Introduction
Hemangioma is a common vascular tumor that develops
during early life, with an incidence in newborns of 1–3%, which
increases to ~10% by 1 year of age (1). Hemangiomas are regarded as benign
neoplasms and are typically characterized by rapid postnatal growth
followed by slow involution. The course of disease is often
uneventful, culminating in a complete regression of the tumor.
However, a number of cases with infantile hemangioma may suffer
from complications, including ulceration, visual and airway
impairment, and residual scarring and disfigurement (2). These complications, along with the
potentially life-threatening consequences, are a definite
indication for treatment. There are several pharmacological
therapies available for patients with problematic hemangiomas,
including oral corticosteroids, interferon α, vincristine and
β-blockers (3,4).
X-chromosome inactivation pattern analysis has led
to the hypothesis that infantile hemangioma arises from the clonal
expansion of endothelial cells (ECs) (5). Drug-induced hemangioma regression can
occur as a result of the induction of apoptosis in proliferating
ECs (6,7). Apoptosis is characterized by numerous
typical morphological features, including cell shrinkage, nuclear
fragmentation, chromatin condensation and the formation of
membrane-bound apoptotic bodies (8). Apoptosis is usually contrasted to
necrosis, which is characterized by cell swelling, disruption of
cellular organelles and plasma membrane rupture. The loss of cell
membrane integrity results in the release of intracellular contents
into the surrounding tissue, consequently leading to the induction
of inflammatory responses. p53 is a pivotal transcription factor
that activates numerous genes to restrict cell growth and induce
apoptosis (9). Dai et al
(10) reported that harmine (a
β-carboline alkaloid) inhibits tumor angiogenesis through
activation of p53 signaling in ECs. Ji et al (11) demonstrated that the pro-apoptotic
effect of propranolol on hemangioma-derived ECs (HemECs) is linked
to the stimulation of p53 and the modulation of the Bcl-2 family.
These findings indicate that pharmacological activation of p53
signaling represents a promising strategy in the treatment of
hemangioma.
Pingyangmycin (also known as Bleomycin A5) is
produced by Streptomyces verticillus var. pingyangensis
n.sp., and has cytotoxic activities against a variety of
tumors (12,13). Several studies have also documented
the therapeutic benefit of pingyangmycin in infantile hemangiomas
(14,15). For example, Hou et al
(14) demonstrated that
pingyangmycin sclerotherapy for infantile hemangiomas in oral and
maxillofacial regions lead to complete resolution or pronounced
improvements in 66 consecutive patients. However, the molecular
mechanism(s) underlying the therapeutic effects of pingyangmycin
against hemangiomas is poorly understood. The present study
profiled the gene expression in HemECs treated with pingyangmycin,
using cDNA microarray technology, and aimed to identify the
important signaling pathways involved in the antitumor activity of
pingyangmycin.
Materials and methods
Isolation and culture of HemECs
The present study was approved by the Ethical
Committee of Xi’an Jiaotong University (Xi’an, Shaanxi, China).
HemECs were previously isolated from a proliferating infantile
hemangioma (16) that was resected
at the Department of Pediatric Surgery, Second Hospital of Xi’an
Jiaotong University. Written informed consent was obatined from the
patient. The cells were cultured in M199 medium supplemented with
10% fetal bovine serum, 100 μg/ml streptomycin and 100 U/ml
penicillin (Invitrogen Life Technologies, Carlsbad, CA, USA) at
37°C with 5% CO2. The culture medium was changed every
two days and the confluent cells were routinely subcultured using
trypsin-EDTA solution (0.05%; Invitrogen Life Technologies). The
cells at passages 6–8 (Invitrogen Life Technologies) were used in
the present study.
Drug treatment
The HemECs were seeded at a density of
2×106 cells/well into 6-well plates. Following
incubation overnight at 37°C, the cells were left untreated as the
control, or treated with different concentrations (100 and 300
μg/ml) or 200 μg/ml (if not stated otherwise) of pingyangmycin
(Tianjin Lisheng Pharmaceutical Co., Ltd., Tianjin, China) for 16
h. The cells were harvested and examined for apoptosis and gene
expression.
Transmission electron microscopy
(TEM)
Following the treatments, the cells were prefixed
2.5% glutaraldehyde in 0.1 M phosphate buffer and postfixed in 1%
osmium tetroxide. The samples were dehydrated in an ascending
series of ethanol to 100%, embedded and cut into ultrathin sections
(50–70 nm). The sections were stained with 0.5% uranyl acetate and
saturated lead citrate and examined on an electron microscope
(H-600; Hitachi, Tokyo, Japan).
