Introduction
Ovarian cancer is one of the most lethal of all
gynecological cancer types, and although its incidence is less
frequent than that of cervical and endometrial cancer, it has the
highest mortality rate (1). In
2010, 21,880 new cases of ovarian cancer and 13,850 mortalities
were estimated in America (2). In
recent decades, although there have been a number of improvements
in surgical techniques and combined chemotherapeutic strategies,
which are the main treatments for ovarian cancer, identifying novel
effective and comprehensive treatment methods remains urgent. As
one of the main, first-line chemotherapeutic treatments for ovarian
cancer, cisplatin has wide clinical applications; however,
cisplatin-resistance is both common and severe, posing a major
challenge for improving treatment efficacy and mortality rates.
Therefore, studies investigating the mechanisms of platinum-based
drug resistance, new anti-tumor drugs and drug resistance-reversal
agents are urgently required.
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL), also known as Apo-2 ligand (Apo2L), is a member of
the tumor necrosis factor superfamily (3,4). It
induces apoptosis selectively in tumor cells through the
caspase-dependent death signaling pathway. TRAIL binds to five
receptors, including DR4 (TRAIL-R1) and DR5 (TRAIL-R2), which
contain a death domain in their cytoplasmic COOH-terminal portions
and are able to induce programmed cell death (5); DcR1 (TRAIL-R3), DcR2 (TRAIL-R4) and
osteoprotegrin, which act as decoy receptors without
intracytoplasmic death domains, prevent cells from TRAIL-mediated
apoptosis. In the majority of tumor cell types, the expression of
DR5, which is rare or even absent in normal cells, is the
predominant death receptor expression type of TRAIL (6) and naturally, DR5 becomes the key
mediator of TRAIL-mediated cell death. Recent findings have
demonstrated the defects of TRAIL, including shorter half-life in
the circulatory system, cytotoxicity in human hepatocytes and
normal brain tissue (7,8), and TRAIL-resistance may compromise
its application clinically. Therefore, to find an alternative to
TRAIL for cancer treatment, the agonistic DR5 antibody, inducing
apoptosis via targeting DR5, has attracted notable attention
worldwide. Similar to the TRAIL-mediated apoptosis mechanism, the
agonistic DR5 antibody may directly bind to DR5 on the cell
membrane, activating caspase-dependent apoptotic pathways to induce
apoptosis of target cells (9,10).
Additionally, these DR5 monoclonal antibodies not only had a longer
half-life in vivo compared with the TRAIL ligand (11), but may have improved safety and
specificity (10). Evidence of
this principle has been reported with murine, rabbit or human
agonistic DR5 antibodies, exhibiting a tumoricidal activity in
vitro and in vivo (12).
Agonistic DR5 antibodies may also interact
synergistically with chemotherapeutic agents with anti-tumor
activities similar to TRAIL. Not only was it reported that several
chemotherapeutic agents upregulate the mRNA expression of DR4 and
DR5 via the p53-dependent or other independent signaling pathways
(13–15), but these DR4 and DR5 monoclonal
antibodies also enhanced the anti-tumor effects of chemotherapeutic
agents (16).
A recent study on the cisplatin-resistance mechanism
determined that the apoptosis-inducing effect of cisplatin on
ovarian cancer mainly proceeded via the intrinsic
mitochondria-dependent apoptosis pathway (17). Since the agonistic DR5 antibody
markedly induced the apoptosis of several ovarian cancer cells via
extrinsic caspase-dependent pathways (9,18), a
more effective ovarian cancer therapeutic strategy for
cisplatin-resistant ovarian cancer may be examined by combination
of the two different anti-tumor agents, due to their different
apoptosis signaling pathways. Such a combination therapy may
improve the crisis of the cisplatin-resistance in ovarian cancer
treatment, change or even enhance the apoptosis-inducing effect of
cisplatin on cisplatin-resistant ovarian cancer.
In the present study, the anti-tumor effect of D-6,
an agonistic DR5 antibody, was investigated as a potential
candidate for TRAIL, alone or accompanied with cisplatin on the C30
cisplatin-resistant ovarian cancer cell line, and the associated
apoptosis mechanism was examined. Furthermore, the present study
focused on whether or not D-6 would also exhibit apoptosis-inducing
activity in C30 xenograft models and the difference in the
anti-tumor effect of cisplatin when in combination with D-6 in C30
cisplatin-resistant ovarian cancer.
Materials and methods
Cell culture and reagents
C30 is a cisplatin-resistant ovarian cancer cell
line, with a tolerance to cisplatin at a concentration of 30
μmol/l, that was generously provided by Kanghong Co. (Chengdu,
China). The C30 cells were cultured in RPM1-1640 culture medium
containing 10% fetal bovine serum and maintained at 37°C in 5%
CO2/95% humidified air. The RPMI-1640 culture medium,
dimethylsulfoxide (DMSO) and fetal bovine serum (FBS) were
purchased from Gibco-BRL (Carlsbad, CA, USA). The
4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene
disulfonate (WST-1) cell proliferation reagent kit for WST-1-based
cell cytotoxicity assay was purchased from Roche Diagnostics GmbH
(Mannheim, Germany). The agonistic DR5 antibody (D-6), caspase 3, 8
and 9 precursor antibodies, and β-actin antibody were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Cisplatin was purchased from Gejiu Bio-Pharmaceutical Co., Ltd.
