Introduction
Colorectal cancer, one of the most common types of
cancer in males and females, is a serious demographic and economic
problem worldwide (1). The
development and progression of colorectal cancer are regulated by a
complex cascade of molecular events, which are involved in cell
proliferation and invasion (2).
Therefore, it is of great importance to explore the molecular
mechanisms underlying colorectal carcinogenesis.
MicroRNAs (miRNAs) are a type of endogenous
non-coding RNA, which act as endogenous suppressors of translation
by directly binding to the 3′ untranslated region (UTR) of target
mRNAs (3). Recently, numerous
miRNAs have been shown to be involved in the development and
progression of colorectal cancer (4). For example, miR-133a has been
observed to suppress colorectal cancer through activating the
p53/p21 signaling pathway (5), and
the inhibition of miR-21 has been found to enhance the
susceptibility of chemoresistant colon cancer cells to therapeutic
treatment (6). Therefore, miRNAs
may be promising candidates or targets for colorectal cancer
therapy.
miR-195, a member of the miR-16/15/195/424/497
family, has been demonstrated to act as a tumor suppressor in
numerous types of malignant human tumors (7). Yang et al (8) found that miR-195 induced apoptosis in
hepatocellular carcinoma cells in vitro through targeting
LATS2 (8). Furthermore, Mao et
al (9) showed that miR-195
suppressed osteosarcoma cell invasion and migration in vitro
through downregulating FASN. Moreover, Ding et al (10) proposed that miR-195 may exert its
tumor suppressive function by downregulating numerous nuclear
factor (NF)-κB) downstream effectors through directly targeting IKK
α and TAB. In addition, miR-195 has been found to enhance the
sensitivity of cancer cells to chemotherapy, with Yang et al
(11) showing that upregulation of
miR-195 increases the sensitivity of breast cancer cells to
adriamycin through targeting Raf-1.
CARMA3, also known as CARD10, is a member of the
CARMA family, as are the other two members CARMA1 (CARD11) and
CARMA2 (CARD14). These three proteins share similar structural
motifs, including CARD, coiled-coil, PDZ, SRC homology 3 and
guanylate kinase-like domains (12). As a scaffold protein, CARMA3 has
been demonstrated to have an important role in tumorigenesis
(13). Recently, CARMA3 was found
to be overexpressed in colorectal cancer and to contribute to
colorectal cancer cell growth by promoting cell cycle progression
through NF-κB-mediated upregulation of cyclin D1 (14).
However, the specific roles and underlying molecular
mechanisms of miR-195 and CARMA3 in colorectal cancer cells are yet
to be elucidated. The present study determined the expression of
miR-195 and CARMA3 in colorectal cancer tissues and their matched
adjacent tissues. CARMA3 was identified to be a novel target of
miR-195. Moreover, the regulatory roles of CARMA3 and miR-195 in
cell proliferation, colony-formation and invasion were investigated
in colorectal cancer cells in vitro.
Materials and methods
Tissue specimen collection
The protocols used in the present study were
approved by the Ethical Committee of Central South University
(Changsha, China). Twenty colorectal cancer and matched adjacent
tissues were obtained from the Department of General Surgery, the
Third Xiangya Hospital of Central South University (Changsha,
China) between September 2012 and March 2013. Informed consent was
obtained from each patient included in this study. Each tissue
sample was immediately frozen in liquid nitrogen following surgical
removal.
Cell culture
SW480 and HT29 human colorectal cancer cell lines
were obtained from the Cell Bank of Central South University
(Changsha, China). Cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum
(FBS), 100 IU/ml penicillin and 100 μg/ml streptomycin sulfate at
37°C in a humidified incubator containing 5% CO2.
RNA extraction and quantitative
polymerase chain reaction (qPCR) analysis
Total RNA was extracted using TRIzol®
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
according to the manufacturer’s instructions. qPCR analysis was
performed to assess mRNA expression using a TaqMan®
Reverse Transcription kit and a Power SYBR® Green kit
(Thermo Fisher Scientific, Waltham, MA, USA).
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an
endogenous control. For the analysis of miRNA expression, 10 ng RNA
was converted to cDNA using the Applied Biosystems®
TaqMan® MicroRNA Reverse Transcription kit (Applied
Biosystems, Carlsbad, CA, USA). qPCR was performed using the
Applied Biosystems 7500 thermocycler (Applied Biosystems). U6
expression was used as an endogenous control. Relative mRNA and
miRNA expression were analyzed using the 2−ΔΔCt method.
