Introduction
Immunoglobulin A (IgA) nephropathy is also termed
chronic glomerulonephritis and is characterized by deposits of IgA
in the mesangial areas of the renal glomeruli and by recurring
hematuria with only partial proteinuria. It occurs more commonly in
young male adults. The clinical and pathological alterations of IgA
nephropathy are diverse. Patient prognosis was initially considered
to be good; however, several studies revealed that 10 years after
the incidence of IgA nephropathy, 15–40% of patients enter the
end-stage of renal failure (1,2).
Tubulointerstitial fibrosis is an important factor
affecting the development and prognosis of IgA nephropathy
(3). Previous studies have
demonstrated that renal tubular epithelial-mesenchymal transition
(EMT) was an important mechanism in tubulointerstitial fibrosis
(4,5). When EMT occurs, renal tubular
epithelial cells lose the characteristics of epithelial cells and
the expression of α-SMA and vimentin, which is usually expressed in
mesenchymal cells. The transformed cells are functionally similar
myofibroblasts (MF), which synthesize the extracellular matrix
(ECM) and contribute to the progressive development of renal
interstitial fibrosis (6,7). Apoptosis of renal tubular cells is
also known to contribute to the regulation of the renal cell number
with an induction and repair of renal injury. The apoptosis of
proximal tubular cells also occurs during renal tubular epithelial
cell-mesenchymal transformation (8). EMT and apoptosis are major mechanisms
of renal injury; however, there have been few studies investigating
the effects of EMT and apoptosis of renal tubular epithelial cells
on the prognosis of IgA nephropathy. In the present study, the
renal biopsy sample tissues with IgA nephropathy were analyzed
using immunohistochemical terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) and Masson staining. The correlation
between the transdifferentiation and apoptosis of renal tubular
epithelial cells and clinical prognosis was analyzed.
Materials and methods
Samples and patients
In total, 74 renal biopsies with IgA nephropathy
were selected, including 32 male cases and 42 female cases aged
between 14 and 51 years (average age, 27.86±9.73 years), and cases
with purpura nephritis, HBV-associated glomerulonephritis and lupus
nephritis were excluded. The histological grading was based on
Lee’s grading (9) and IgA
nephropathy was further divided into the following groups: A, the
mild mesangial proliferative group (Lee grade: level I–II, 27
cases); B, the focal hyperplasia group (Lee grade: level III, 28
cases); and C, the proliferative sclerosis group (Lee grade: level
IV–V, 19 cases). Three normal renal tissue samples far from tumors
on nephrectomy biopsies were selected as the control (9). All experiments were performed in the
Laboratory of Medical Biomechanics, Hubei University of Medicine
(Shiyan, China). The samples were pathologically confirmed by Taihe
Hospital of Hubei, University of Medicine (Shiyan, China). The
Institutional Ethics Committee of Hubei University of Medicine
(Shiyan, China) approved the project and all the patients gave
informed consent prior to participation.
Staining of collagen fibers
Collagen fibers in samples of renal needle biopsy
and normal control samples were detected by Masson trichrome
staining (Masson trichrome kit; Fuzhou Maixin Biotechnology Co.,
Ltd., Fuzhou, Fujian, China) with collagen fibers staining blue.
Under the microscope, the ratio between renal interstitial areas
with blue staining and total areas in the same visual field was
calculated.
Immunohistochemical staining
The samples were fixed in 4% paraformaldehyde and
embedded in paraffin. The sections were cut into 4-μm thick slices
from paraffin blocks. Following deparaffinization and rehydration,
the sections were boiled with citrate buffer for antigen retrieval.
Following washing, they were immersed in 3%
H2O2 in methanol to inhibit endogenous
peroxidase. Anti-α-SMA and anti-vimentin (Fuzhou Maixin
Biotechnology Co., Ltd.) were applied as primary antibodies.