(Yunnan, China).
WST-1-based cytotoxicity assay
The C30 cells were counted and cultured in two
96-well, flat bottom plates at a concentration of 5×104
cells/well and a volume of 100 μl/well in 35 wells in each plate.
In one plate, 30 wells were randomized into six groups (five
wells/group as five replicates) and to every well, 100 μl RPMI-1640
culture medium + 10% FBS, containing various amounts of D-6 (final
concentration, e.g. 0.125, 0.25, 0.5, 1, 2 and 4 μg/ml) was added,
while the remaining five wells as the control group were cultured
in medium only. The other plate was treated as the above one,
except for that cisplatin (2.5 μg/ml) was added to every well with
the six serial concentrations of D-6. Following 48 h culture of the
cells at 37°C in 5% CO2/95% humidified air, 10 μl WST-1
was added to each well. The plates were then incubated for an
additional 4 h. The plates were then thoroughly agitated for 1 min,
and the absorbance values of the sample in each well were measured
at 440 nm by a Varioskan Flash reader (Thermo Fisher Scientific,
Waltham, MA, USA). The cell growth inhibition rate was calculated
as follows: [1-(OD test-OD blank)/(OD control-OD blank)] × 100%
(19).
Western blot analyses
The C30 cells were counted and cultured in 25
cm2 culture flasks at a concentration of
1×106 cells/flask. Following 12 h of culture in the
incubator, the supernatant was extracted and the four flasks were
randomized into four groups: The control group, where 5 ml culture
medium was added; the cisplatin group, where 5 ml culture medium
containing 2.5 μgml cisplatin was added; the D-6 group, where 5 ml
culture medium containing 2 μg/ml D-6 was added; the combination
group, where 5 ml culture medium containing 2.5 μgml cisplatin and
2 μg/ml D-6 was added. Following 48 h in the incubator, the cells
from each group were collected into four different tubes and then
protein of the cells was extracted using a Total Protein Extraction
kit (Millipore Chemicon, MA, USA), and then determined with a
Bradford Protein Assay kit (Bio-Rad, Hercules, CA, USA) and
measured at 595 μm using a Varioskan Flash reader (Thermo Fisher
Scientific). Similarly, the xenograft tumors from the four
different groups were homogenized using a technical homogenizer and
protein extraction was performed as for the cells. The protein (40
μgwell) extracted from the cells or tissues of each group was
subjected to SDS-PAGE for 30 min at 80 V, followed by 50 min at 110
V. Then, the gels were electroblotted on polyvinylidene fluride
membranes (Bio-Rad) for 1.5 h at 250 mA. The membranes were
subsequently incubated in 5% non-fat milk powder in Tris-buffered
saline (TBS) containing 0.05% Tween-20 (TBS-T) for 1–2 h to block
non-specific binding sites and then incubated in the appropriate
primary antibody overnight. The concentration of these primary
antibodies were as follows: Procaspase-3 (dilution, 1:250),
procaspase-8 (dilution, 1:500), procaspase-9 (dilution, 1:500) and
β-actin (dilution, 1:1,000). The following morning, the membranes
were incubated at room temperature for 1 h in secondary antibody
(Zsgb-bio, Beijing, China), after rinsing three times in TBS-T.
Finally, the membranes were incubated with enhanced
chemoluminescence western blotting detection reagent (Bio-Rad) for
1–2 min and exposed to a ChemiDoc XRS system (Bio-Rad).
Electron microscopic analysis of
morphological changes during apoptosis
The C30 cells from the four different groups were
treated as described above in the western blot analyses. Following
24 h in the incubator, the cells from each group were collected
into four different centrifuge tubes, washed in phosphate-buffered
saline (PBS; pH 7.4) and then carefully transferred into 0.5%
glutaraldehyde using a pipette. Then, they were incubated for 10
min at 4°C. Subsequently, the samples were fixed in 3%
glutaraldehyde and fixed again with 1% osmiumtetroxide. The fixed
samples were dehydrated with a gradient series of acetone and then
embedded in Epon-812 agar (Shell Chemicals, Deer Park, TX, USA).
Finally, the embedded samples, which were constructed into
ultrathin sections by automatic semi-thin rotary microtome (Leica,
Wetzlar, Germany) and stained with uranyl acetate and lead citrate,
were observed under a Hitachi H-600IV transmission electron
microscope (Hitachi, Tokyo, Japan) and images were also captured by
the transmission electron microscope.