Apoptosis analysis
Following treatment, the cells were collected,
washed and subjected to apoptosis analysis using an Annexin V-FITC
kit (Trevigen, Gaithersburg, MD, USA), according to the
manufacturer’s instructions. The cells were analyzed on a FACScan
flow cytometer with CellQuest software (BD Biosciences, San Jose,
CA, USA).
cDNA microarray analysis
For microarray analysis, the Whole Human Genome
Microarray kit (4×44K; Agilent Technologies, Santa Clara, CA, USA)
was used. The sample preparation and microarray hybridization were
performed according to the manufacturer’s instructions. Briefly,
the total RNA from the HemECs with or without pingyangmycin
treatment was amplified and transcribed into fluorescent
complementary RNA (cRNA) using the Quick Amp Labeling (one color)
kit (Agilent Technologies). The Cy3-labeled cRNAs were hybridized
onto the Agilent Whole Human Genome Microarray. Following washing,
the arrays were scanned on a DNA microarray scanner (G2565BA;
Agilent Technologies).
Feature Extraction software (version 10.7.3.1;
Agilent Technologies) was used to analyze the acquired array
images. Quantile normalization and subsequent data processing were
performed using the GeneSpring GX v11.5.1 software package (Agilent
Technologies). Differentially-expressed genes were identified with
at least 2-fold changes between the control and
pingyangmycin-treated cells. Pathway and gene ontology analysis of
differentially-expressed genes was conducted.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted with TRIzol according to the
manufacturer’s instructions (Invitrogen Life Technologies). Reverse
transcription was performed using the AMV First Strand cDNA
Synthesis kit (Bio Basic, Inc., Amhurst, NY, USA). qPCR
amplification was conducted on an Applied Biosystems StepOnePlus
Real-Time PCR system (Applied Biosystems, Foster City, CA, USA)
using the SYBR Green PCR Master Mix (Applied Biosystems). The PCR
primers used are listed in Table
I. As an internal quantitative control, glyceraldehyde
3-phosphate dehydrogenase (GAPDH) was amplified in a parallel
reaction. All assays were performed in triplicate, and the
threshold cycle (Ct) was calculated. The relative mRNA expression
level normalized by that of GAPDH was determined using the
2−ΔΔCt method (17).
 | Table IPrimers used for qPCR. |
Table I
Primers used for qPCR.
| Gene | Primer sequence |
|---|
| p53 | F:
5′-GGAAATCTCACCCCATCCCA-3′
R: 5′-CAGTAAGCCAAGATCACGCC-3′ |
| PIDD | F:
5′-CATCAAGCTGCCGAGACTTC-3′
