that SPAG9 upregulates PODXL expression in human
astrocytoma cells and that a major part of the promoting effect of
SPAG9 on astrocytoma cell invasion is mediated by PODXL, most
likely through the upregulation of MMP-9 expression.
SPAG9 is overexpressed in 60% of human astrocytomas
and is associated with higher tumor grades. In the present study,
several human astrocytoma cell lines were assessed and it was found
that, while high levels of SPAG9 were expressed in U87 cells, SPAG9
was expressed at a relatively low constitutive level in SW1783
cells. Thus, knockdown and overexpression of SPAG9 were
respectively performed in the two cell lines in the context of the
study aims.
SPAG9 has been suggested to be involved in the
activation of the JNK pathway, and JNK signaling is considered to
be involved in SPAG9-enhanced tumor cell invasion (10). In the present study, PODXL
expression at the mRNA and protein levels was significantly
increased and decreased in parallel with the overexpression and
knockdown, respectively, of SPAG9 in astrocytoma cells, and
these effects were blocked by the selective JNK inhibitor and
restored by the JNK agonist, respectively. The results suggest that
SPAG9 expression may affect PODXL expression in human astrocytoma
cells at the gene transcription level through a JNK-dependent
mechanism. Luciferase assays confirmed that SPAG9 may enhance
PODXL gene promoter/transcriptional activities in
astrocytoma cells through a JNK-dependent mechanism. However, how
SPAG9 modulates PODXL promoter activities has yet to be
elucidated and requires further analysis.
It has been shown that SPAG9 in human astrocytoma
cells promotes cell invasion, possibly through the regulation of
MMP-9 transcription (10). A
recent study has shown that PODXL promotes cell invasion through
upregulating MMP-9 expression in human astrocytoma cells (15). This suggests that the interaction
between SPAG9 and PODXL may be important in promoting astrocytoma
cell invasion. The results from the present study suggested that
PODXL is a downstream effector of SPAG9/JNK signaling; therefore,
the functional role of PODXL in SPAG9-enhanced astrocytoma cell
invasion and MMP-9 expression was investigated. It was found that
PODXL-knockdown almost eliminated the increased cell
invasion and MMP-9 expression caused by SPAG9 overexpression
in SW1783 cells, while PODXL overexpression completely
restored the decreased cell invasion and MMP-9 expression caused by
SPAG9-knockdown in U87 cells. The results indicate that
PODXL is a critical mediator of the promoting effect of SPAG9 on
astrocytoma cell invasion, most likely through the upregulation of
MMP-9 expression. Consistent with a previous study (15), TIMP1 and TIMP2 were not found to be
involved in the SPAG9/PODXL/astrocytoma cell invasion process.
In conclusion, it was demonstrated in the present
study that SPAG9 upregulates PODXL expression in human astrocytoma
cells at the PODXL gene promoter/transcriptional level
through a JNK-dependent mechanism and that PODXL is a critical
mediator of the promoting effect of SPAG9 on astrocytoma cell
invasion, most likely through the upregulation of MMP-9 expression.
This study provides novel insights into the molecular mechanisms of
astrocytoma invasion.
Acknowledgements
This study was supported by Hunan Provincial Natural
Science Foundation (grant nos. 12H5438 and 13A1116), Hunan,
China.
References
|
1
|
Ohgaki H and Kleihues P: Genetic
alterations and signaling pathways in the evolution of gliomas.
Cancer Sci. 100:2235–2241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Ohgaki H, Wiestler OD, et al:
The 2007 WHO classification of tumours of the central nervous
system. Acta Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giese A, Bjerkvig R, Berens ME and
Westphal M: Cost of migration: invasion of malignant gliomas and
implications for treatment. J Clin Oncol. 21:1624–1636. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ando K, Uemura K, Kuzuya A, et al:
N-cadherin regulates p38 MAPK signaling via association with
JNK-associated leucine zipper protein: implications for
neurodegeneration in Alzheimer disease. J Biol Chem. 286:7619–7628.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanojia D, Garg M, Gupta S, Gupta A and
Suri A: Sperm-associated antigen 9, a novel biomarker for early
detection of breast cancer. Cancer Epidemiol Biomarkers Prev.
18:630–639. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garg M, Kanojia D, Suri S and Suri A:
Small interfering RNAmediated down-regulation of SPAG9 inhibits
cervical tumor growth. Cancer. 115:5688–5699. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garg M, Kanojia D, Salhan S, et al:
Sperm-associated antigen 9 is a biomarker for early cervical
carcinoma. Cancer. 115:2671–2683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garg M, Kanojia D, Suri S, Gupta S, Gupta
A and Suri A: Sperm-associated antigen 9: a novel diagnostic marker
for thyroid cancer. J Clin Endocrinol Metab. 94:4613–4618. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanojia D, Garg M, Gupta S, Gupta A and
Suri A: Sperm-associated antigen 9 is a novel biomarker for
colorectal cancer and is involved in tumor growth and
tumorigenicity. Am J Pathol. 178:1009–1020. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yi F, Ni W, Liu W, et al: SPAG9 is
overexpressed in human astrocytoma and promotes cell proliferation
and invasion. Tumour Biol. 34:2849–2855. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nielsen JS and McNagny KM: The role of
podocalyxin in health and disease. J Am Soc Nephrol. 20:1669–1676.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Riccioni R, Calzolari A, Biffoni M, et al:
Podocalyxin is expressed in normal and leukemic monocytes. Blood
Cells Mol Dis. 37:218–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sizemore S, Cicek M, Sizemore N, Ng KP and
Casey G: Podocalyxin increases the aggressive phenotype of breast
and prostate cancer cells in vitro through its interaction with
ezrin. Cancer Res. 67:6183–6191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayatsu N, Kaneko MK, Mishima K, Nishikawa
R, Matsutani M, Price JE and Kato Y: Podocalyxin expression in
malignant astrocytic tumors. Biochem Biophys Res Commun.
374:394–398. 2008. View Article : Google Scholar
|
|
15
|
Wu H, Yang L, Liao D, Chen Y, Wang W and
Fang J: Podocalyxin regulates astrocytoma cell invasion and
survival against temozolomide. Exp Ther Med. 5:1025–1029.
2013.PubMed/NCBI
|
|
16
|
Butta N, Larrucea S, Alonso S, et al: Role
of transcription factor Sp1 and CpG methylation on the regulation
of the human podocalyxin gene promoter. BMC Mol Biol. 7:172006.
View Article : Google Scholar : PubMed/NCBI
|