Introduction
Bronchopulmonary dysplasia (BPD) is the most common
chronic lung disease in preterm infants (1). In recent years, with the development
of perinatal medicine, the survival rate of very low birth weight
infants (VLBWI) has been continuously improving, however, the
incidence of BPD is gradually on the rise and the disease exhibits
a number of novel features. Infant patients initially have minimal
or absent signs of respiratory distress syndrome but subsequently
develop oxygen dependency and require a ventilator within the first
two weeks of life, which may last for weeks and months, termed ‘new
BPD’. A large number of these infants may have been exposed to an
intrauterine inflammatory environment, such as chorioamnionitis,
and are subsequently born with inflammation of the lungs. Following
birth, various risk factors, including pulmonary or systemic
infections, high concentrations of oxygen inspiration and
mechanical ventilation, may act synergistically, amplifying the
inflammation and subsequently affecting normal alveolarization in
preterm infants (2). In view of
the central role of inflammation in the development of BPD and the
known anti-inflammatory properties of corticosteroids, these drugs
have been used in the prevention and treatment of BPD for decades.
However, a number of studies have demonstrated that postnatal
corticosteroid treatment, mainly with dexamethasone in relatively
high doses, may result in long-term adverse neurocognitive
outcomes, brain growth reduction and hippocampus degeneration
(3,4). Therefore, the present study aimed to
identify the efficacy of another clinical drug to suppress the
effects of chorioamnionitis on neonatal lung development with
minimal side effects.
Theophylline, a classical bronchodilator drug for
the treatment of chronic lung diseases, including asthma and
chronic obstructive pulmonary disease (COPD), inhibits
phosphodiesterase (PDEs) activity, antagonizes adenosine receptors
and increases intracellular cyclic adenosine monophosphate (cAMP)
levels (5). Theophylline at high
concentrations is associated with numerous side effects, including
headache and nausea mediated by PDE inhibition, and cardiac
arrhythmias and seizures due to adenosine receptor antagonism
(5). Therefore, the clinical use
of theophylline for the treatment of respiratory diseases has been
rapidly declining. Previously, numerous studies have demonstrated
that physiological doses of theophylline have anti-inflammatory
effects by inhibiting inflammatory factors and increasing
anti-inflammatory cytokine secretion to suppress the development of
inflammatory responses (2,6). Our previous studies have demonstrated
that theophylline restored histone deacetylase (HDAC) activity to
inhibit inflammatory factor IL-8 expression and improve alveolar
development in the lungs of neonatal rats induced by hyperoxia
(7). Therefore, it was
hypothesized that theophylline may also ameliorate the
pathophysiological changes in BPD induced by chorioamnionitis
through inflammatory regulation.
LPS is a potent pro-inflammatory stimulating agent
associated with intrauterine inflammatory processes, including
chorioamnionitis (1,2). Intra-amniotic LPS administration in
pregnant rodents may mimic BPD in human premature infants,
resulting in lung development inhibition, fewer alveoli and
secondary septa in the lungs (8).
In the present study, LPS was injected into the amniotic cavity of
Sprague-Dawley (SD) pregnant rats (E16.5) and theophylline was
administered into neonatal rats to examine the hypothesis that
theophylline may restore the inflammatory equilibrium in the lungs
of neonatal rats exposed to chorioamnionitis, to improve disrupted
alveolarization in BPD.
Materials and methods
Intra-amniotic LPS administration
Pregnant rats on embryonal day 16.5 (E16.5) that
were used in the present study were purchased from the Shanghai
Laboratory Animal Center (Shanghai, China). The experimental
procedures involving rats were performed under the guidelines and
permission of the Experts Committee of Laboratory Animal Sciences
of the Shanghai Jiaotong University School of Medicine (Shanghai,
China). On gestation day 16.5, gravid females were anesthetized
with an intraperitoneal injection of 10% chloral hydrate at a dose
of 3.5 ml/kg body weight. Following midline abdominal section, 1 μg
lipopolysaccharide (LPS; 055: B5, Sigma-Aldrich, St. Louis, MO,
USA) at a concentration of 0.2 μg/μl, solubilized in saline, was
injected into the amniotic sac of each fetus. The same volume of
sterile, endotoxin-free saline without LPS was injected in the
saline control groups. The pups were delivered spontaneously 3–4
days following these injections.