The following primers were used in the qPCR analysis: Forward:
5′-CTGTGGGAGCGAATCGAGG-3′ and reverse: 5′-CAG CGCAAGATGTCCATCA-3′
for CARMA3; and forward: 5′-CTGGGCTACACTGAGCACC-3′ and reverse:
5′-AAG TGGTCGTTGAGGGCAATG-3′ for GAPDH. The primer sequences used
to detect anti-matrix metalloproteinase (MMP)-9 were as follows:
Forward: 5′-TGTACCGCTATG GTTACACTCG-3′; and reverse:
5′-GGCAGGGACAGTTGCT TCT-3′.
Western blot analysis
Tissues and cells were lysed in cold
radioimmunoprecipitation assay lysis buffer. Proteins were
separated using 10% SDS-PAGE and transferred onto polyvinylidene
difluoride (PVDF) membranes (Invitrogen Life Technologies), which
were then incubated with tris-buffered saline and Tween-20
containing 5% skimmed milk at 4°C overnight. Membranes were then
incubated with anti-CARMA3, and anti-GAPDH primary antibodies
(Abcam PLC., Cambridge, UK), at room temperature for 3 h.
Subsequent to washing with phosphate-buffered saline and Tween-20
three times, PVDF membranes was incubated for 1 h with
corresponding rabbit anti-mouse horseradish peroxidase-conjugated
secondary antibodies (Abcam PLC.). Chemiluminescent detection was
performed using an enhanced chemiluminescence kit (Pierce Chemical
Co., Rockford, IL, USA). Image-Pro Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA) was used to analyze the
relative target gene protein expression, which was normalized using
GAPDH protein expression.
Transfection
Transfection was performed using
Lipofectamine® 2000 (Invitrogen Life Technologies)
according to the manufacturer’s instructions. For the functional
analysis of miR-195, SW480 and HT29 cells were transfected with
scrambled miRNA as a negative control (NC), miR-195 mimics, or an
miR-195 inhibitor (Invitrogen Life Technologies). For the
functional analysis of CARMA3, SW480 and HT29 cells were
transfected with CARMA3-pcDNA3.1.
Dual Luciferase reporter assays
A Quick-Change® Site-Directed Mutagenesis
kit (Stratagene California, La Jolla, CA, USA) was used to generate
a mutant 3′ UTR of CARMA3. The wild type 3′ UTR of CARMA3 and the
mutant 3′ UTR of CARMA3 were then inserted into the psiCHECK™-2
Vector (Promega Corporation, Madison, WI, USA). SW480 and HT29
cells were cultured to approximately 50–60% confluence on 24-well
plates, prior to co-transfection with psiCHECK™-2-CARMA3-3′ UTR or
psiCHECK™-2-mutant CARMA3-3′ UTR vector, with or without 50 nM
miR-195, using the Cellfectin® II Reagent (Invitrogen
Life Technologies). The luciferase activity was assessed 48 h
following transfection using a Beckman Coulter LD 400 luminometer
(Beckman Coulter Inc., Fullerton, CA, USA). Renilla
luciferase activity was normalized to firefly luciferase
activity.
Cell proliferation assay
The MTT assay was used to measure cell
proliferation. At 48 h post-transfection, the transfection medium
in each well was replaced with 100 μl fresh serum-free medium
containing 0.5 g/l MTT. Subsequent to incubation at 37°C for 4 h,
the MTT medium was removed by aspiration and 50 μl
dimethylsulfoxide was added to each well. Following incubation at
37°C for a further 10 min, the optical density at 570 nm was
measured using the Bio-Tek™ ELX-800™ Absorbance Microplate reader
(Biotek, Winooski, VT, USA). This experiment was repeated three
times.
Cell invasion assay
A cell suspension containing 5×105
cells/ml was prepared using serum-free DMEM. For the cell invasion
assay, 500 μl DMEM containing 10% FBS was added to the upper
chamber. Following incubation at 37°C with 5% CO2 for 24
h, the non-invading cells and the extracellular matrix gel were
gently removed. Cells that had invaded through the membrane were
stained for 20 min, washed with water and then dried in air. Five
fields were randomly selected and the number of cells which had
invaded through the membrane were counted using a microscope
(MVX10; Olympus, Tokyo, Japan).
Statistical analysis
All data are presented as the mean ± standard
deviation of at least three independent samples. Statistical
analysis was performed using one-way analysis of variance or
Student’s t-tests using SPSS 19.0 (SPSS, Inc., IBM, Armonk, NY,
USA) software. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-195 expression is downregulated in
colorectal cancer tissues
miR-195 expression was determined in human
colorectal cancer tissues and their matched adjacent tissues. As
shown in Fig. 1, the expression of
miR-195 was found to be increased in colorectal cancer tissues
compared with their matched adjacent tissues. This finding suggests
that miR-195 upregulation may have a role in colorectal cancer.