Creating C30 cisplatin-resistant ovarian
cancer xenografts in vivo
All animal procedures were approved by the Animal
Care and Scientific Committee of Sichuan University (Chengdu,
China). A total of 24 4–5-week old female BALB/C (nu/nu) nude mice
were purchased from the Experimental Animal Center of Sichuan
University (Chengdu, China) and then raised in a specific
pathogen-free animal house. Every nude mouse was inoculated
subcutaneously with C30 cells (1×107). When the
xenograft tumor volume reached ~100 mm3, the nude mice
were randomized into four groups. Each group contained six nude
mice and was subject to the following treatment: The control group
with normal saline (300 μl/mouse); the cisplatin group with
cisplatin (3 mg/kg); the D-6 group with D-6 (3 mg/kg); the
combination group with cisplatin (3 mg/kg) and D-6 (3 mg/kg).
Administration by abdominal subcutaneous injection was twice a week
for 2 weeks, and the nude mice were sacrificed three days following
the last administration. During the treatment time, the weight of
the animals and the xenograft tumor volume were measured at the
time-point of each administration and at the termination of the
study. The xenograft tumor volume was measured with a vernier
caliper and calculated by the product of the maximum transverse
diameter, the maximum width and height. The xenograft tumor of each
nude mouse, which was completely stripped, was divided into two
parts; one was stored in 4% paraformaldehyde and the other was
snap-frozen in liquid nitrogen for several subsequent assays.
Terminal transferase dUTP nick end
labeling (TUNEL) assay
Apoptosis in the xenograft tumors from nude mice was
detected by an in situ cell death detection kit, alkaline
phosphatase (AP) (Roche Diagnostics GmbH) according to
manufacturer’s instructions. The xenograft tumors, immersed in 4%
polyformaldehyde, were embedded in paraffin, and then dewaxed with
xylene twice for 10 min and alcohol at 100, 90 and 80%
concentration once for 2 min. The sections were subsequently
digested with proteinase K (20 μg/ml) for 20 min, rinsed with
distilled water, treated in 3% hydrogen peroxide for 10 min, rinsed
with distilled water again and PBS twice for 5 min. The TUNEL
reaction mixture (1:10 dilution; Roche Diagnostics GmbH) was added
dropwise to the sections at 30 μl/section which were then
maintained at a constant temperature of 37°C for 1 h. Following
rinsing with PBS twice for 3 min, anti-fluorescein isothiocyanate
(FITC)-AP (ready-to-use) was added dropwise at 20–30 μl/section and
sections were maintained at a constant temperature of 37°C for 30
min. Finally, following rinsing with PBS twice for 3 min, the
sections were developed with AP-red and observed under a light
microscope (Nikon, Tokyo, Japan). Apoptotic cells stained red in
the sections.
Statistical analyses
Values are expressed as the mean ± standard
deviation. The differences in cell growth inhibition rates,
xenograft tumor volumes and wet weights, and apoptosis rates of
xenograft tumors among the four groups, were analyzed by one-way
analysis of variance. All statistical analyses were performed by
the SPSS 17.0 software package (SPSS, Inc., Chicago, IL, USA) and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cell growth inhibition rate
The C30 cell growth inhibition rates were examined
by an WST-1-based cytotoxicity assay. As shown in Fig. 1, treatment of C30 cells with D-6
alone or in combination with cisplatin produced dose-dependent cell
growth inhibition rates for D-6 concentrations >0.25 μg/ml
(P<0.05). These rates were more significant in the combination
group than in the D-6 alone group (P<0.05). However, the cell
growth inhibition rates decreased following treatment with D-6
concentrations of <0.25 μg/ml in the D-6 group.
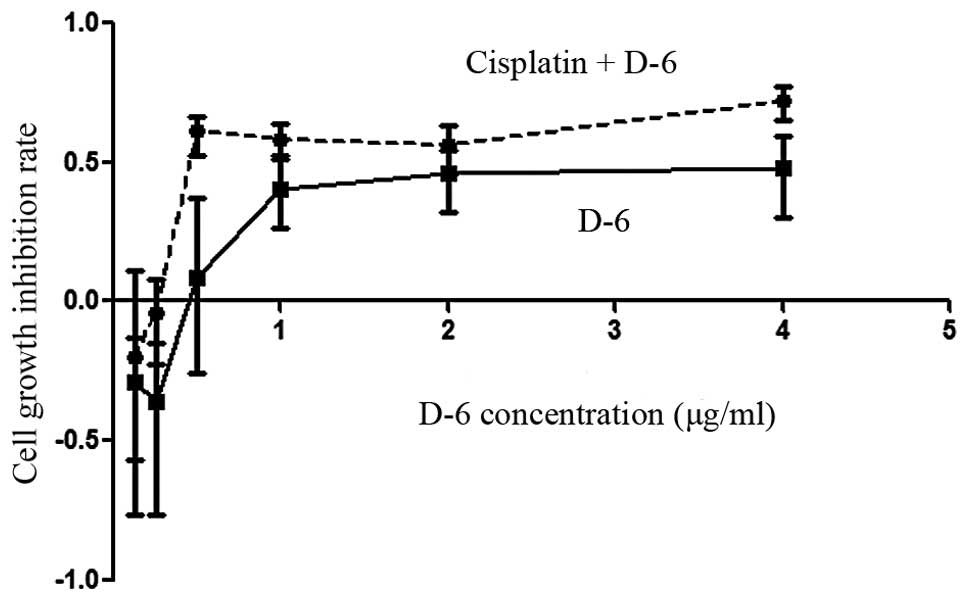 | Figure 1Cell growth inhibition rate of C30
cisplatin-resistant ovarian cancer cells analyzed by WST-1-based
cytotoxicity assay. (--●--) Combination group: Cells were treated
with various concentrations (0.125, 0.25, 0.5, 1, 2 and 4 μg/ml) of
D-6 and cisplatin at a constant concentration (2.5 μg/ml).