Theophylline drug administration
Following delivery, the pups of the same litter with
intra-amniotic LPS injection were randomly divided into two groups:
The LPS+theo group and the LPS group. In the LPS+theo group pups
were injected with theophylline (20 mg/kg/day; Sigma, St. Louis,
MO, USA) and the LPS group were treated with the saline of the same
volume. Theophylline was dissolved in saline to a final
concentration of 5 mg/ml and injected subcutaneously into the neck
of the pups, first within 6 h after birth and then once a day until
postnatal day 7 (P7). Pups of all groups obtained identical
postnatal care following drug administration.
Lung processing
At P7, all of the surviving rat pups were sacrificed
by an intraperitoneal injection of 10% chloral hydrate. The lung
and trachea were exposed by thoracotomy, and the right bronchus and
trachea were ligated. The pups then received an intratracheal
instillation of buffered formaldehyde (4% paraformaldehyde
solubilized in PBS; pH 7.4) at a pressure of 20 cm H2O
for 20 min. For histological analysis, the left lung was further
fixed in buffered formaldehyde for 24 h at 4°C, and followed by
hematoxylin and eosin staining. For the antibody array analysis,
the right lung without perfusion was excised, frozen in liquid
nitrogen and stored at −80°C.
Lung morphometry
Five pups were selected in each group and five
random non-overlapping fields in one distal lung section per pup
were utilized for the morphometric examinations. The terminal air
spaces and alveolar secondary septa were counted manually at a
magnification of ×200.
Cytokine antibody array
Protein extraction from the four right lungs of the
saline group, LPS group and LPS+theo group were pooled respectively
for one antibody array (RayBio® Biotin Label-based Rat
Antibody Array I; RayBiotech, Norcross, GA, USA), including 90
cytokines. Briefly, 2 ml of 1X blocking buffer was added to the
array membranes and incubated at room temperature for 30 min. The
blocked membranes were then incubated with 1 ml protein extraction
of pooled lung tissues at room temperature for 1–2 h. Thereafter,
the membranes were washed three times with 2 ml of 1X wash buffer I
and twice with 2 ml of 1X wash buffer II (RayBiotech). Following
this, 1 ml diluted biotin-conjugated anti-cytokines antibodies
(RayBiotech) against cytokines extracted from samples, which were
captured by the antibodies fixed in the cytokine antibody arrays,
were added to each membrane and incubated at room temperature for
1–2 h. Membranes were washed once more as mentioned previously and
then each incubated with 2 ml of 1,000-fold diluted horseradish
peroxidase-conjugated streptavidin at room temperature for 2 h. The
intensities of the signals were quantified by densitometric
analysis. The internal positive controls of each membrane were used
to normalize the relative expression levels of 90 cytokines from
the different membranes being compared.
Statistical analysis
The results are expressed as the mean ± standard
deviation. Statistical analysis was performed with one-way analysis
of variance followed by Student-Newman-Keuls test for multiple
comparisons using SPSS 18.0 (IBM, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference. In
the cytokine antibody array detection, an expression alteration of
≥2-fold was considered to be significant.
Results
Theophylline improves alveolarization in
newborn rats with intra-amniotic LPS injection
Gravid rats were injected with LPS or saline into
the amniotic sacs at E16.5. Histological analysis revealed marked
differences in the lung structure of neonatal rats in the three
groups (Fig. 1A). Pups at 7
days-old in the LPS group demonstrated notably fewer terminal
spaces and secondary septa than the saline group (41±8 vs. 75±7,
13±5 vs. 26±6, respectively), suggesting successful induction of
rodent BPD by LPS. Furthermore, it was identified that the terminal
air space count and secondary septa number in the LPS+theo group
increased significantly compared with the LPS group (65±7 vs. 41±8,
20±6 vs. 13±5, respectively), although these parameters did not
completely return to the levels of the saline group (Fig. 1B and C). These data suggest that
theophylline may improve the lung development/function and restore
alveolarization in LPS-induced rodent BPD.