R: 5′-TGCTCATCCAGATCATCCCG-3′ |
| PIG3 | F:
5′-AGTGACCGAAATCCAGGAGG-3′
R: 5′-GCTTTAAACGGCTCTGGAGG-3′ |
| PUMA | F:
5′-CCCACCACCATCTCAGGAAA-3′
R: 5′-GTGGTCACGTTTGGCTCATT-3′ |
| MDM2 | F:
5′-TCCCAGCCTAGGTTTCAGAC-3′
R: 5′-AACACGGAGCTTGAGAGGAA-3′ |
| Bax | F:
5′-AAGAAGCTGAGCGAGTGTCT-3′
R: 5′-GTTCTGATCAGTTCCGGCAC-3′ |
| GAPDH | F:
5′-TGGGTGTGAACCATGAGAAGT-3′
R: 5′-TGAGTCCTTCCACGATACCAA-3′ |
Statistical analysis
The statistical differences between the groups were
calculated using Student’s t-test. One-way analysis of variance,
followed by Tukey’s post-hoc test, was used to examine the
differences among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Pingyangmycin induces apoptosis in
HemECs
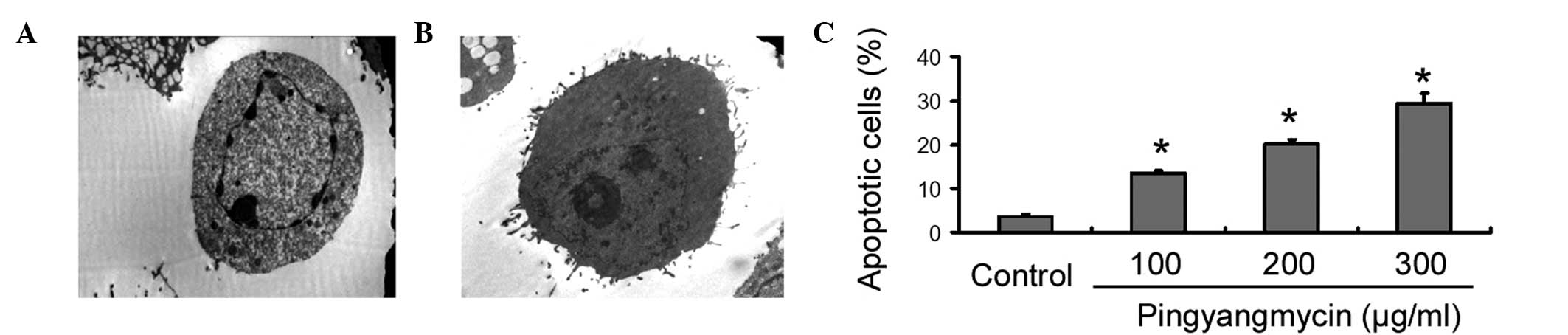
TEM examination revealed that the
pingyangmycin-treated HemECs exhibited typical apoptotic
characteristics, i.e., intact cell membranes, chromatin
condensation and the formation of apoptotic bodies (Fig. 1A). By contrast, the untreated
control cells had a normal morphology (Fig. 1B). Flow cytometric analysis further
demonstrated that the treatment with pingyangmycin for 16 h
resulted in a dose-dependent induction of apoptosis in the HemECs
(Fig. 1C). Pingyangmycin at 300
μg/ml caused an ~2-fold increase in the number of apoptotic cells
compared with pingyangmycin at 100 μg/ml (29.2±2.4 vs. 13.5±0.5%,
respectively).
Regulation of mRNA expression by
pingyangmycin
Microarray expression analysis of the
pingyangmycin-treated and untreated HemECs was performed to
determine the differentially-expressed genes. It was identified
that 4,752 genes demonstrated at least 2-fold expressional changes
at the mRNA level. Among them, 2,544 were upregulated and 2,208
were downregulated by pingyangmycin (data not shown). Pathway
analysis of the differentially-expressed genes revealed that the
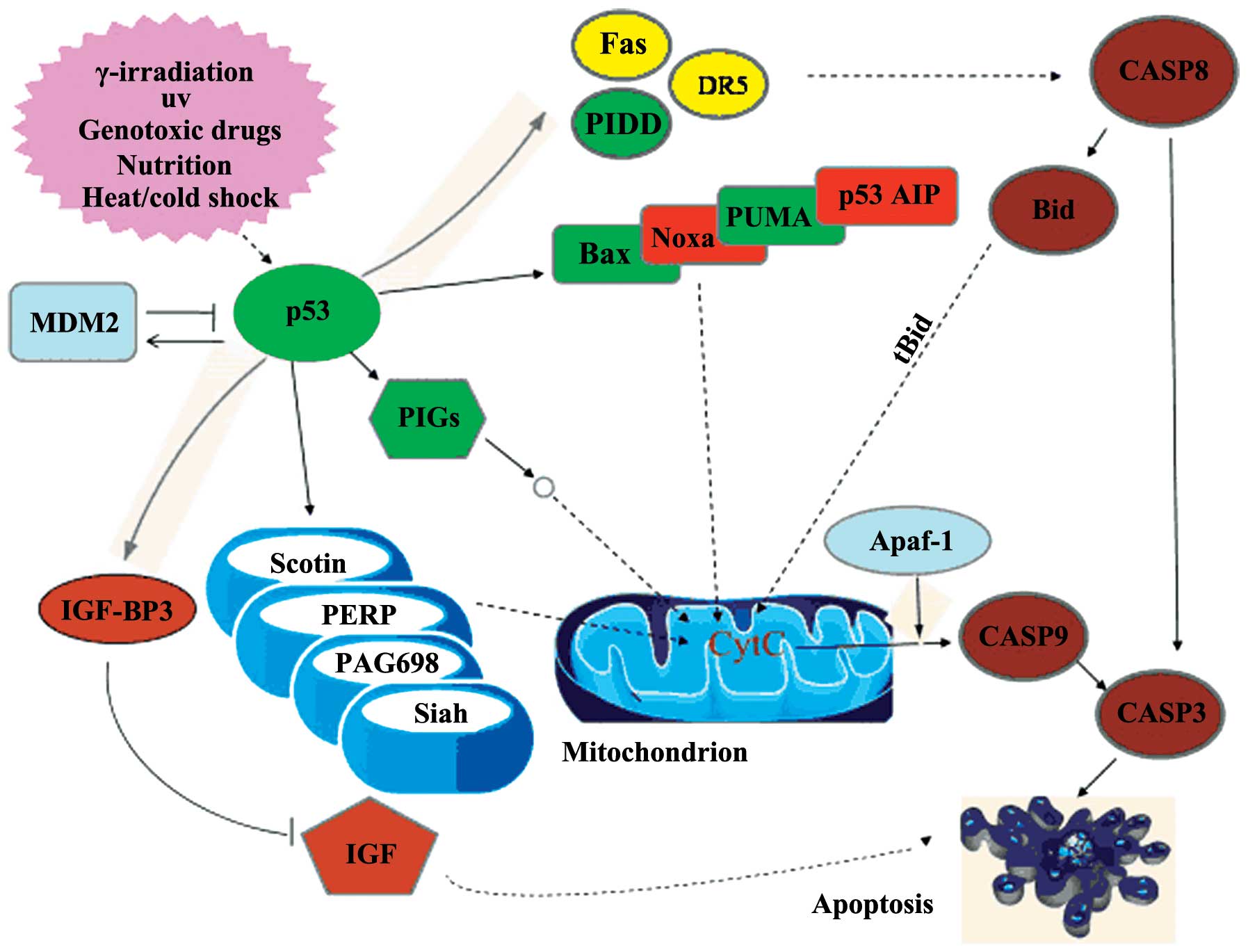
p53 pathway appeared to be a central pathway involved in the action
of pingyangmycin in the HemECs (Fig.
2).
Verification of differentially-expressed
genes using qPCR
To improve the quantitative verification of the gene
expression alterations determined by the microarray, six genes of
the p53 pathway were selected for qPCR analysis. It was identified
that pingyangmycin treatment significantly (P<0.05) raised the
mRNA abundance of p53, p53-induced protein with death domain
(PIDD), Bax, p53 upregulated modulator of apoptosis (PUMA) and p53
inducible gene 3 (PIG3), and decreased the mRNA level of murine
double minute 2 (MDM2) compared with the untreated control cells