Phosphate-buffered saline (PBS) was used to replace the primary
antibody in the negative controls and then the sections were
incubated at 4°C overnight. The biotin-labeled secondary antibody
and streptavidin-peroxidase were also added drop by drop, with
diaminobenzidine (DAB) color development. All the reagents were
purchased from Fuzhou Maixin Biotechnology Co., Ltd..
The Motic Med 6.0 pathological image analysis
software from Beijing University of Aeronautics and Astronautics
(Beijing, China) was selected to collect five visions
(magnification, ×400) for each sample. The integrated optical
density of tubulointerstitial α-SMA and vimentin-positive areas was
examined and the average value was obtained.
Detection of apoptotic cells by
TUNEL
The TUNEL kit purchased from Boehringer Mannheim
GMBH (Mannheim, Germany) was used to label the apoptotic cells. The
paraffin sections were processed by conventional dewaxing to water
and the proteinase K (mass concentration of proteinase K was 20
mg/l and 10 mmol/l in Tris-HCl; pH 8.0) was then added for 15–25
min of digestion at 37°C. The sections were then washed in PBS and
the methanol solution of H2O2 with the mass
concentration of 3 g/l was used for sealing, which was followed by
storing for 30 min at room temperature. Following this, the
sections were washed in PBS, TUNEL reaction solution was added, the
negative and positive controls were established and they were
incubated for 30 min at 37°C. Then, the steps including washing in
PBS, adding the conversion agent-POD and incubation for 30 min at
37°C were conducted. Following which, the sections were washed in
PBS and DAB staining was performed for 3 min. In addition,
distilled water was selected for washing and hematoxylin was
selected for re-staining. Finally, dehydration, transparency
processing and observation using a light microscope (BX51; Olympus
Optical Co., Ltd. Tokyo, Japan) were performed. The cells with
brown particles in the nucleus were the apoptotic cells.
Under a high-power microscope (magnification, ×400),
five visions were randomly collected and analyzed by pathological
image software (Motic Med 6.0; Beijing University of Aeronautics
and Astronautics), and the positive cells were counted, with the
average value of five visions as the index of cell apoptosis.
Statistical analysis
Data are shown as the mean ± standard error. The
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA) was used for
statistical analysis. Statistical analyses were performed using
analysis of variance and linear trend. Individual comparisons were
made using Fisher’s least significant difference. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical indicators
The blood pressure, serum creatinine and 24 h
urinary protein excretion of patients with IgA nephropathy in three
groups were analyzed. The clinical indices of the proliferative
sclerosis group were significantly higher than those of the mild
proliferation group and focal hyperplasia group (P<0.01). All
clinical indices of the focal hyperplasia group were higher than
those of the mild proliferation group, while the difference in 24 h
urinary protein excretion between these two groups was
statistically significant (P<0.01; Table I).
 | Table IClinical pathological features of
immunoglobulin A nephropathy. |
Table I
Clinical pathological features of
immunoglobulin A nephropathy.
| Group | Cases (n) | SBP (mm Hgc) | DBP (mm Hg) | Scr (μmol/l) | Upro (g/24 h) |
|---|
| Mild mesangial
proliferation (A) | 27 | 117.85±14.81 | 74.30±7.29 | 70.70±13.21 | 0.49±0.60 |
| Hyperplasia (B) | 28 | 124.82±16.74 | 77.93±10.28 | 95.22±25.41 | 1.70±1.60a,b |
| Proliferative
sclerosis (C) | 19 | 141.74±31.67a,b | 94.47±19.37a,b | 150.11±109.80a,b | 2.86±1.65a,b |
Pathological indices
Apoptosis
TUNEL-positive cells were distributed in tubules and
the interstitium of IgA nephropathy tissue, with single, scattered
or small cluster-like models, when those cells were rare in normal
renal tissue (Fig. 1). The
tubulointerstitial apoptotic index of IgA nephropathy in different
pathological grades increased significantly (P<0.01; Table II).