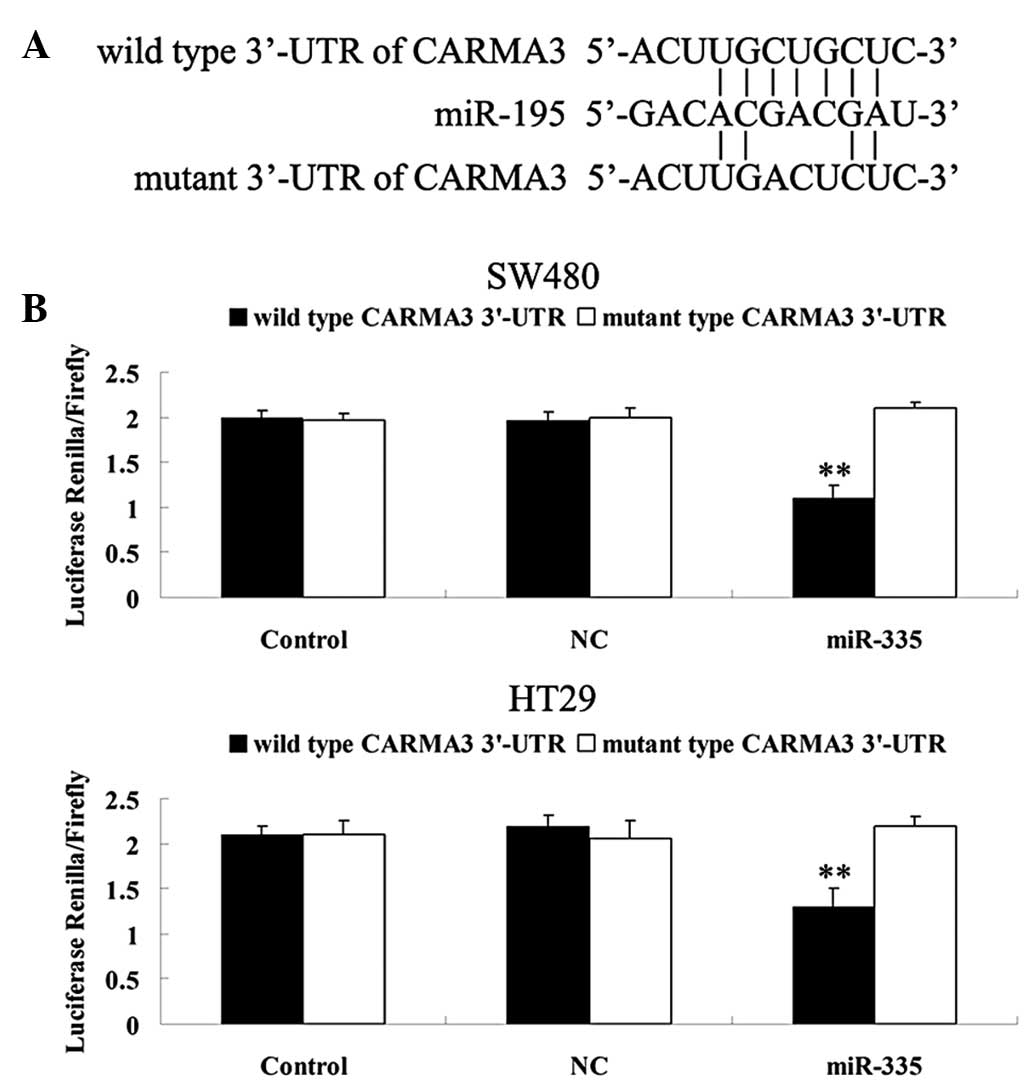
CARMA3 is a novel target of miR-195
Bioinformatic predication showed that the putative
binding site for miR-195 at the 3′ UTR of CARMA3 is highly
conserved. In the present study, a mutant CARMA3 3′ UTR was
generated (Fig. 2A). As shown in
Fig. 2B, luciferase activity was
significantly reduced following co-transfection with the wild type
CARMA3 3′ UTR and miR-195 in SW480 and HT29 cells (P<0.01).
However, the luciferase activity in other groups showed no
significant differences. These findings suggest that CARMA3 is a
novel target of miR-195 in SW480 and HT29 colorectal cancer
cells.