(----■----) D-6 group: Cells were treated with various
concentrations (0.125, 0.25, 0.5, 1, 2 and 4 μg/ml) of D-6. WST-1,
4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene
disulfonate. |
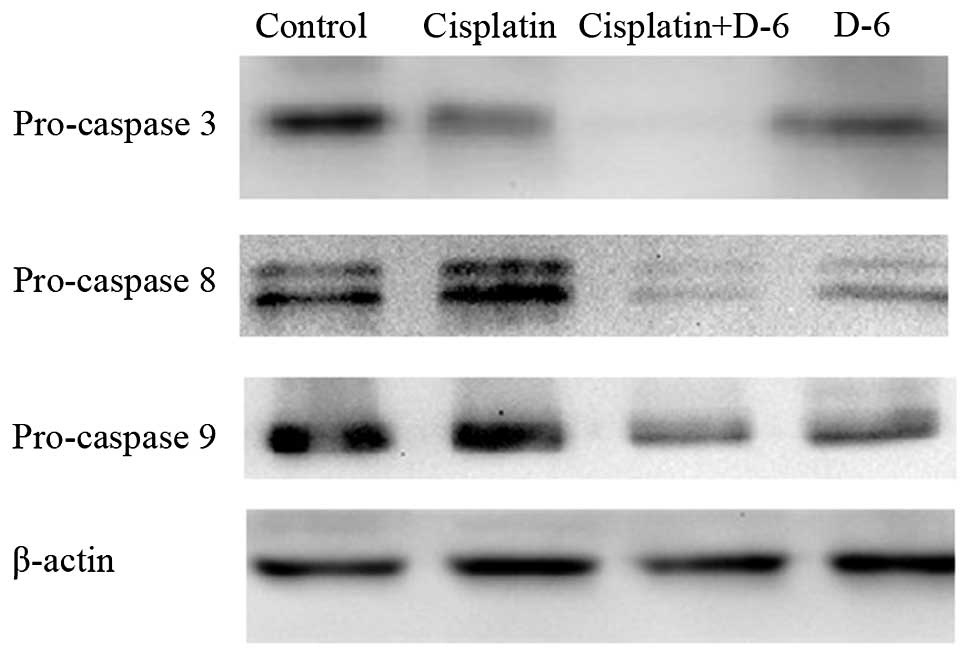
Caspase cascade changes in vitro
As TRAIL activates caspases in various tumor cells,
it was investigated whether the agonistic DR5 antibody D-6 would
also activate these apoptotic reactions. In Fig. 2, 48 h following treatment, the
expression of caspase 3, 8 and 9 precursors in C30 cells was
evidently decreased in the D-6 and combination groups compared with
the control and cisplatin groups by western blot analysis, and this
decrease was more evident in the combination group. The
downregulation of caspase 3, 8 and 9 precursors results in the
upregulation of caspase 3, 8 and 9, which are key factors in the
caspase-dependent and -independent death signaling pathway. The
results suggested that cisplatin induced weak or no cleavage of
caspase 3, 8 and 9, whereas D-6 triggered marked caspase
activation, with a substantially enhanced cleavage of caspase 3, 8
and 9 when accompanied with cisplatin in vitro.
Morphological changes during
apoptosis
As demonstrated in Fig.
3, 24 h following treatment, the C30 cells in the control group
demonstrated a normal morphology, without any damage or significant
morphological-apoptotic changes, as observed using electron
microscopy. Cisplatin-only treatment resulted in mild chromatin
condensation and organelle swelling in the C30 cells. By contrast,
the C30 cells from the D-6 and combination groups demonstrated
morphological changes characteristic for apoptosis, including
nucleus pycnosis, significant chromatin condensation and
marginalization, cell membrane integrity, reduction in cell volume
and the appearance of typical apoptotic bodies.