(Fig. 3). The results from the
qPCR analysis are in agreement with the cDNA microarray data.
Discussion
Pingyangmycin has been shown to exhibit cytotoxicity
against numerous types of tumor cells, including tongue carcinoma,
sacrococcygeal chordoma and oral carcinoma cells (18,19).
The induction of apoptosis constitutes an important mechanism for
the eradication of tumor cells by therapeutic agents. Several
drugs, including propranolol (11)
and interferon α (20), have
demonstrated pro-apoptotic activity in infantile hemangiomas. The
retention of plasma membrane integrity during apoptosis prevents
the onset of an inflammatory response that can favor tumor
progression (21). Therefore,
drug-induced apoptosis may represent a preferred and superior
strategy for eradicating tumor cells. Notably, the present data
demonstrated that pingyangmycin induced significant apoptosis in
the HemECs. Furthermore, this pro-apoptotic effect was
dose-dependent. These findings provide an explanation for the
clinical benefits of pingyangmycin in infantile hemangiomas
(14,15).
As an active suicidal response, apoptosis is
characterized by nuclear condensation and fragmentation, cellular
shrinkage without the loss of plasma membrane integrity and
apoptotic body formation. The TEM examination in the present study
revealed typical apoptotic characteristics in the
pingyangmycin-treated HemECs, including shrinkage of the cytoplasm,
chromatin condensation and formation of apoptotic bodies. Gene
expression profiling studies have indicated that apoptotic
morphological changes are a consequence of the coordinated
regulation of a large number of genes (22). Consistent with this view, the
present study demonstrated that pingyangmycin treatment upregulated
2,544 genes and downregulated 2,208 genes in the HemECs. The
network pathway analysis indicated that the p53 pathway is involved
in the pro-apoptotic effects of pingyangmycin in HemECs. To
validate the microarray data, qPCR analysis was performed to
examine the expressional changes of several key components of the
p53 pathway. It was identified that pingyangmycin treatment
significantly increased the mRNA expression levels of p53, PIDD,
PIG3 and PUMA, and decreased the MDM2 mRNA level compared with the
untreated control cells. p53 is a well-established tumor suppressor
that is able to induce cell cycle arrest and apoptosis (23). Therefore, reactivation of p53
represents a promising therapeutic strategy against tumor growth.