LPS increases the expression levels of
pro-inflammatory cytokines
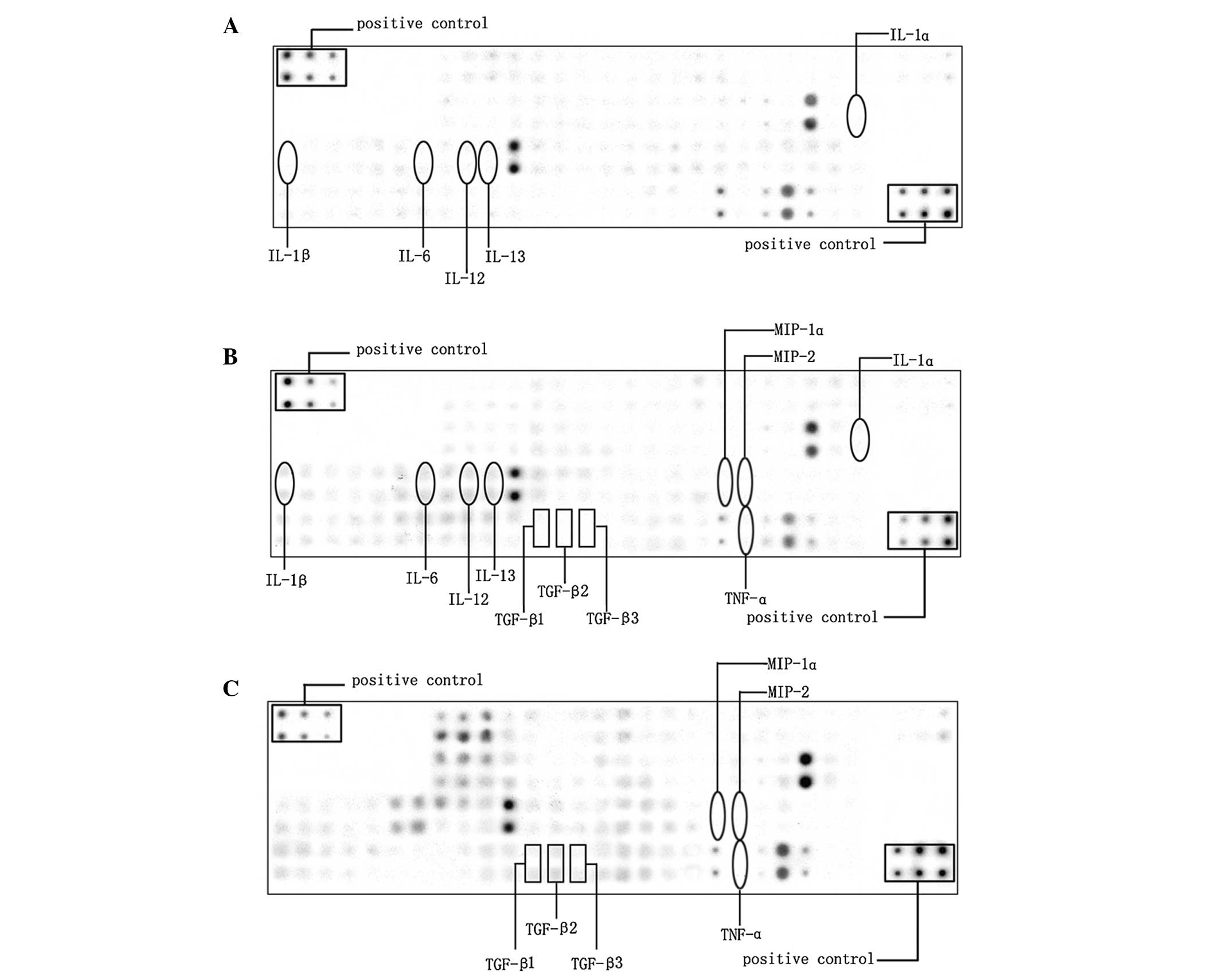
In the present study, 90 cytokines in the lungs of
the rats in the saline and LPS groups were examined by cytokine
antibody array (Fig. 2A and B), in
which the levels of 28 cytokines had changed significantly
(>2-fold; Table I). It is
well-established that LPS is a potent agent that induces the
expression of pro-inflammatory cytokines in various systems
(1,2). Among the upregulated factors, a
series of pro-inflammatory cytokines, including interleukin
(IL)-1α, IL-1β, IL-6, IL-12 and IL-13 were increased by 2.6, 44.5,
3.6, 4.4 and 3.6 times, respectively.
 | Table ICytokine expression upregulated by
≥2-fold in the LPS group compared with the saline group. |
Table I
Cytokine expression upregulated by
≥2-fold in the LPS group compared with the saline group.