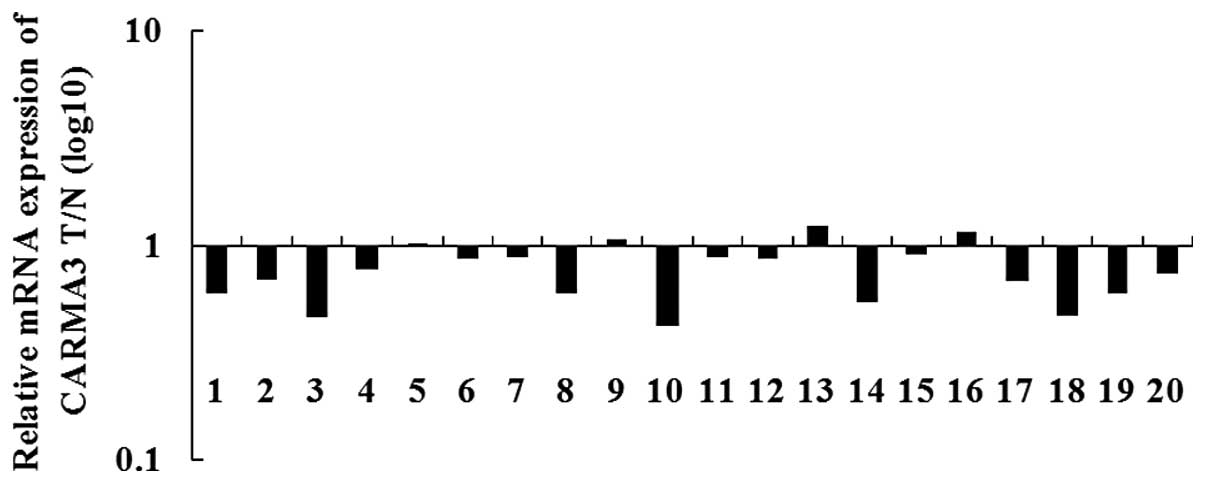
CARMA3 expression is increased in
colorectal cancer tissues
qPCR analysis was performed in order to determine
the expression of CARMA3 in human colorectal cancer and matched
adjacent normal tissues. As shown in Fig. 3, CARMA3 mRNA expression was
observed to be downregulated in colorectal cancer tissues compared
with the matched adjacent tissues. This suggests that CARMA3 may
have a suppressive role in colorectal cancer.
miR-195 negatively regulates CARMA3
protein expression in SW480 and HT29 cells
The effect of miR-195 expression on CARMA3 protein
expression in SW480 and HT29 cells was investigated. Subsequent to
the transfection of SW480 and HT29 cells with an miR-195 mimic or
inhibitor, the level of miR-195 expression was determined. As
demonstrated in Fig. 4A, compared
with the control group, miR-195 expression was significantly
upregulated and downregulated following transfection with the
miR-195 mimic and inhibitor, respectively (P<0.01). Therefore,
the transfection efficiency was condsidered satisfactory. CARMA3
protein expression was then assessed using western blot analysis.
CARMA3 protein expression was observed to be reduced in the cells
overexpressing miR-195, but increased in the cells with
downregulated miR-195 expression (Fig.
4B). These findings suggest that miR-195 negatively regulates
the protein expression of CARMA3 in colorectal cancer cells.
miR-195 downregulates colorectal cancer
cell proliferation through targeting CARMA3
To investigate the roles of CARMA3 and miR-195 in
colorectal cancer cells, SW480 and HT29 cells were either
transfected with an miR-195 mimic or inhibitor, or co-transfected
with an miR-195 mimic and CARMA3-pcDNA3.1. As shown in Fig. 5A, transfection with CARMA3-pcDNA3.1
was found to reverse the inhibitory effect of miR-195 on CARMA3
protein expression in SW480 and HT29 cells. Moreover, the cell
proliferation assay revealed that miR-195-overexpression suppressed
proliferation in colorectal cancer cells, but that downregulation
of miR-195 promoted colorectal cancer cell proliferation.
Furthermore, overexpression of CARMA3 was observed to reverse the
inhibitory effect of miR-195 on SW480 and HT29 cell proliferation
(Fig. 5B). These findings suggest
that miR-195 may have an inhibitory role in colorectal cancer cell
proliferation through directly inhibiting CARMA3 expression.