Indeed, upregulation of p53 is causally linked to the
apoptosis-inductive effects of propranolol in HemECs (11).
The p53-dependent induction of apoptosis is largely
associated with its transcriptional regulation of numerous target
genes. PIDD is a downstream target gene of p53 and mediates
p53-dependent apoptosis through interactions with components of the
mitochondrial and death receptor signaling pathways (24). In addition to PIDD, the PUMA, PIG3
and Bax genes are another three targets of p53. Compelling evidence
indicates that PUMA and PIG3 also function as critical mediators of
p53-dependent apoptosis in a variety of cells (25,26).
PUMA may bind to the anti-apoptotic protein Bcl-2 and induce
cytochrome c release from the mitochondria, consequently
leading to the activation of caspases 9 and 3 and the induction of
caspase-dependent apoptosis. PIG3 has been indicated to be involved
in the accumulation of reactive oxygen species and the induction of
apoptosis (27). A conserved
intronic p53-response element has been identified in the promoter
of the Bax gene, which is sufficient to mediate p53-dependent
transcriptional activation (28).
In response to pro-apoptotic stimuli, Bax forms a homodimer and
releases cytochrome c from the mitochondria, consequently
resulting in caspase-9 activation (29). Yang et al (30) reported that the Bax level is
significantly higher in the ECs of involutive hemangioma than in
that of proliferating hemangioma and normal skin tissue, indicating
that this gene is involved in the involution of hemangioma through
the induction of apoptosis. By contrast, MDM2 is a major negative
regulator of p53. This protein interferes with the p53 activity in
two ways. Firstly, through the ubiquitination of p53, signaling for
its degradation by the proteasome, and secondly, through directly
binding to p53, masking its transactivation domain (23). Taken together, these data indicate
that the pro-apoptotic effect of pingyangmycin on HemECs is largely
mediated through the coordinated regulation of the p53 pathway
components, particularly p53, PIDD, PUMA, PIG3, Bax and MDM2.
However, there are certain limitations to the
present study that should be noted. Firstly, there is no direct
evidence for the role of the p53 pathway in the induction of
apoptosis by pingyangmycin. Genetic manipulation of the key p53
pathway components is valuable to determine to what extent the
activation of the p53 pathway mediates the apoptosis-inductive
effect of pingyangmycin in HemECs. Additionally, it remains unclear
whether the findings of the present study may be translated into
the in vivo setting.
In conclusion, the present study demonstrated that
pingyangmycin is capable of inducing apoptosis in HemECs, coupled
with the upregulation of p53, PIDD, PUMA, PIG3 and Bax, and the
downregulation of MDM2. These data indicate that the therapeutic
role of pingyangmycin against infantile hemangiomas is associated
with the induction of p53-dependent apoptosis.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 30700954).
References
|
1
|
Mendiratta V and Jabeen M: Infantile
hemangioma: an update. Indian J Dermatol Venereol Leprol.
76:469–475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kwon EK, Seefeldt M and Drolet BA:
Infantile hemangiomas: an update. Am J Clin Dermatol. 14:111–123.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eivazi B and Werner JA: Management of
vascular malformations and hemangiomas of the head and neck - an
update. Curr Opin Otolaryngol Head Neck Surg. 21:157–163. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Itinteang T, Withers AH, Leadbitter P, et
al: Pharmacologic therapies for infantile hemangioma: is there a
rational basis? Plast Reconstr Surg. 128:499–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bischoff J: Monoclonal expansion of
endothelial cells in hemangioma: an intrinsic defect with extrinsic
consequences? Trends Cardiovasc Med. 12:220–224. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng Q, Liu W, Zhou F, et al: An
experimental study on the therapy of infantile hemangioma with
recombinant interferon γ. Pediatr Surg. 46:496–501. 2011.PubMed/NCBI
|
|
7
|
Storch CH and Hoeger PH: Propranolol for
infantile haemangiomas: insights into the molecular mechanisms of
action. Br J Dermatol. 163:269–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ouyang L, Shi Z, Zhao S, et al: Programmed
cell death pathways in cancer: a review of apoptosis, autophagy and
programmed necrosis. Cell Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haupt S, Berger M, Goldberg Z and Haupt Y:
Apoptosis - the p53 network. J Cell Sci. 116:4077–4085. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai F, Chen Y, Song Y, et al: A natural
small molecule harmine inhibits angiogenesis and suppresses tumour
growth through activation of p53 in endothelial cells. PLoS One.