| Cytokine | LPS | Saline | Fold change |
|---|
| β-Catenin | 0.0652770 | 0.0206126 | 3.1668524 |
| b-FGF | 0.1367899 | 0.0196013 | 6.9786044 |
| CCR4 | 0.0931056 | 0.0444458 | 2.0948132 |
| CINC-3 | 0.0955325 | 0.0273635 | 3.4912371 |
| CNTF | 0.1087187 | 0.0034210 | 31.7798012 |
| CNTF R α | 0.0898292 | 0.0010705 | 83.9133115 |
| CXCR4 | 0.2192642 | 0.0165675 | 13.2345979 |
| EGFR | 0.1818090 | 0.0226351 | 8.0321594 |
| E-Selectin | 0.1881998 | 0.0601068 | 3.1310900 |
| FADD | 0.1466997 | 0.0676230 | 2.1693774 |
| IFN-γ | 0.1696340 | 0.0206126 | 8.2296299 |
| IL-1 α | 0.0958965 | 0.0364103 | 2.6337766 |
| IL-1 β | 0.3738986 | 0.0083954 | 44.5363370 |
| IL-1 R6/IL-1 R
rp2 | 0.2595509 | 0.0738272 | 3.5156518 |
| IL-2 | 0.1620297 | 0.0789109 | 2.0533247 |
| IL-6 | 0.3586091 | 0.0986990 | 3.6333606 |
| IL-10 | 0.3855882 | 0.1265773 | 3.0462677 |
| IL-12/IL-23 p40 | 0.4204143 | 0.0962392 | 4.3684326 |
| IL-13 | 0.3703392 | 0.1019515 | 3.6325041 |
| Leptin (OB) | 0.2086668 | 0.0723787 | 2.8829868 |
| NGFR | 0.2634339 | 0.1211929 | 2.1736741 |
| Orexin A | 0.2017905 | 0.0795122 | 2.5378554 |
|
Osteopotin/SPP1 | 0.2419963 | 0.1153440 | 2.0980401 |
| RALT/MIG-6 | 0.3737368 | 0.1330002 | 2.8100473 |
| RELM β | 0.4076326 | 0.1191157 | 3.4221561 |
| Resistin | 0.3374141 | 0.1454634 | 2.3195808 |
| TIE-2 | 0.1393786 | 0.0640699 | 2.1754157 |
| TIMP-3 | 0.0981212 | 0.0029564 | 33.1894196 |
Theophylline regulates inflammatory
balance
To examine the hypothesis that theophylline
treatment prevents LPS-induced inflammation, cytokine antibody
arrays of the lung samples from the LPS+theo group were performed
and compared with those of the LPS group (Fig. 2B and C). It was identified that the
expression levels of 36 cytokines altered significantly in the
LPS+theo group as compared with the LPS group, with 20 cytokines
upregulated (Table II) and 16
cytokines downregulated (Table
III). A larger number of the cytokines were considered to be
associated with inflammation. In particular, the pro-inflammatory
cytokines tumor necrosis factor (TNF)-α, macrophage inflammatory
protein (MIP)-1α and MIP-2 were significantly decreased 37.4, 12.5
and 8.6 times, respectively (Table
III) and the anti-inflammatory cytokine tumor growth factor
(TGF)-β family members TGF-β1, TGF-β2 and TGF-β3 were elevated 2.4,
56.9 and 17.8 times, respectively (Table II). These data suggest that
theophylline may reduce the inflammatory responses induced by LPS
and therefore improve the alveolarization in the rodent BPD
lung.
 | Table IICytokine expression upregulated by
≥2-fold in the LPS+theo group compared with the LPS group. |
Table II
Cytokine expression upregulated by
≥2-fold in the LPS+theo group compared with the LPS group.