Anti-tumor effect of D-6 and cisplatin in
xenograft models
To examine the anti-tumor activity of D-6 in
vivo, the present study investigated its efficacy in xenograft
models, in which C30 cells were inoculated into nude mice
subcutaneously. Xenograft tumors were established at a mean volume
of 100 mm3 prior to initiating the administration. For
each group, the xenograft tumor volumes were measured prior to each
administration and before the mice were sacrificed (Table I). The tumors grew rapidly in the
control group, whereas the tumors in the other three groups
advanced significant slower and demonstrated delayed trends in
progression. As compared with the control group, the inhibition
rates (i.e. the control group volume minus the treated group
volume/the control group volume at the last measured time-point) in
the cisplatin, D-6 and combination groups were 37.56, 70.99 and
72.47% respectively. As shown in Fig.
4B, the administration of D-6 alone or in combination with
cisplatin resulted in the significant repression of tumor growth
(P<0.05), and the evident gross change of the xenograft tumor
volume is also illustrated in Fig.
4A. However, the xenograft tumors in the combination group
demonstrated higher inhibition rates and smaller tumor volume
compared with the D-6 group with no significance.
 | Table ISummary of xenograft tumor volume in
each group (mm3). |
Table I
Summary of xenograft tumor volume in
each group (mm3).
| Administration time
(days) | Control group | Cisplatin group | D-6 groupa | Cisplatin + D-6
groupa |
|---|
| 1 | 112.7±56.3 | 136.4±73.1 | 99.7±54.1 | 145.95±48.2 |
| 4 | 362.2±215.2 | 278.1±157.2 | 139.8±81.8 | 215.31±90.4 |
| 7 | 748.2±633.1 | 427.1±266.1 | 210.1±127.1 | 286.53±173.4 |
| 11 | 1376.8±939.3 | 832.7±625.4 | 340.7±210.0 | 395.5±264.8 |
| 14 | 2243.8±935.6 | 1401.0±995.7 | 650.9±295.6 | 617.6±370.5 |
Following sacrificing the mice, the entire xenograft
tumors were completely removed and weighed and the results of the
wet weights are illustrated in Table
II. The tumor wet weights in the D-6 and combination groups
were the smallest among the four groups, consistent with the
changes of the tumor volumes.
 | Table IISummary of xenograft tumor wet weight
in each group (g). |
Table II
Summary of xenograft tumor wet weight
in each group (g).
| Control group | Cisplatin
group | D-6 groupa | Cisplatin+ D-6
groupa |
|---|
| Wet weight | 1.35±0.7 | 0.91±0.62 | 0.38±0.19 | 0.27±0.11 |
Caspase cascade changes in vivo
Following sacrificing the xenograft mice, western
blot analyses were performed on the xenograft tumors to detect
changes in the expression of the caspase precursors. The expression
of caspase 3, 8 and 9 precursors changed in accordance with the
results in C30 cells following 48 h treatment in vitro
(Fig. 5). D-6 induced the cleavage
of caspase 3, 8 and 9, and when in combination with cisplatin,
caused a markedly greater caspase activation. However, weak or no
caspase activation reactions occurred in the cisplatin-treated
xenograft tumors.
Assessment of apoptosis by TUNEL
staining
TUNEL-positive cells, stained red, were observed in
the xenograft tumor sections among the four different groups. As
shown in Fig. 6A, there were
notably more red stained cells in the D-6 and the combination
groups as compared with the cisplatin and control groups. The rates
of apoptosis of the four groups are illustrated in Fig. 6B, demonstrating that D-6 induced
substantial apoptosis, with the rate of apoptosis being
17.32±3.67%, and this rate was increased in the combination group
with a rate of 18.91±4.68%. The increases in rates of apoptosis in
the D-6 and combination groups compared with those of the control
and the cisplatin groups were statistically significant
(P<0.05).
Discussion
Ovarian cancer is associated with poor prognosis and
represents a severe threat to female reproductive health and
quality of life. A large number of patients with ovarian cancer
present in the advanced ovarian cancer stages at first diagnosis.
As a result, developing effective treatment strategies to improve
the prognosis of ovarian cancer is highly important. The majority
of ovarian cancer cases are sensitive to platinum-based
chemotherapy in the initial treatment stages; however, later
recurrence of the disease, as is common for most patients, is
associated with the development of platinum resistance (18). Therefore, the identification of
novel anti-tumor drugs and drug resistance-reversal agents is
urgently required. TRAIL/TRAIL receptors, as members of the tumor
necrosis factor superfamily, have an important role in regulating
apoptosis of malignant neoplasms. They exhibit notable anti-tumor
effects via activation of caspase-dependent and -independent
pathways (10,12), which is an effect that is enhanced
by exposure to chemotherapy, including cisplatin, adriamycin,
BisVIII and others (20,21). The present study investigated the
in vitro tumoricidal activity of an agonistic antibody to
DR5, D-6, alone or in combination with cisplatin, against the C30
cisplatin-resistant ovarian cancer cell line, which is resistant to
cisplatin at a high concentration of 30 μmol/l. Additionally,
xenograft models based on C30 cells were established to further
examine these effects in vivo. It was identified that D-6
not only induced apoptosis in tumor cells in vitro and
inhibited cisplatin-resistant ovarian tumor growth in nude mice,
but also enhanced the activity of cisplatin in the
platinum-resistant cancer cell line.