 | Table IITubulointerstitial cell apoptotic
index and expression of α-SMA,
vimentin and collagen fibers in immunoglobulin A nephropathy in
different pathological grades. |
Table II
Tubulointerstitial cell apoptotic
index and expression of α-SMA,
vimentin and collagen fibers in immunoglobulin A nephropathy in
different pathological grades.
| Group | Cases (n) | Apoptotic index | α-SMA | Vimentin | Collagen fibers |
|---|
| Mild mesangial
proliferation (A) | 27 | 18.08±5.31 | 8.43±3.09 | 9.79±3.25 | 12.51±3.46 |
| Hyperplasia (B) | 28 | 73.52±12.25a | 15.29±3.55a | 17.05±3.51a | 24.47±6.44a |
| Proliferative
sclerosis (C) | 19 | 48.79±11.06a,c | 13.13±3.66a,b | 19.50±4.13a,b | 31.16±6.43a,c |
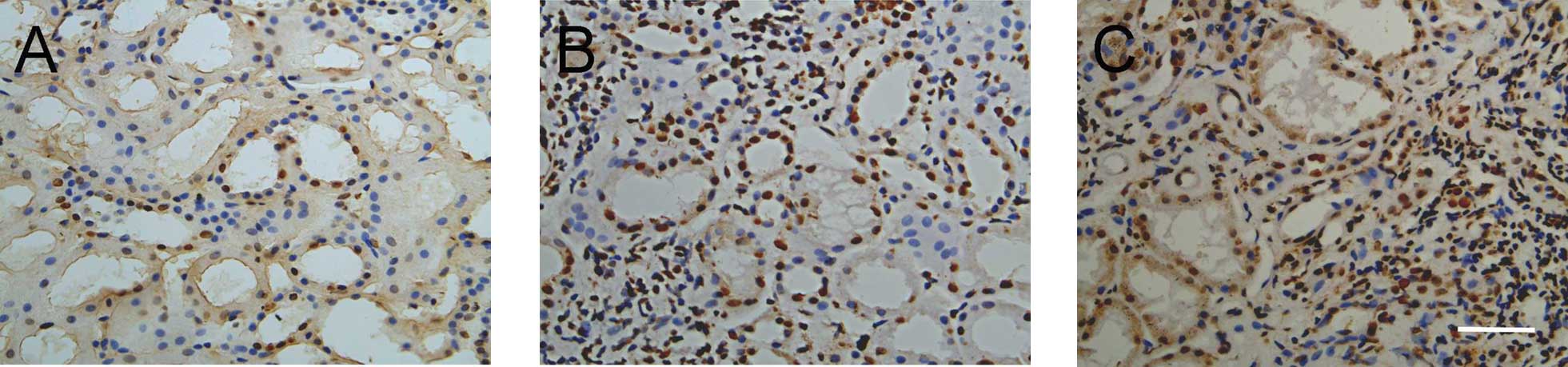
Expression and distribution of α-SMA
In normal renal tissue, α-SMA staining was positive
only in vascular smooth muscle cells and not present in glomerular
and tubulointerstitial cells. In IgA nephropathy, α-SMA expression
was increased in renal tubular epithelial cells and renal
interstitial cells (Fig. 2), and
without changes in the glomerulus. Quantitatively, the expression
of α-SMA increased in tubulointerstitial cells in hyperplasia and
the proliferative sclerosis groups compared with that in the mild
mesangial proliferation group (Table
II).