7:e521622012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji Y, Li K, Xiao X, et al: Effects of
propranolol on the proliferation and apoptosis of
hemangioma-derived endothelial cells. J Pediatr Surg. 47:2216–2223.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen P, Liu B and Hu M: The effect of
hydroxycamptothecin and pingyangmycin on human squamous cell
carcinoma of the tongue. Oncol Lett. 5:947–952. 2013.PubMed/NCBI
|
|
13
|
Gong JH, Liu XJ, Li Y and Zhen YS:
Pingyangmycin downregulates the expression of EGFR and enhances the
effects of cetuximab on esophageal cancer cells and the xenograft
in athymic mice. Cancer Chemother Pharmacol. 69:1323–1332. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hou J, Wang M, Tang H, et al:
Pingyangmycin sclerotherapy for infantile hemangiomas in oral and
maxillofacial regions: an evaluation of 66 consecutive patients.
Int J Oral Maxillofac Surg. 40:1246–1251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo QF and Zhao FY: The effects of
Bleomycin A5 on infantile maxillofacial haemangioma. Head Face Med.
7:112011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tu JB, Dong Q, Hu XY, et al: Proteomic
analysis of mitochondria from infantile hemangioma endothelial
cells treated with sodium morrhuate and its liposomal formulation.
J Biochem Mol Toxicol. 26:374–380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
18
|
Guan JY, He XF, Chen Y, et al:
Percutaneous intratumoral injection with pingyangmycin lipiodol
emulsion for the treatment of recurrent sacrococcygeal chordomas. J
Vasc Interv Radiol. 22:1216–1220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ho YC, Tai KW and Chang YC: Synergistic
effects of verapamil on pingyangmycin-induced cytotoxicity and
apoptosis in KB cells. Oral Dis. 13:40–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sgonc R, Fuerhapter C, Boeck G, et al:
Induction of apoptosis in human dermal microvascular endothelial
cells and infantile hemangiomas by interferon-alpha. Int Arch
Allergy Immunol. 117:209–214. 1998.PubMed/NCBI
|
|
21
|
Sethi G, Shanmugam MK, Ramachandran L, et
al: Multifaceted link between cancer and inflammation. Biosci Rep.
32:1–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lindgren T, Stigbrand T, Riklund K, et al:
Gene expression profiling in MOLT-4 cells during
gamma-radiation-induced apoptosis. Tumour Biol. 33:689–700. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Di J, Zhang Y and Zheng J: Reactivation of
p53 by inhibiting Mdm2 E3 ligase: a novel antitumor approach. Curr
Cancer Drug Targets. 11:987–994. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bradley G, Tremblay S, Irish J, et al: The
expression of p53-induced protein with death domain (Pidd) and
apoptosis in oral squamous cell carcinoma. Br J Cancer.
96:1425–1432. 2007.PubMed/NCBI
|
|
25
|
Nakano K and Vousden KH: PUMA, a novel
proapoptotic gene, is induced by p53. Mol Cell. 7:683–694. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nicholls CD, Shields MA, Lee PW, et al:
UV-dependent alternative splicing uncouples p53 activity and PIG3
gene function through rapid proteolytic degradation. J Biol Chem.
279:24171–24178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JH, Kang Y, Khare V, et al: The
p53-inducible gene 3 (PIG3) contributes to early cellular response
to DNA damage. Oncogene. 29:1431–1450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thornborrow EC, Patel S, Mastropietro AE,
et al: A conserved intronic response element mediates direct
p53-dependent transcriptional activation of both the human and
murine bax genes. Oncogene. 21:990–999. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haupt S, Berger M, Goldberg Z and Haupt Y:
Apoptosis - the p53 network. J Cell Sci. 116:4077–4085. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang H, Deng C, Shen S, et al: Expression
and significance of Bcl-2, Bax, Fas and caspase-3 in different
phases of human hemangioma. J Huazhong Univ Sci Technolog Med Sci.
26:402–404. 2006. View Article : Google Scholar : PubMed/NCBI
|