| Cytokine | LPS+theo | LPS | Fold change |
|---|
| TGF-β2 | 0.3311682 | 0.0058178 | 56.9229390 |
| Thrombospondin | 0.2600238 | 0.0116020 | 22.4119807 |
| TGF-β3 | 0.1512473 | 0.0084874 | 17.8202160 |
| ACTH | 0.9745741 | 0.0705353 | 13.8168279 |
| ADFP | 1.0446070 | 0.1205296 | 8.6668081 |
| Activin A | 0.7720283 | 0.1109029 | 6.9613009 |
| ICK | 0.2347178 | 0.0435158 | 5.3938547 |
| GFR α-1 | 0.2692111 | 0.0643467 | 4.1837572 |
| MuSK | 0.0252079 | 0.0060605 | 4.1593573 |
| E-Selectin | 0.7515612 | 0.1881998 | 3.9934209 |
| FADD | 0.5765773 | 0.1466997 | 3.9303222 |
|
L-Selectin/CD62L | 0.4030973 | 0.1185881 | 3.3991385 |
| Hepassocin | 0.1511165 | 0.0447292 | 3.3784738 |
| TIE-2 | 0.4536439 | 0.1393786 | 3.2547603 |
| β-Catenin | 0.2105234 | 0.0652770 | 3.2250766 |
| EG-VEGF/PK1 | 0.6236254 | 0.2039343 | 3.0579726 |
| GFR α-2 | 0.2219340 | 0.0861484 | 2.5761818 |
| TGF-β1 | 0.2109158 | 0.0885753 | 2.3812022 |
| TIMP-1 | 0.3927330 | 0.1782495 | 2.2032764 |
| β-NGF | 0.1306167 | 0.0624861 | 2.0903328 |
 | Table IIICytokine expression downregulated by
≥2-fold in the LPS+theo group compared with the LPS group. |
Table III
Cytokine expression downregulated by
≥2-fold in the LPS+theo group compared with the LPS group.
| Cytokine | LPS+theo | LPS | Fold change |
|---|
| TNF-α | 0.0036945 | 0.1380842 | 37.3756124 |
| EGFR | 0.0077487 | 0.1818090 | 23.4631616 |
| VEGF | 0.0089257 | 0.1235228 | 14.9920866 |
| MIP-1α | 0.0082392 | 0.1033795 | 12.5472740 |
| B7-1/CD80 | 0.0102989 | 0.1287002 | 12.4964996 |
| CINC-2 α/β | 0.0105605 | 0.1288620 | 12.2022631 |
| RALT/MIG-6 | 0.0428305 | 0.3737368 | 8.7259500 |
| MIP-2 | 0.0068333 | 0.0589671 | 8.6293738 |
| RAGE | 0.0547642 | 0.3713908 | 6.7816347 |
| CXCR4 | 0.0402149 | 0.2192642 | 5.4523125 |
| Prolactin R | 0.0683000 | 0.3356344 | 4.9141201 |
| CD106 | 0.0651285 | 0.2237945 | 3.4361992 |
| RELM β | 0.1340824 | 0.4076326 | 3.0401649 |
| CSK | 0.0662075 | 0.1999703 | 3.0203572 |
| FGF-BP | 0.0609436 | 0.1841550 | 3.0217283 |
| VEGF-C | 0.0142550 | 0.0328374 | 2.3035707 |
Discussion
Human lung development is a complex process, which
is characterized by five stages of development based on
histological appearance, including embryonic, pseudoglandular,
canalicular, saccular and alveolar stages (9). Premature infants delivered at 26–36
weeks of gestation are born in the saccular phase of lung
development and are particularly susceptible to BPD. Disrupted
saccular phase development results in pulmonary interstitium
remolding and morphological changes in preterm infants with BPD
(10). Lung development during the
saccular phase involves a number of vital morphological processes,
including growth and widening of distal airways, further division
of the distal airspaces by primary septation, thinning of the
air-blood barrier and formation of the double capillary network
(11). Therefore, the saccular
phase has a key role in preparing the distal lung for the
subsequent formation of alveoli and gas exchange at birth.
In the present study, an intra-amniotic injection of
LPS was administered to pregnant SD rats at E16.5, a transitional
period from the canalicular to saccular stage. Newborn rats exposed
in utero to LPS demonstrated arrested lung development,
characterized by fewer numbers of alveoli and secondary septa
counts. The results unexpectedly revealed that theophylline
improved alveolarization and partly ameliorated the process of
alveolar arrest. These data suggest the potential benefits of
theophylline in the treatment of BPD infants; however, elucidating
the mechanisms underlying these effects requires further study. In
the present study, the antibody cytokine arrays demonstrated that a
series of pro-inflammatory cytokines were upregulated in the LPS
group, as compared with the saline group, including IL-1α, IL-1β,
IL-6, IL-12 and IL-13, implying that inflammation may have a vital
role in the development of LPS-induced BPD. Numerous studies have
provided epidemiological data indicating a strong correlation
between the exposure in utero to chorioamnionitis and the
occurrence of ‘new BPD’. High concentrations of pro-inflammatory
cytokines in human amniotic fluid have been identified as one of
risk factors for BPD (2,12,13,14).