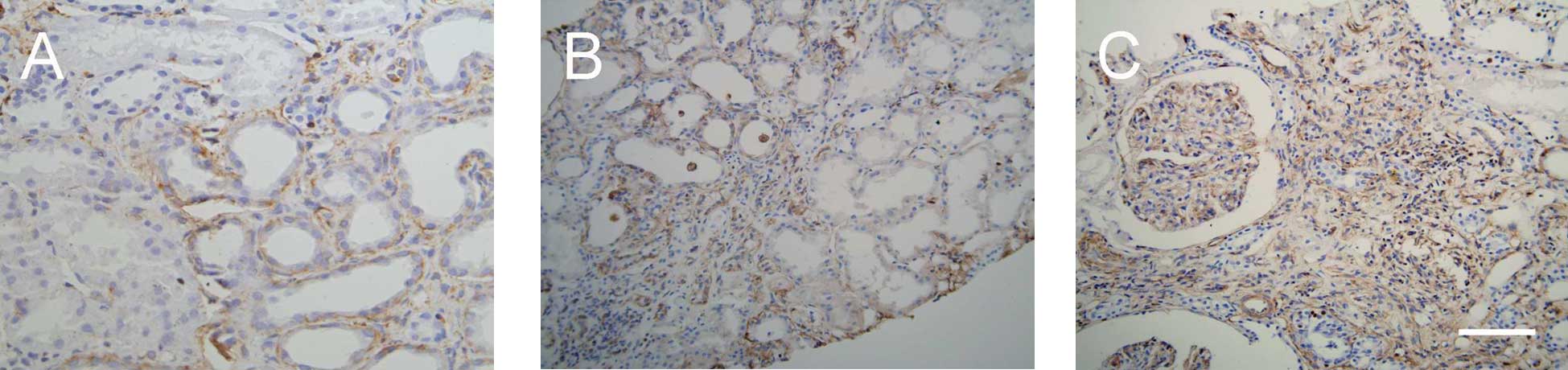
Expression and distribution of
vimentin
Vimentin was expressed in glomerular mesangial and
certain renal interstitial cells of normal renal tissue; however,
it was not expressed in renal tubular epithelial cells. In IgA
nephropathy tissue, vimentin levels were higher in the glomerulus
and expressed in renal tubular epithelial and interstitial cells
(Fig. 3). Quantitative data
demonstrated that the tubulointerstitial expression of vimentin was
different in groups with different pathological grades (P<0.05
or P<0.01; Table II).
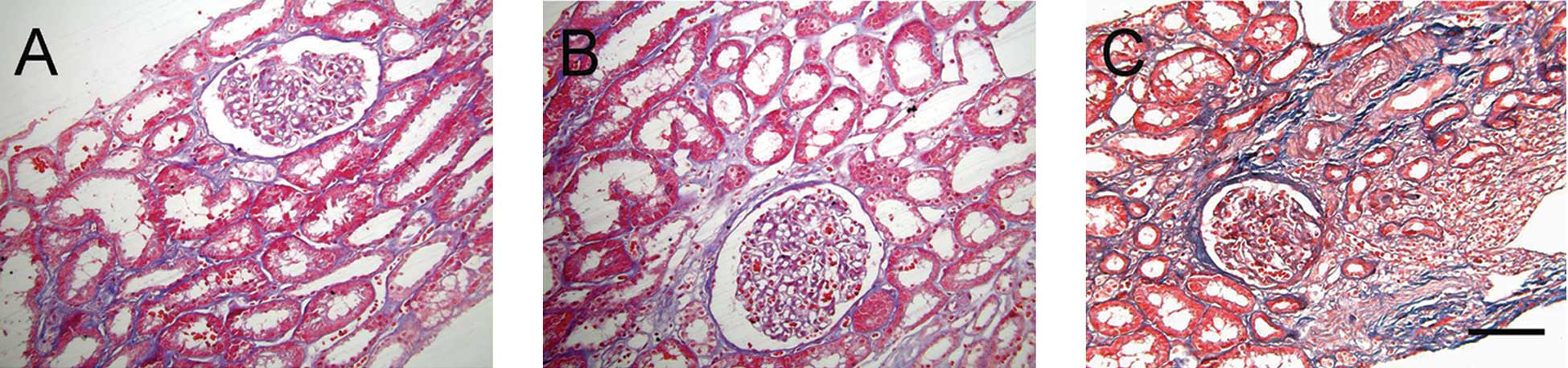
Expression of collagen fibers
Masson staining showed collagen fibers in the renal
interstitium. Interstitial collagen fibers increased in the renal
interstitium of patients with IgA nephropathy with increasing
pathological grading (Fig. 4).