Over the past several decades, numerous in-depth
studies have investigated the efficacy and mechanisms underlying
TRAIL-based cancer therapies. While the TRAIL-inducing apoptosis
pathways are more thoroughly understood, its various shortcomings
appear to be restricting its clinical application. Agonistic
antibodies against DR4 or DR5, as potent candidates for TRAIL, have
been produced in recent years, including TRA-8, CS-1008, apomab and
HGS-ETR1 (12,22–25).
These antibodies generally have a longer plasma half-life,
increased anti-tumor activity without decoy receptors, specificity
without exogenous cross-linking, and the ability to overcome
TRAIL-associated cytotoxicity and resistance. Among these
antibodies, the murine and human monoclonal antibodies targeting
DR5 have exerted marked tumoricidal activity in vitro and
in vivo (9,25–27).
The human monoclonal antibody conatumumab (AMG655) has been
investigated in phase II clinical trials in patients with
metastatic pancreatic cancer, demonstrating marked efficacy in
improving the six-month survival rate and overall survival
(28). D-6, a murine agonistic
antibody to DR5, which was investigated in our previous study,
exhibited strong anti-tumor efficacy towards the
cisplatin-sensitive ovarian cancer cell line A2780 in vitro,
with an enhanced effect in combination with cisplatin (9). In the present study, it was
demonstrated that D-6 was also effective in triggering apoptosis of
the cisplatin-resistant ovarian cancer cell line C30 in
vitro and this effect was improved when in combination with
cisplatin. Under an electron microscope, significant and typical
morphological apoptosis changes were observed in the C30 cells
treated with D-6, and the most evident programed cell death changes
were found in the C30 cells treated with D-6 plus cisplatin. These
results suggested that D-6 and cisplatin acted synergistically to
induce apoptosis in ovarian cancer cells.
The results of the WST-1-based cytotoxicity assay
in vitro suggested that D-6 inhibited the growth of C30
cells in a dose-dependent manner, and this inhibitory effect was
enhanced by cisplatin. Studies of other agonistic DR5 antibodies in
combination with chemotherapy on other tumor cells have revealed a
correlation between agonistic DR5 antibodies and cisplatin
(18,27). Similar to TRAIL, numerous TRAIL
receptors induce apoptosis via the cell-extrinsic pathway,
involving the recruitment and activation of caspase 8, followed by
ligand binding to DR4 or DR5. Of note, several recent studies also
reported that a number of the TRAIL receptors not only induce
caspase-dependent cell death, but also regulate a
caspase-independent cell death mechanism (10,12).
Concurrently, numerous studies have demonstrated that the majority
of chemotherapeutic agents trigger apoptosis through the
cell-intrinsic pathway, by activating pro-apoptotic B-cell lymphoma
2 family members, releasing cytochrome C and apoptotic protease
activating factor 1, and thereafter activating caspase 9 (29). Although caspase 8 and 9 are
activated by means of two independent pathways, in turn, they
activate the same downstream effectors, including caspase 3, 6 and
7, and subsequently stimulate programmed cell death. In the present
study, the internal reaction in the C30 cisplatin-resistant ovarian
cancer cells following treatment was examined by investigating the
expression of caspase 3, 8 and 9 precursors via western blot
analysis. D-6 alone or in combination with cisplatin resulted in
caspase cascade expression changes in C30 cells, substantially
downregulating the caspase 3, 8 and 9 precursors and subsequently
enhancing the activation of the associated caspases (i.e., highly
activating the associated caspases, including caspase 3, 8 and 9).
The data suggested that the apoptosis of C30 cells in the present
study occurred in a caspase-dependent and -independent manner.
Cisplatin alone induced weak or no activation of caspase 3, 8 and
9; however, D-6 triggered detectable processing of caspase 3, 8 and
9, and enhanced cleavage of these caspases when in combination with
cisplatin.
TRAIL/TRAIL receptors exhibit tumoricidual efficacy
in xenograft models, consistent with in vitro evidence. The
agonistic DR5 antibodies, including AD5-10 and Apomab, exhibited a
marked anti-tumor effect on xenograft models based on a variety of
tumor cell types (10,25). During these investigations, it was
demonstrated that the agonistic DR5 antibodies they had examined
exhibited the capability to inhibit tumor growth and even regress
xenograft tumor types, including lung, liver and breast cancer
(10,25,30).