A number of studies also demonstrated that intra-amnionitic
injection of a high dose of endotoxin may induce more pronounced
expression of cytokines and notably longer existence of
inflammatory cells in the lungs than in chorioamnionitis (2,15,16,17).
Premature infants at various stages of the development of BPD have
notably higher and persisting numbers of neutrophils and
macrophages, both of which have essential roles in pulmonary
inflammatory responses (18,19,20).
Furthermore, elevated protein and mRNA levels of certain
pro-inflammatory cytokines and chemokines have also been detected
in the airway secretions and bronchoalveolar cells of infants with
developing BPD; however, cellular mRNA for IL-10, a classical
anti-inflammatory cytokine, was undetectable in the majority of
airway samples (21). This
evidence suggests that BPD results, at least in part, from a
persistent imbalance between pro-inflammatory and anti-inflammatory
mechanisms, which favors pro-inflammatory processes.
Theophylline was initially used in the treatment of
respiratory diseases, including bronchial asthma and COPD,
attributed to its beneficial effects on bronchodilation and
anti-inflammatory activity (22,23).
Consensus is held that theophylline at a high dose inhibits PDEs,
synergizes with the activators of adenylatecyclase, antagonizes
adenosine receptors, increases cellular cAMP concentration and
inhibits Ca2+ influxes into the cell, relaxing airway
smooth muscle (6,22,24).
However, the reported frequent side effects associated with high
therapeutic doses limit its clinical use. Furthermore, to date, the
anti-inflammatory mechanisms of theophylline at physiological
concentrations remain ambiguous. The proposed mechanisms vary from
inducing IL-10 release to mediator inhibition or increased
apoptosis of inflammatory cells (22,24,25).
In recent years, a considerable number of studies have indicated
that it is more likely that the transcription-dependent mechanism
at relative low dose-physiological concentrations, by inhibition of
PI3K, inhibition of transcription factor NF-κB and induction of
HDAC activity, perhaps with histone acetylation/deacetylation as
the point of convergence, is involved in preventing the
transcription of pro-inflammatory cytokines (26). Most importantly, the minimal
detrimental effects associated with physiological doses improve the
clinical applications of theophylline and provide more choices for
BPD therapy.
To improve the understanding of the effect of
theophylline on the inflammatory regulation in developing BPD
induced by chorioamnionitis, a cytokine antibody array for the
multiplex analysis of 90 rat cytokines was conducted to detect the
differential expression pattern between the LPS+theo and LPS
groups. Utilizing high-throughput screening of the protein
expression levels, it was identified that the inflammatory
cytokines TNF-α, MIP-1α and MIP-2 had a similar decreasing trend in
the LPS+theo group compared with the LPS group. The
anti-inflammatory cytokine TGF-β family members TGF-β1, TGF-β2 and
TGF-β3 increased markedly, particularly TGF-β2 and TGF-β3. As a
result, the cytokine profiling confirmed the hypothesis that
theophylline ameliorates the inflammatory cytokine imbalance in BPD
of neonatal rats induced by chorioamnionitis.
In addition to the effect of theophylline in
directly suppressing the expression of inflammatory cytokines,
previous studies have reported that theophylline may also increase
the production of IL-10, a well-established cytokine to indirectly
downregulate the release of several pro-inflammatory cytokines,
including as TNF-α, IL-1β, IL-6, IL-8, MIP-1α and granulocyte
macrophage-colony stimulating factor from monocytes and alveolar
macrophages (5,27). The cytokine profiling of the
antibody array of the present study indicated that, in this BPD
model, theophylline upregulated the expression levels of another
anti-inflammatory cytokine, TGF-β, but not IL-10, which is a result
that, to the best of our knowledge, has not been previously
reported. It is well established that TGF-β and IL-10 are Th3
cytokines and thus exert immunosuppressive actions. TGF-β is not
only a regulator of cell proliferation, differentiation and
migration, but also an important immunomodulator (28). Previous studies have reported that
TGF-β1-deficient mice spontaneously developed autoimmune diseases
with uncontrollable systemic inflammation and died in utero
or perinatally due to widespread inflammation (29). Another study revealed a protective
role of TGF-β signaling in nuclear factor (NF)-κB-mediated acute
peritonitis. The upregulation of endogenous peritoneal TGF-β1 and
the activation of TGF-β signaling were closely associated with a
significant inhibition of NF-κB activation and mitigation of acute
peritoneal inflammation following E. coli infection
(30). Kitamura et al
(31) demonstrated that cAMP
induced the secretion 10-fold of bioactive TGF-β2 in PC-3 cells.