Quantitative data demonstrated that interstitial collagen fibers
increased more in the proliferative sclerosis group than those in
the mild proliferation group and the focal hyperplasia group
(P<0.01), and those in the focal hyperplasia group were higher
than those in the mild proliferation group (P<0.01; Table II).
Relevance between the expression of
vimentin, α-SMA and collagen fibers and serum creatinine and 24 h
urinary protein excretion
Statistical analysis demonstrated that serum
creatinine and 24 h urinary protein excretion was moderately
correlated with the apoptotic index, expression of vimentin, α-SMA
and collagen fibers (0.4≤r≤0.7; P<0.01; Table III).
 | Table IIIRelevance between the expression of
apoptotic indexes, vimentin, α-SMA and collagen fibers in
immunoglobulin A nephropathy tissue (relevance coefficient r;
n=74). |
Table III
Relevance between the expression of
apoptotic indexes, vimentin, α-SMA and collagen fibers in
immunoglobulin A nephropathy tissue (relevance coefficient r;
n=74).
| Indeces | Apoptotic index | α-SMA | Vimentin | Collagen fibers |
|---|
| Creatinine | 0.521a | 0.408a | 0.533a | 0.529a |
| Urinary protein | 0.457a | 0.466a | 0.548a | 0.574a |
Discussion
IgA nephropathy is a chronic renal disease and is
the most common form of primary glomerular disease in the world,
with ~20–40% of patients eventually entering into the end-stage of
renal failure within 10–20 years (1–3,10).
Currently, the most common clinical features of IgA nephropathy
include blood pressure, serum creatinine and 24 h urinary protein
excretion, which are closely correlated with histological grading;
however, they are not associated with the renal prognosis (11,12).
To further verify the association of blood pressure, serum
creatinine and 24 h urinary protein excretion with the histological
pathological alterations in patients with IgA nephropathy, previous
studies grouped the patients according to the pathological
diagnosis of renal biopsy tissue, which is different from the
Oxford classification, by detailing the immunohistology of IgA
nephropathy (13,14). The present study grouped the
patients by a modification of Lee’s method, in which IgA
nephropathy was further divided into the following groups: The mild
mesangial proliferative group (Lee grade: level I–II), the focal
hyperplasia group (Lee grade: level III) and the proliferative
sclerosis group (Lee grade: level IV–V) (9). The results demonstrated that blood
pressure, serum creatinine and 24 h urinary protein excretion of
patients with IgA nephropathy worsened with increasing grade. Each
clinical indicator of patients in the proliferative sclerosis group
was significantly higher than that in the mild proliferation and
local hyperplasia groups (P<0.01). In addition, 24 h urinary
protein excretion of the focal hyperplasia group was significantly
higher than that of the mild proliferation group (P<0.01).
Collagen fibers, indicating the degree of fibrosis between
different pathological grading, also ascended as the clinical
stages gradually increased. Collagen fibers in the proliferative
sclerosis group significantly deteriorated compared with those in
the mild proliferation and local hyperplasia groups (P<0.01),
and apparently increased in the focal hyperplasia group compared
with the mild proliferation group (P<0.01). Serum creatinine and
24 h urinary protein excretion of patients were interrelated with
the expression of collagen fibers (P<0.01). These results
confirmed that the degree of interstitial fibrosis gradually
worsened with the different pathological grading of IgA
nephropathy, and proteinuria in patients increased, renal function
weakened and the prognosis gradually deteriorated.