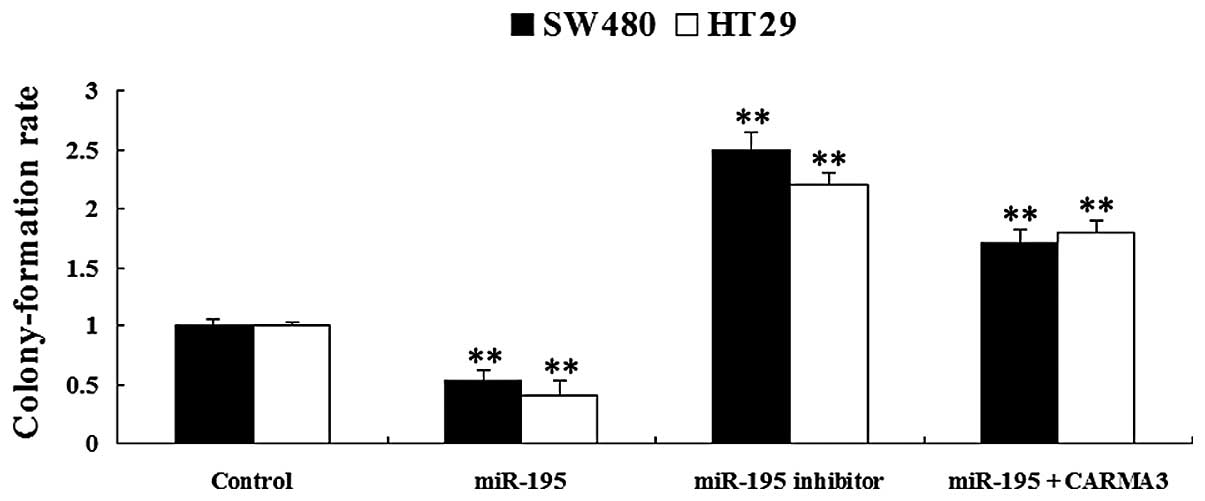
miR-195 suppresses colony formation in
colorectal cancer cells through inhibiting CARMA3
The roles of miR-195 and CARMA3 in the regulation of
colony formation in SW480 and HT29 colorectal cancer cells were
investigated. As shown in Fig. 6,
overexpression of miR-195 was found to reduce colony formation in
SW480 and HT29 cells compared with the control group. However,
downregulation of miR-195 was observed to promote colony formation
in SW480 and HT29 cells. Moreover, the suppressive effect of
miR-195 on colony formation in colorectal cancer cells was reversed
with the overexpression of CARMA3. These findings suggest that
miR-195 downregulates the malignant characteristics of colorectal
cancer cells through inhibiting CARMA3.
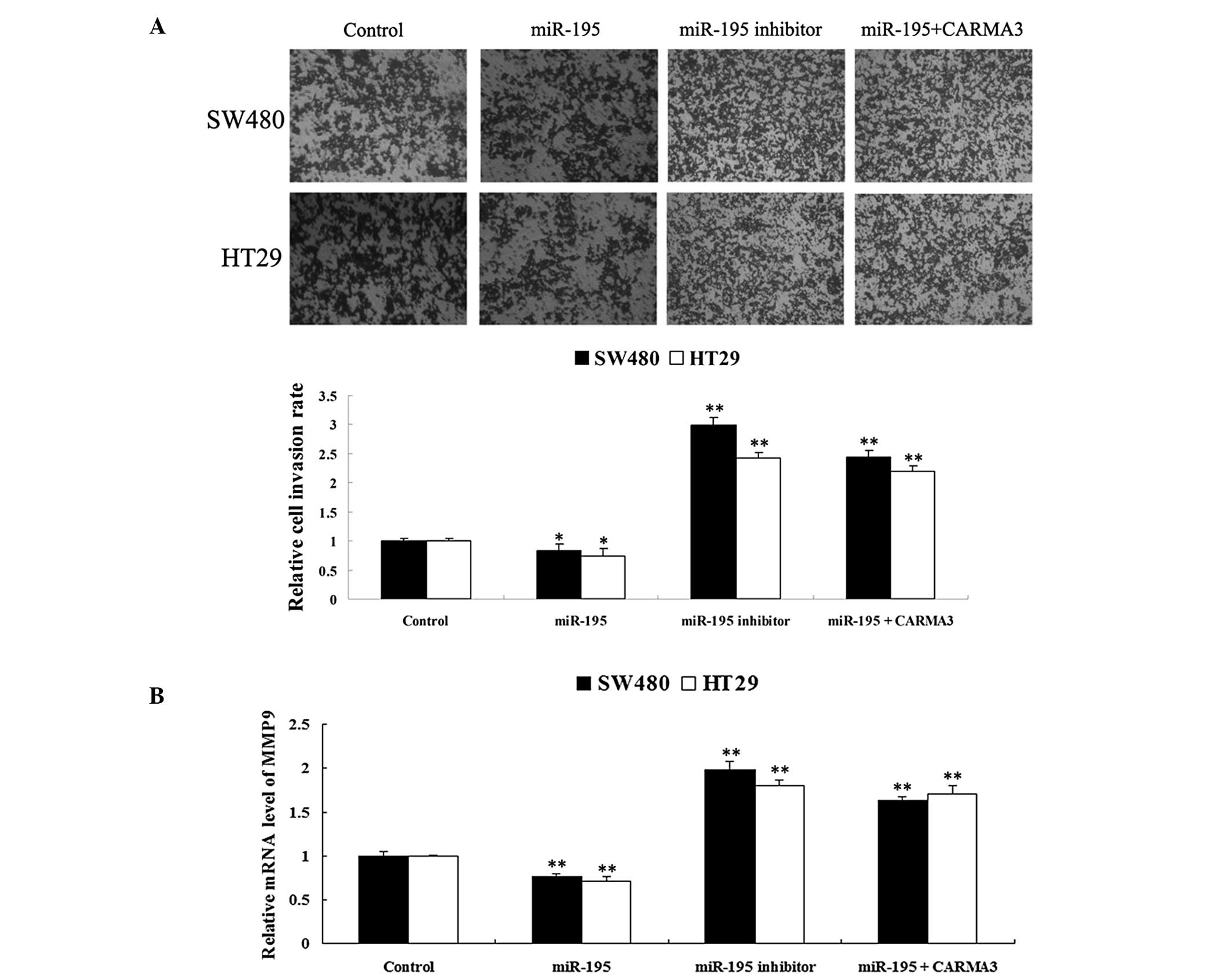
miR-195 inhibits colorectal cancer cell
invasion through targeting CARMA3
The roles of CARMA3 and miR-195 in the regulation of
invasion in SW480 and HT29 cells were investigated. As shown in
Fig. 7A, overexpression of miR-195
significantly inhibited SW480 and HT29 cell invasion, whereas
downregulation of miR-195 significantly promoted SW480 and HT29
cell invasion. Moreover, the inhibitory effect of miR-195 on cell
invasion was reversed with the overexpression of CARMA3, suggesting
that miR-195 has an inhibitory role in the regulation of colorectal
cancer cell invasion through downregulating CARMA3.
Recently, Feng et al (15) reported that CARMA3 promoted glioma
cell invasion through MMP-9, a key tumor invasion factor. Thus, to
investigate the mechanism by which miR-195 regulates colorectal
cancer cell invasion, MMP-9 mRNA expression was assessed in each
group. As shown in Fig. 7B, MMP-9
expression was significantly reduced in the SW480 and HT29 cells
overexpressing miR-195, while significantly increased in the cells
with downregulated miR-195 expression. Furthermore, CARMA3
overexpression was found to reverse the suppressive effect of
miR-195 on MMP-9 expression in SW480 and HT29 cells. These findings
suggest that the effect of miR-195 on colorectal cancer cell