In the present study, it was demonstrated that the volume of the
xenograft tumors in nude mice increased by 19.9-fold two weeks
following subcutaneous injection in the control group. This
aggressive tumor growth was inhibited by D-6 alone or in
combination with cisplatin, and combination treatment demonstrated
a more evident tumor growth repression than D-6 alone, although
this difference was no statistically significant. Indeed, both the
D-6 and combination groups reached a high tumor inhibition rate of
>70%. Similar to the changes of tumor volume, the tumor wet
weights in the D-6 and combination groups were significantly lower
than those in the other two groups. Taken together, D-6 repressed
the growth of xenograft tumors in nude mice, as demonstrated by the
evident inhibition of the increases in both the tumor volume and
wet weight. The internal reaction proteins in the processes of
apoptosis in vivo were also detected, with the activation of
caspase 3, 8 and 9. Caspase-dependent and -independent apoptosis
occurred both in vitro and in vivo, further providing
evidence suggesting a mechanism of apoptosis-induction by D-6.
Additionally, in order to observe the macroscopic apoptotic cells
in xenograft tumor sections, apoptosis analyses were conducted by a
TUNEL assay. The results demonstrated there were more macroscopic
apoptotic cells in the D-6 and combination treatment groups as
compared with the other two groups, which further confirmed the
marked anti-tumor effect of D-6 in xenograft tumors. It was
hypothesized that D-6 had a role in C30 growth inhibition and was
able to trigger apoptosis in C30 cisplatin-resistant ovarian cancer
via regulating the relative caspases. Furthermore, D-6 treatment in
combination with cisplatin activated more caspases (3, 8 and 9)
than D-6 or cisplatin treatment alone. Therefore, D-6 may be able
to overcome the cisplatin-resistance in C30 cisplatin-resistant
ovarian cancer by changing the apoptosis signaling pathways of
cisplatin, and exerting a synergistic function on apoptosis in
combination with cisplatin. However, further studies are required
to elucidate the underlying synergitistic mechanisms of apoptosis
induction by DR5 monoclonal antibodies with chemotherapeutic agents
in drug-resistant cancer types.
In conclusion, the agonistic DR5 antibody D-6 is a
potential anti-tumor agent that triggers apoptosis via
caspase-dependent and -independent pathways in C30
cisplatin-resistant ovarian cancer. Furthermore, the reported
anti-tumor effect of DR5 may be enhanced when concomitantly treated
with the chemotherapeutic agent cisplatin. These results suggested
that D-6 may represent a novel candidate for overcoming the
cisplatin-resistance of the cell line C30. Therefore, these
preclinical data may facilitate further large-scale clinical
investigations for the development of novel therapeutic strategies
for the treatment of cisplatin-resistant ovarian cancer.
Acknowledgements
The present study was supported by grants from the
National Natural Scientific Foundation of China (no. 30973192).
References
|
1
|
Fialka I, Pasquali C, Kurzbauer R,
Lottspeich F and Huber LA: Loss of epithelial polarity is
accompanied by differential association of proteins with
intracellular membranes. Electrophoresis. 20:331–343. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
3
|
Pitti RM, Marsters SA, Ruppert S, Donahue
CJ, Moore A and Ashkenazi A: Induction of apoptosis by Apo-2
ligand, a new member of the tumor necrosis factor cytokine family.
J Biol Chem. 271:12687–12690. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wiley SR, Schooley K, Smolak PJ, et al:
Identification and characterization of a new member of the TNF
family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lacour S, Hammann A, Wotawa A, Corcos L,
Solary E and Dimanche-Boitrel MT: Anticancer agents sensitize tumor
cells to tumor necrosis factor-related apoptosis-inducing
ligand-mediated caspase-8 activation and apoptosis. Cancer Res.
61:1645–1651. 2001.
|
|
6
|
Ma Y, Yang D and Chen Y: Analysis of TRAIL
receptor expression using anti-TRAIL death receptor-5 monoclonal
antibodies. Chin Med J (Engl). 116:947–950. 2003.PubMed/NCBI
|
|
7
|
Jo M, Kim TH, Seol DW, et al: Apoptosis
induced in normal human hepatocytes by tumor necrosis
factor-related apoptosis-inducing ligand. Nat Med. 6:564–567. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nitsch R, Bechmann I, Deisz RA, et al:
Human brain-cell death induced by tumour-necrosis-factor-related
apoptosis-inducing ligand (TRAIL). Lancet. 356:827–828. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang Q, Zhu H, Liang B, Huang Y and Li C:
Apoptosis-inducing effect of the DR5 monoclonal antibody, D-6,
alone or in combination with cisplatin, on A2780 ovarian cancer
cells. Mol Med Rep. 6:316–320. 2012.PubMed/NCBI
|
|
10
|
Guo Y, Chen C, Zheng Y, et al: A novel
anti-human DR5 monoclonal antibody with tumoricidal activity
induces caspase-dependent and caspase-independent cell death. J
Biol Chem. 280:41940–41952. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yagita H, Takeda K, Hayakawa Y, Smyth MJ
and Okumura K: TRAIL and its receptors as targets for cancer
therapy. Cancer Sci. 95:777–783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pukac L, Kanakaraj P, Humphreys R, et al:
HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody,
induces cell death in multiple tumour types in vitro and in vivo.