Therefore, it is hypothesized that theophylline may increase
cellular cAMP levels by PDE inhibition and adenosine receptor
antagonism to induce bioactive TGF-β2 at the level of either
transcription or messenger stability.
In conclusion, theophylline may restore the balance
between pro-and anti-inflammatory mechanisms by downregulating the
expression of the pro-inflammatory cytokines, TGF-α, MIP-1α and
MIP-2 and upregulating anti-inflammatory cytokines including TGF-β
family members to improve alveolarization in BPD of neonatal rats
induced by chorioamnionitis. Furthermore, other categories of
cytokines in the arrays, including growth factor signaling protein
nerve growth factor receptor, basic fibroblast growth factor, cell
adhesion and motility related protein E-Selectin, C-src tyrosine
kinase and cell apoptosis related protein FADD, were also altered
by >2-fold, which may contribute to the arrested alveolar
development in BPD. Thus, we propose that theophylline may
ameliorate alveolarization disruption of BPD through regulating the
balance between pro- and anti-inflammatory mechanisms, which
provides a novel indication of clinical therapy for BPD.
Acknowledgements
The present study was supported by the Shanghai
Natural Science Foundation (grant no. 11ZR1423800) and National
Natural Science Foundation of China (grant no. 81270729) (to Y.
Zhang).
References
|
1
|
Choi CW, Kim BI, Hong JS, Kim EK, Kim HS
and Choi JH: Bronchopulmonary dysplasia in a rat model induced by
intra-amniotic inflammation and postnatal hyperoxia: morphometric
aspects. Pediatr Res. 65:323–327. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Speer CP: Inflammation and
bronchopulmonary dysplasia: A continuing story. Semin Fetal
Neonatal Med. 11:354–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jarreau PH, Fayon M, Baud O, Autret-Leca
E, Danan M, de Verdelhan A and Castot A: The use of postnatal
corticosteroid therapy in premature infants to prevent or treat
bronchopulmonary dysplasia: current situation and recommendations.
Arch Pediatr. 17:1480–1487. 2010.(In French).
|
|
4
|
Grier DG and Halliday HL: Corticosteroids
in the prevention and management of bronchopulmonary dysplasia.
Semin Neonatol. 8:83–91. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
To Y, Ito K, Kizawa Y, Failla M, Ito M,
Kusama T, Elliott WM, Hogg JC, Adcock IM and Barnes PJ: Targeting
phosphoinositide-3-kinase-delta with theophylline reverses
corticosteroid insensitivity in chronic obstructive pulmonary
disease. Am J Respir Crit Care Med. 182:897–904. 2010. View Article : Google Scholar
|
|
6
|
Mascali JJ, Cvietusa P, Negri J and Borish
L: Anti-inflammatory effects of theophylline: modulation of
cytokine production. Ann Allergy Asthma Immunol. 77:34–38. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu L, Li H, Tang J, Zhu J and Zhang Y:
Hyperoxia arrests alveolar development through suppression of
histone deacetylases in neonatal rats. Pediatr Pulmonol.
47:264–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coalson JJ: Pathology of bronchopulmonary
dysplasia. Semin Perinatol. 30:179–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schreiber T, Niemann C, Schmidt B and
Karzai W: A novel model of selective lung ventilation to
investigate the long-term effects of ventilation-induced lung
injury. Shock. 26:50–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kramer EL, Deutsch GH, Sartor MA, Hardie
WD, Ikegami M, Korfhagen TR and Le Cras TD: Perinatal increases in
TGF-α disrupt the saccular phase of lung morphogenesis and cause
remodeling: microarray analysis. Am J Physiol Lung Cell Mol
Physiol. 293:L314–L327. 2007.
|
|
11
|
Kim N and Vu TH: Parabronchial smooth
muscle cells and alveolar myofibroblasts in lung development. Birth
Defects Res C Embryo Today. 78:80–89. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bland RD: Neonatal chronic lung disease in
the post-surfactant era. Biol Neonate. 88:181–191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vohr BR, Wright LL, Dusick AM, et al:
Neurodevelopmental and functional outcomes of extremely low birth
weight infants in the National Institute of Child Health and Human
Development Neonatal Research Network, 1993–1994. Pediatrics.