invasion may involve the modulation of MMP-9 through targeting
CARMA3.
Discussion
It is well established that miRNAs have a crucial
role in the regulation of various human malignancies, due to their
capacity to regulate the expression of numerous target genes, which
are associated with tumorigenesis, tumor progression, metastasis
and sensitivity to chemotherapy (16–18).
The development and progression of colorectal cancer has been shown
to be mediated by complex molecular events, which monitor cell
proliferation and motility (19).
In the present study, miR-195 downregulation was found to correlate
with CARMA3 upregulation in colorectal cancer tissues. Furthermore,
CARMA3 was identified to be a novel target of miR-195, with miR-195
negatively regulating the protein expression of CARMA3 in
colorectal cancer cells. Functional analyses demonstrated that
miR-195 had an inhibitory effect on cell proliferation, colony
formation and invasion in colorectal cancer cells, at least in part
through targeting CARMA3.
It has been demonstrated that miR-195 is involved in
colorectal cancer (20–23). Wang et al (21) showed that miR-195 was downregulated
in colorectal cancer tissues, which is consistent with the findings
of the present study. Moreover, Wang et al (21) showed that the downregulation of
miR-195 in colorectal cancer was correlated with lymph node
metastasis and poor prognosis, indicating that miR-195 may have the
potential to be an independent marker for the predication of
clinical outcome in patients with colorectal cancer. In addition,
miR-195 has been found to promote apoptosis and suppress
tumorigenicity in human colorectal cancer cells through targeting
Bcl-2, an important anti-apoptotic gene (22). Furthermore, miR-195 has been
reported to enhance the susceptibility of colorectal cancer cells
to the chemotherapeutic drug doxorubicin through directly
inhibiting the expression of the BCL2L2(23). These findings suggest that miR-195
has an inhibitory role in colorectal cancer and may be a promising
therapeutic candidate for colorectal cancer.