Br J Cancer. 92:1430–1441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gibson SB, Oyer R, Spalding AC, Anderson
SM and Johnson GL: Increased expression of death receptors 4 and 5
synergizes the apoptosis response to combined treatment with
etoposide and TRAIL. Mol Cell Biol. 20:205–212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu GS, Burns TF, McDonald ER III, et al:
KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor
gene. Nat Genet. 17:141–143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sheikh MS, Burns TF, Huang Y, et al:
p53-dependent and -independent regulation of the death receptor
KILLER/DR5 gene expression in response to genotoxic stress and
tumor necrosis factor alpha. Cancer Res. 58:1593–1598.
1998.PubMed/NCBI
|
|
16
|
Rajeshkumar NV, Rasheed ZA, García-García
E, et al: A combination of DR5 agonistic monoclonal antibody with
gemcitabine targets pancreatic cancer stem cells and results in
long-term disease control in human pancreatic cancer model. Mol
Cancer Ther. 9:2582–2592. 2010. View Article : Google Scholar
|
|
17
|
Er E, Oliver L, Cartron PF, Juin P, Manon
S and Vallette FM: Mitochondria as the target of the pro-apoptotic
protein Bax. Biochim Biophys Acta. 1757:1301–1311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bevis KS, McNally LR, Sellers JC, et al:
Anti-tumor activity of an anti-DR5 monoclonal antibody, TRA-8, in
combination with taxane/platinum-based chemotherapy in an ovarian
cancer model. Gynecol Oncol. 121:193–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W, Wang S, Chen C and Zhuang G:
Induction of tumor cell apoptosis via Fas/DR5. Cell Mol Immunol.
3:467–471. 2006.PubMed/NCBI
|
|
20
|
Ohtsuka T, Buchsbaum D, Oliver P, Makhija
S, Kimberly R and Zhou T: Synergistic induction of tumor cell
apoptosis by death receptor antibody and chemotherapy agent through
JNK/p38 and mitochondrial death pathway. Oncogene. 22:2034–2044.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohtsuka T and Zhou T: Bisindolylmaleimide
VIII enhances DR5-mediated apoptosis through the MKK4/JNK/p38
kinase and the mitochondrial pathways. J Biol Chem.
277:29294–29303. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Estes JM, Oliver PG, Straughn JM Jr, et
al: Efficacy of anti-death receptor 5 (DR5) antibody (TRA-8)
against primary human ovarian carcinoma using a novel ex vivo
tissue slice model. Gynecol Oncol. 105:291–298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang Z, Chen JJ, Yu Y, et al: Drozitumab,
a human antibody to death receptor 5, has potent antitumor activity
against rhabdomyosarcoma with the expression of caspase-8
predictive of response. Clin Cancer Res. 17:3181–3192. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yada A, Yazawa M, Ishida S, et al: A novel
humanized anti-human death receptor 5 antibody CS-1008 induces
apoptosis in tumor cells without toxicity in hepatocytes. Ann
Oncol. 19:1060–1067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin H, Yang R, Ross J, et al: Cooperation
of the agonistic DR5 antibody apomab with chemotherapy to inhibit
orthotopic lung tumor growth and improve survival. Clin Cancer Res.
14:7733–7740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Straughn JM Jr, Oliver PG, Zhou T, et al:
Anti-tumor activity of TRA-8 anti-death receptor 5 (DR5) monoclonal
antibody in combination with chemotherapy and radiation therapy in
a cervical cancer model. Gynecol Oncol. 101:46–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaplan-Lefko PJ, Graves JD, Zoog SJ, et
al: Conatumumab, a fully human agonist antibody to death receptor
5, induces apoptosis via caspase activation in multiple tumor
types. Cancer Biol Ther. 9:618–631. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kindler HL, Richards DA, Garbo LE, et al:
A randomized, placebo-controlled phase 2 study of ganitumab (AMG
479) or conatumumab (AMG 655) in combination with gemcitabine in
patients with metastatic pancreatic cancer. Ann Oncol.
23:2834–2842. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chaudhary PM, Eby M, Jasmin A, Bookwalter
A, Murray J and Hood L: Death receptor 5, a new member of the TNFR
family, and DR4 induce FADD-dependent apoptosis and activate the
NF-kappaB pathway. Immunity. 7:821–830. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Buchsbaum DJ, Zhou T, Grizzle WE, et al:
Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or
in combination with chemotherapy and/or radiation therapy in a
human breast cancer model. Clin Cancer Res. 9:3731–3741.
2003.PubMed/NCBI
|