Introduction
In recent years, obesity has become a global public
health problem, associated with a number of complications and
increasing prevalence among children and adolescents (1,2).
Obesity is characterized as an excessive accumulation of adipose
tissue due to severe energy imbalance (3) and it is associated with dyslipidemia,
type-2 diabetes mellitus, cardiovascular disease, cancer and
obstructive sleep apnea (4,5). As
energy-source materials, free fatty acids (FFAs) and glucose are
important in obesity and obesity-related insulin sensitivity.
Furthermore, studies have indicated that glucocorticoids and growth
hormone (GH) are also closely associated with obesity and insulin
resistance (6,7). However, the molecular mechanisms
underlying the effects of FFAs, glucose, glucocorticoids and GH in
obesity and insulin sensitivity, have not yet been fully
clarified.
MicroRNAs (miRNAs) are endogenous ~22 nucleotide
RNAs that have important regulatory roles in animal and plant
physiology, by targeting mRNAs for cleavage or translational
repression (8). Emerging evidence
has suggested that a number of miRNAs are important in the
regulation of adipocyte differentiation, including the miR-143
(9) and miR-1792 clusters
(10). miR-26b is an intronic
miRNA encoded in the carboxy-terminal domain, RNA polymerase II,
polypeptide A, small phosphatase 1 (CTDSP1) gene that is
important in a variety of different diseases, including lung
carcinoma, breast cancer and cardiac hypertrophy (11–13).
However, there have been few studies investigating the role of
miR-26b in obesity and insulin sensitivity. Previously, Klöting
et al (14) indicated that
miR-26b is closely correlated with the number of infiltrating
macrophages in abdominal subcutaneous (SC) adipose tissue. In
addition, it appears that miR-26b is upregulated during
adipogenesis in mice and humans (15,16).
In our preliminary study, it was identified that miR-26b is
increased during adipogenesis of human multipotent adipose-derived
stem cells and preadipocytes screened from miRNA microarray
experiments (unpublished data). Therefore, it is likely that
miR-26b is associated with obesity and obesity-related insulin
resistance. Investigation into the correlation between miR-26b and
energy-source materials or hormones that are associated with
obesity and obesity-related insulin resistance, may be critical for
enhancing our understanding of the mechanisms of obesity
development and may aid in the development of strategies that
promote obesity prevention and control.
The aim of the present study was to examine the
effects of energy-source materials (FFAs and glucose) or hormones
(glucocorticoids and GH) associated with obesity and
obesity-related insulin resistance on miR-26b expression in human
adipocytes, and to clarify the role of miR-26b in regulating
obesity development and insulin resistance.
Materials and methods
Cell culture and differentiation
Human preadipocytes (ScienCell Research
Laboratories, San Diego, CA, USA) were maintained in preadipocyte
medium (PAM; ScienCell Research Laboratories) supplemented with 5%
fetal bovine serum (FBS), 1% preadipocyte growth supplement and 1%
penicillin/streptomycin solution (P/S) at 37°C in a humidified
atmosphere of 5% CO2. To induce differentiation,
confluent human preadipocytes (day 0) were cultured in serum-free
PAM containing 50 nM insulin, 100 nM dexamethasone (DEX), 0.5 mM
3-isobutyl-1-methylxanthine and 100 μM rosiglitazone. The medium
was changed every two days for the first four days. Following this,
the medium was replaced with serum-free PAM containing 50 nM
insulin, which was changed every two days until the accumulation of
lipid droplets was observed (day 15). Subsequently, when >75% of
the cells exhibited the morphological and biochemical properties of
adipocytes, the cells were selected to be used in the
experiments.
Oil red O staining
For oil red O staining, cell culture plates were
retrieved and the medium was removed. Adipocytes were washed three
times with phosphate-buffered saline (PBS) and fixed with 4%
formalin in phosphate buffer for 30 min at room temperature.
Following fixation, cells were washed twice with PBS and stained
with 0.6% (w/v) filtered oil red O solution (60% isopropanol, 40%
water) for 30 min at room temperature. Following this, cells were
washed with tap water to remove unbound dye, visualized by light
microscopy (Olympus, Tokyo, Japan) and photographed (Olympus,
Tokyo, Japan).
Interference of adipocytes with FFAs,
glucose, glucocorticoids and GH
Following overnight incubation in serum-free PAM,
human adipocytes were treated with different adipokines,
respectively, including 1 mmol/l FFA cocktail (lauric, myristic,
linoleic, oleic and arachidonic acids), glucose (5 or 25 mmol/l), 1
mmol/l DEX and 100 nmol/l GH (Sigma, St. Louis, MO, USA) for
different durations of time (4, 8 and 24 or 48 h). Adipocytes were
collected at each time-point and prepared for further
investigation.
RNA isolation and quantitative
(q)PCR
Total RNA was extracted from adipocytes using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and
quantified spectrophotometrically (NanoDrop Technology, Rockland,
DE, USA) at 260 nm. miRNA quantification was performed via
TaqMan miRNA analysis of miR-26b. Generation of cDNA was
performed using the TaqMan microRNA reverse transcription
kit (Applied Biosystems, Foster City, CA, USA) in 15 μl reverse
transcriptase reactions containing 70 ng total RNA, 50 nM stem-loop
RT primer, 1X RT buffer, 0.25 mM each dNTP, 3.33 U/ml Multi-Scribe
RT and 0.25 U/ml RNase inhibitor. Reaction conditions were as
previously described (16). For
qPCR, 1.33 μl (dilution, 1:15) cDNA, 0.2 mM TaqMan probe,
1.5 mM forward primer, 0.7 mM reverse primer and TaqMan
Universal PCR Master mix (Applied Biosystems) were included in 20
μl reactions. The RT primer, PCR primer and Taq Man probe for
miR-26b were purchased from Applied Biosystems. Reaction conditions
were as previously described (16). The qPCR results were analyzed and
expressed relative to the miRNA expression of CT (threshold cycle)
value. The RT primer, PCR primer and TaqMan probe for
miR-26b were purchased from (Applied Biosystems). U6 small
nucleolar RNA (snRU6) and miR-103 were used as references to obtain
the relative fold-change in expression in target samples, using the
comparative CT method.