A single miRNA is capable of regulating numerous
target genes; therefore, in the present study it was hypothesized
that other genes may also be involved in miR-195-mediated
biological processes in colorectal cancer cells. In order to
investigate this hypothesis, bioinformatic analyses were performed
and CARMA3 was identified to be a target of miR-195. A luciferase
reporter assay further confirmed that CARMA3 was a target of
miR-195 in colorectal cancer cells. CARMA3 has recently been shown
to have an oncogenic role in the progression of several human
malignancies (12–14,24,25).
Wu et al (24) showed that
CARMA3 was upregulated in renal cell carcinoma and that its
overexpression may be an independent prognostic indicator of
survival in patients with renal cell carcinoma. Furthermore, Li
et al (12) reported that
overexpression of CARMA3 in non-small-cell lung cancer is
associated with tumor progression. Recently, Miao et al
(14) reported that CARMA3 was
overexpressed in colorectal cancer, consistent with the findings of
the present study. Miao et al (14) also found that the overexpression of
CARMA3 in colorectal cancer was associated with
tumor-node-metastasis stage, lymph node metastasis and Ki67
proliferation index, indicating that CARMA3 may act as an oncogene
in colorectal cancer. CARMA3 was also found to enhance colorectal
cancer cell proliferation in vitro, by promoting cell cycle
progression through NF-κB-induced upregulation of cyclin D1
(14). However, the role of CARMA3
and miRNAs in the regulation of colorectal cancer is yet to be
elucidated. Therefore, in the present study, the interelation
between miR-195 and CARMA3 was investigated in colorectal cancer
cells. miR-195 overexpression was found to significantly
downregulate CARMA3 protein expression, while inhibition of miR-195
was observed to significantly promote CARMA3 protein expression in
colorectal cancer cells. These findings suggest that miR-195
negatively regulates CARMA3. Moreover, these findings suggest that
miR-195 inhibited colorectal cancer cell proliferation,
colony-formation and invasion through directly suppressing CARMA3
expression.
CARMA3 has previously been reported to be required
for G protein-coupled receptor induced NF-κB activation, which
participates in the regulation of MMP-9, a key promoter of cancer
cell invasion (26,27). Therefore, in the present study the
expression of MMP-9 was assessed. MMP-9 mRNA expression was found
to correlate with colorectal cancer cell invasion, indicating that
the inhibitory effect of miR-195 on colorectal cancer cell invasion
may at least partially be mediated through the downregulation of
MMP-9, by directly targeting CARMA3. In accordance with these
findings, Feng et al (15)
reported that CARMA3 promoted glioma cell invasion through
upregulating the expression of MMP-9.
In conclusion, the present study demonstrates that
miR-195 inhibits colorectal cancer cell proliferation, colony
formation and invasion, at least partially through targeting
CARMA3. These findings suggest that miR-195 may be a potential
candidate for the treatment of colorectal cancer.
References
|
1
|
Boncheva V, Bonney SA, Brooks SE, et al:
New targets for the immunotherapy of colon cancer-does reactive
disease hold the answer? Cancer Gene Ther. 20:157–168. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang N, Li X, Wu CW, et al: microRNA-7 is
a novel inhibitor of YY1 contributing to colorectal tumorigenesis.
Oncogene. 32:5078–5088. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dassow H and Aigner A: MicroRNAs (miRNAs)
in colorectal cancer: from aberrant expression towards therapy.
Curr Pharm Des. 19:1242–1252. 2013.PubMed/NCBI
|
|
5
|
Dong Y, Zhao J, Wu CW, et al: MiR-133a
activates the p53/p21 pathway and functions as a tumor suppressor
in colorectal cancer by repressing RFFL. Mol Cancer Res.
11:1051–1060. 2013. View Article : Google Scholar
|
|
6
|
Yu Y, Sarkar FH and Majumdar AP:
Down-regulation of miR-21 induces differentiation of chemoresistant
colon cancer cells and enhances susceptibility to therapeutic
regimens. Transl Oncol. 6:180–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He JF, Luo YM, Wan XH and Jiang D:
Biogenesis of MiRNA-195 and its role in biogenesis, the cell cycle,
and apoptosis. J Biochem Mol Toxicol. 25:404–408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang X, Yu J, Yin J, Xiang Q, Tang H and
Lei X: MiR-195 regulates cell apoptosis of human hepatocellular
carcinoma cells by targeting LATS2. Pharmazie. 67:645–651.