The main pathological symptoms of renal
tubulointerstitial fibrosis include renal interstitial ECM
accumulation, renal tubular atrophy and increased MFs in the renal
interstitium. The pivotal step in renal interstitial fibrosis is
the activation of fibroblasts, whose activated form is MFs. The
quantity of collagen secreted by MFs is four- to five-fold higher
compared with that secreted by fibroblasts, exacerbating ECM
extracellular deposition and leading to renal structural remodeling
due to its strong capacity to contract. Under pathological
conditions, renal tubular epithelial cells possessed the capacity
to transform into mesenchymal cells. In 1995, it was reported for
the first time by Strutz et al (15) that EMT appears in renal fibrosis,
followed by studies by Okada et al (16) and Fan et al (17), respectively, reporting that mice
and rat renal tubular epithelial cells are able to
transdifferentiate into MFs in experiments in vitro.
Additionally, analysis of renal biopsies of 133 patients with
different types of nephropathy revealed that the quantity of renal
tubular epithelial cells with EMT characteristics was closely
associated with the concentration of serum creatinine, as well as
the degree of renal interstitial damage (18), suggesting that EMT is involved in
the process of renal fibrosis (15). In the present study, it was
observed that α-SMA and vimentin were expressed in renal tubular
epithelial cells of IgA nephropathy renal biopsies. Among IgA
nephropathy with different pathological grades, with the
development of the disease, tubulointerstitial α-SMA and vimentin
expression gradually increased (P<0.05 or P<0.01). This
suggested that the phenotypic transformation indeed occurred in
renal tubular epithelial cells of IgA nephropathy and, with the
development of IgA nephropathy, transdifferentiation of tubule
epithelial cells gradually increased. In addition, renal
tubulointerstitial α-SMA and vimentin expression correlated with
the expression of collagen fibers (P<0.01), and closely
correlated with serum creatinine and 24 h urinary protein excretion
of patients (P<0.01). These results lead to the conclusion that
transdifferentiation of tubule epithelial cells is involved in the
progression of the renal interstitial fibrosis lesion, relevant to
the alterations in serum creatinine and 24 h urinary protein
excretion of patients. Accordingly, it was verified that in IgA
nephropathy, the transdifferentiation degree of renal tubular
epithelial cells is associated with renal and tubulointerstitial
fiber dysfunction, and the transdifferentiation of renal tubular
epithelial cells may be one of the key factors leading to poor
renal prognosis. Certainly, the detection of α-SMA and vimentin may
be used as one of the clinical indicators in the assessment of IgA
nephropathy prognosis.
Renal interstitial fibrosis lesions are usually
paralleled with renal tubular atrophy, due to the involvement of
renal tubular epithelial cells in the process of renal interstitial
fibrosis through apoptosis. The present study showed that, in IgA
nephropathy, apoptosis can be detected mainly in renal tubules and
interstitium, particularly at sites where MFs have infiltrated,
which is consistent with previous studies (16). It was also revealed that apoptosis
in tissues increased with the development of the lesion. The
apoptotic index was relatively low in the mild proliferation group
and the highest in the focal hyperplasia group and decreased
slightly in the proliferative sclerosis group (P<0.01).
Therefore, it is hypothesized that at the early stages of lesions,
the rate of apoptosis was relatively low, and as the lesion
developed, apoptosis of tubule epithelial cells and interstitial
cells increased. However, at the end-stage of lesions, the majority
of tubule atrophy and the involvement of fibrosis in a relatively
large area reduced the amount of apoptosis. Furthermore, it was
also demonstrated that the apoptotic index of the
tubulointerstitial region was moderately correlated with the degree
of expression of interstitial MFs, fibrosis and clinical prognosis
(P<0.01). In conclusion, tubulointerstitial cell apoptosis may
be one of the factors leading to a poor prognosis in IgA
nephropathy.
The present study demonstrated that α-SMA and
vimentin expression of tubule epithelial cells, interstitial MF
accumulation, collagen deposition, apoptosis of tubular epithelial
and interstitial cells, interstitial fibrosis and renal dysfunction
have a significant relevance in IgA nephropathy. Thus, it may be
concluded that renal tubular epithelial cells in IgA nephropathy
generate MFs through transdifferentiation, secreting a large
quantity of collagen and finally leading to fibrosis, apoptosis of
certain tubular epithelial cells, tubular atrophy and renal
failure. Therefore, the transdifferentiation and apoptosis of renal
tubular epithelial cells reflected the clinical severity of IgA
nephropathy, and the detection of the expression of α-SMA, vimentin
and apoptosis may be useful as an important index for evaluating
the prognosis of IgA nephropathy.