105:1216–1226. 2000.PubMed/NCBI
|
|
14
|
Yoon BH, Romero R, Jun JK, et al: Amniotic
fluid cytokines (interleukin-6, tumor necrosis factor-alpha,
interleukin-1 beta, and interleukin-8) and the risk for the
development of bronchopulmonary dysplasia. Am J Obstet Gynecol.
177:825–830. 1997. View Article : Google Scholar
|
|
15
|
Speer CP, Pabst MJ, Hedegaard HB, Rest RF
and Johnston RB Jr: Enhanced release of oxygen metabolites by
monocyte-derived macrophages exposed to proteolytic enzymes:
activity of neutrophil elastase and cathepsin G. J Immunol.
133:2151–2156. 1984.
|
|
16
|
Yoon BH, Romero R, Jun JK, et al: Amniotic
fluid cytokines (interleukin-6, tumor necrosis factor-alpha,
interleukin-1 beta, and interleukin-8) and the risk for the
development of bronchopulmonary dysplasia. Am J Obstet Gynecol.
177:825–830. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gomez R, Romero R, Ghezzi F, Yoon BH,
Mazor M and Berry SM: The fetal inflammatory response syndrome. Am
J Obstet Gynecol. 179:194–202. 1998. View Article : Google Scholar
|
|
18
|
Arnon S, Grigg J and Silverman M:
Pulmonary inflammatory cells in ventilated preterm infants: effect
of surfactant treatment. Arch Dis Child. 69:44–48. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kotecha S, Chan B, Azam N, Silverman M and
Shaw RJ: Increase in interleukin-8 and soluble intercellular
adhesion molecule-1 in bronchoalveolar lavage of premature infants
with chronic lung disease. Arch Dis Child Fetal Neonatal Ed.
72:F90–F96. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kotecha S, Mildner RJ, Prince LR, et al:
The role of neutrophil apoptosis in the resolution of acute lung
injury in newborn infants. Thorax. 58:961–967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones CA, Cayabyab RG, Kwong KY, et al:
Undetectable interleukin (IL)-10 and persistent IL-8 expression
early in hyaline membrane disease: a possible developmental basis
for the predisposition to chronic lung inflammation in preterm
newborns. Pediatr Res. 39:966–975. 1996. View Article : Google Scholar
|
|
22
|
Kaneko Y, Takashima K, Suzuki N and Yamana
K: Effects of theophylline on chronic inflammatory lung injury
induced by LPS exposure in guinea pigs. Allergol Int. 56:445–456.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ito K, Lim S, Caramori G, et al: A
molecular mechanism of action of theophylline: Induction of histone
deacetylase activity to decrease inflammatory gene expression. Proc
Natl Acad Sci USA. 99:8921–8926. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang B, Jiang S, Zhang Z, et al:
Anti-inflammatory effects of theophylline: modulation of immune
functions during murine leukemia virus infection. Immunopharmacol
Immunotoxicol. 23:307–319. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ezeamuzie CI and Shihab PK: Interactions
between theophylline and salbutamol on cytokine release in human
monocytes. J Pharmacol Exp Ther. 334:302–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marwick JA, Caramori G, Stevenson CS, et
al: Inhibition of PI3Kdelta restores glucocorticoid function in
smoking-induced airway inflammation in mice. Am J Respir Crit Care
Med. 179:542–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bonner JC: The epidermal growth factor
receptor at the crossroads of airway remodeling. Am J Physiol Lung
Cell Mol Physiol. 283:L528–L530. 2002.PubMed/NCBI
|
|
28
|
Lutgens E, Gijbels M, Smook M, Heeringa P,
Gotwals P, Koteliansky VE and Daemen MJ: Transforming growth
factor-beta mediates balance between inflammation and fibrosis
during plaque progression. Arterioscler Thromb Vasc Biol.
22:975–982. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shull MM, Ormsby I, Kier AB, et al:
Targeted disruption of the mouse transforming growth factor-beta 1
gene results in multifocal inflammatory disease. Nature.
359:693–699. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lan HY: Diverse roles of TGF-β/Smads in
renal fibrosis and inflammation. Int J Biol Sci. 7:1056–1067.
2011.
|
|
31
|
Kitamura H, Cambier S, Somanath S, et al:
Mouse and human lung fibroblasts regulate dendritic cell
trafficking, airway inflammation, and fibrosis through integrin
αvβ8-mediated activation of TGF-β. J Clin Invest. 121:2863–2875.
2011.PubMed/NCBI
|