2012.PubMed/NCBI
|
|
9
|
Mao JH, Zhou RP, Peng AF, et al:
microRNA-195 suppresses osteosarcoma cell invasion and migration
in vitro by targeting FASN. Oncol Lett. 4:1125–1129.
2012.PubMed/NCBI
|
|
10
|
Ding J, Huang S, Wang Y, et al:
Genome-wide screening reveals that miR-195 targets the TNF-α/NF-κB
pathway by down-regulating IκB kinase alpha and TAB3 in
hepatocellular carcinoma. Hepatology. 58:654–666. 2013.PubMed/NCBI
|
|
11
|
Yang G, Wu D, Zhu J, et al: Upregulation
of miR-195 increases the sensitivity of breast cancer cells to
Adriamycin treatment through inhibition of Raf-1. Oncol Rep.
30:877–889. 2013.PubMed/NCBI
|
|
12
|
Li Z, Qu L, Dong Q, et al: Overexpression
of CARMA3 in non-small-cell lung cancer is linked for tumor
progression. PLoS One. 7:e369032012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao T, Miao Z, Wang Z, et al: CARMA3
overexpression accelerates cell proliferation and inhibits
paclitaxel-induced apoptosis through NF-κB regulation in breast
cancer cells. Tumour Biol. 34:3041–3047. 2013.PubMed/NCBI
|
|
14
|
Miao Z, Zhao T, Wang Z, et al: CARMA3 is
overexpressed in colon cancer and regulates NF-κB activity and
cyclin D1 expression. Biochem Biophys Res Commun. 425:781–787.
2012.PubMed/NCBI
|
|
15
|
Feng X, Miao G, Han Y and Xu Y: CARMA3 is
overexpressed in human glioma and promotes cell invasion through
MMP9 regulation in A172 cell line. Tumour Biol. July 27–2013.(Epub
ahead of print).
|
|
16
|
Callegari E, Elamin BK, Sabbioni S,
Gramantieri L and Negrini M: Role of microRNAs in hepatocellular
carcinoma: a clinical perspective. Onco Targets Ther. 6:1167–1178.
2013.PubMed/NCBI
|
|
17
|
Chou J, Shahi P and Werb Z:
microRNA-mediated regulation of the tumor microenvironment. Cell
Cycle. 12:3262–3271. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bienertova-Vasku J, Sana J and Slaby O:
The role of microRNAs in mitochondria in cancer. Cancer Lett.
336:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hammoud SS, Cairns BR and Jones DA:
Epigenetic regulation of colon cancer and intestinal stem cells.
Curr Opin Cell Biol. 25:177–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Piepoli A, Tavano F, Copetti M, et al:
Mirna expression profiles identify drivers in colorectal and
pancreatic cancers. PLoS One. 7:e336632012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Wang J, Ma H, Zhang J and Zhou X:
Downregulation of miR-195 correlates with lymph node metastasis and
poor prognosis in colorectal cancer. Med Oncol. 29:919–927. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu L, Chen L, Xu Y, Li R and Du X:
microRNA-195 promotes apoptosis and suppresses tumorigenicity of
human colorectal cancer cells. Biochem Biophys Res Commun.
400:236–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qu J, Zhao L, Zhang P, et al: MicroRNA-195
chemosensitizes colon cancer cells to the chemotherapeutic drug
doxorubicin by targeting the first binding site of BCL2L2 mRNA. J
Cell Physiol. March 22–2013.(Epub ahead of print).
|
|
24
|
Wu GL, Yuan JL, Huang XD, et al:
Evaluating the expression of CARMA3 as a prognostic tumor marker in
renal cell carcinoma. Tumour Biol. 34:3431–3435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rehman AO and Wang CY: CXCL12/SDF-1 alpha
activates NF-kappaB and promotes oral cancer invasion through the
Carma3/Bcl10/Malt1 complex. Int J Oral Sci. 1:105–118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun J: CARMA3: A novel scaffold protein in
regulation of NF-κB activation and diseases. World J Biol Chem.
1:353–361. 2010.
|
|
27
|
Dilly AK, Ekambaram P, Guo Y, et al:
Platelet-type 12-lipoxygenase induces MMP9 expression and cellular
invasion via activation of PI3K/Akt/NF-κB. Int J Cancer.
133:1784–1791. 2013.PubMed/NCBI
|