References
|
1
|
Berger J and Hinglais N: Intercapillary
deposits of IgA-IgG. J Urol Nephrol (Paris). 74:694–695. 1968.(In
French).
|
|
2
|
Mubarak M: IgA nephropathy: an update on
pathogenesis and classification. J Coll Physicians Surg Pak.
21:230–233. 2011.PubMed/NCBI
|
|
3
|
Bogenschütz O, Bohle A, Batz C, et al: IgA
nephritis: on the importance of morphological and clinical
parameters in the long-term prognosis of 239 patients. Am J
Nephrol. 10:137–147. 1990.PubMed/NCBI
|
|
4
|
Liu Y: Renal fibrosis: new insights into
the pathogenesis and therapeutics. Kidney Int. 69:213–217. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeisberg M and Kalluri R: The role of
epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med
(Berl). 82:175–181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chevalier RL: Obstructive nephropathy:
towards biomarker discovery and gene therapy. Nat Clin Pract
Nephrol. 2:157–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hills CE and Squires PE: TGF-beta1-induced
epithelial-to-mesenchymal transition and therapeutic intervention
in diabetic nephropathy. Am J Nephrol. 31:68–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pozdzik AA, Salmon IJ, Debelle FD, et al:
Aristolochic acid induces proximal tubule apoptosis and epithelial
to mesenchymal transformation. Kidney Int. 73:595–607. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee HS, Lee MS, Lee SM, et al:
Histological grading of IgA nephropathy predicting renal outcome:
revisiting H. S. Lee’s glomerular grading system. Nephrol Dial
Transplant. 20:342–348. 2005.PubMed/NCBI
|
|
10
|
Glassock RJ: The pathogenesis of IgA
nephropathy. Curr Opin Nephrol Hypertens. 20:153–160. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li PK, Ho KK, Szeto CC, Yu L and Lai FM:
Prognostic indicators of IgA nephropathy in the Chinese - clinical
and pathological perspectives. Nephrol Dial Transplant. 17:64–69.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manno C, Strippoli GF, D’Altri C, Torres
D, Rossini M and Schena FP: A novel simpler histological
classification for renal survival in IgA nephropathy: a
retrospective study. Am J Kidney Dis. 49:763–775. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bellur SS, Troyanov S, Cook HT and Roberts
IS; Working Group of International IgA Nephropathy Network and
Renal Pathology Society. Immunostaining findings in IgA
nephropathy: correlation with histology and clinical outcome in the
Oxford classification patient cohort. Nephrol Dial Transplant.
26:2533–2536. 2011. View Article : Google Scholar
|
|
14
|
D’Amico G: Natural history of idiopathic
IgA nephropathy and factors predictive of disease outcome. Semin
Nephrol. 24:179–196. 2004.PubMed/NCBI
|
|
15
|
Strutz F, Okada H, Lo CW, et al:
Identification and characterization of a fibroblast marker: FSP1. J
Cell Biol. 130:393–405. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okada H, Danoff TM, Kalluri R and Neilson
EG: Early role of Fsp1 in epithelial-mesenchymal transformation. Am
J Physiol. 273:F563–F274. 1997.PubMed/NCBI
|
|
17
|
Fan JM, Ng YY, Hill PA, et al:
Transforming growth factor-beta regulates tubular
epithelial-myofibroblast transdifferentiation in vitro. Kidney Int.
56:1455–1467. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rastaldi MP, Ferrario F, Giardino L, et
al: Epithelial-mesenchymal transition of tubular epithelial cells
in human renal biopsies. Kidney Int. 62:137–146. 2002. View Article : Google Scholar : PubMed/NCBI
|