Statistical analysis
The data are presented as the mean ± standard error
of the mean. Statistical analysis was performed using one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
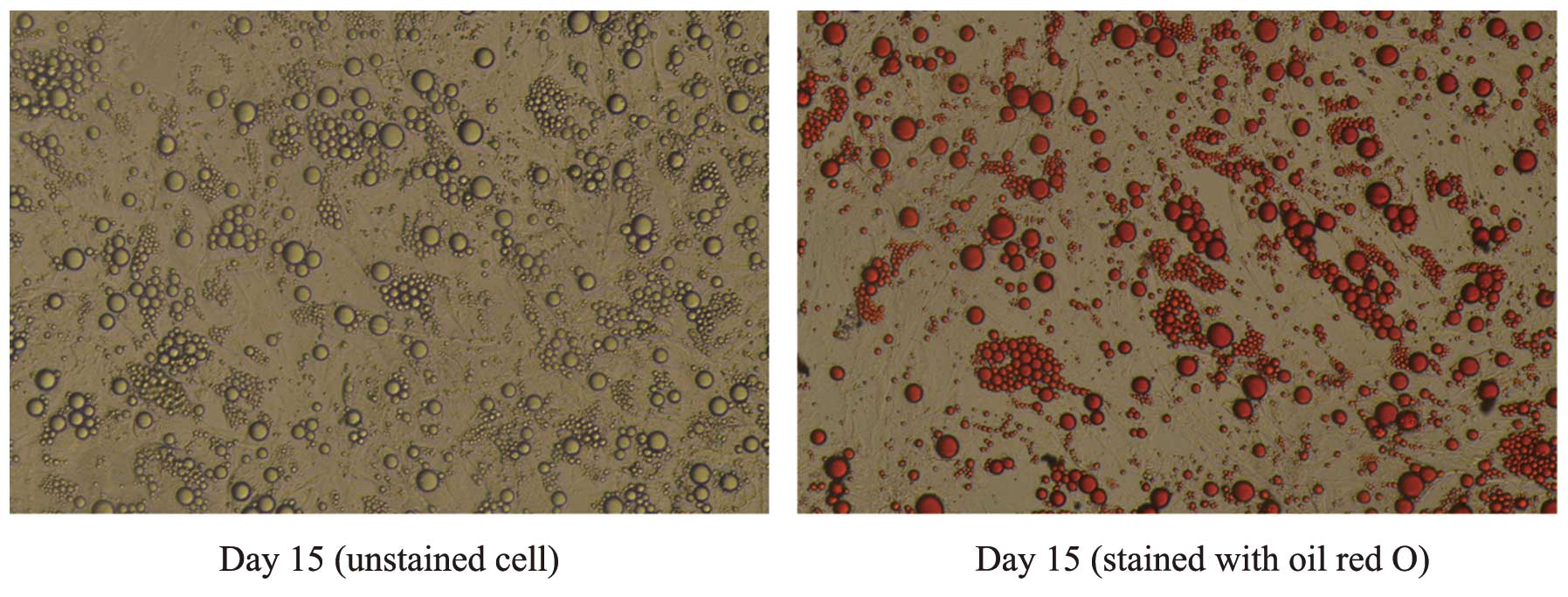
Assessment of lipid accumulation
Through oil red O staining, it was observed that
>75% of the human preadipocytes differentiated into mature
adipocytes, which were large, round and filled with fat droplets.
These data suggested that the majority of the adipocytes had
matured (Fig. 1).
Effects of FFAs on miR-26b expression in
human adipocytes
The effects of 1 mmol/l FFAs on miR-26b expression
in cultured human adipocytes were analyzed using qPCR
(TaqMan probe method). Differentiation of human
preadipocytes was induced and adipocyte cultures were prepared for
use in experiments as described in the Materials and methods.
Adipocytes were cultured in the presence of 1 mmol/l FFAs.
Expression of miR-26b was significantly downregulated in a
time-dependent manner from 4 h following the initiation of
stimulation by FFAs. This effect was maintained for up to 24 h
(Fig. 2).
Effects of glucose on miR-26b expression
in human adipocytes
To assess the effects of glucose on miR-26b
expression, the miR-26b expression in human adipocytes treated with
5 or 25 mmol/l glucose for different durations (4, 8, 24 and 48 h)
was examined on the 15th day of differentiation. It was identified
that glucose concentrations of 5 or 25 mmol/l led to a
time-dependent decrease in the miR-26b expression and this effect
was more potently induced by 25 mmol/l glucose. As shown in
Fig. 3, miR-26b expression
significantly decreased after only 12 h of exposure. Following
this, the levels of miR-26b expression remained relatively low at
24–48 h, at which time the expression of miR-26b was ~3-fold lower
than that in the control (P<0.05).
Effects of DEX on miR-26b expression in
human adipocytes
The effects of 1 mmol/l DEX on miR-26b expression in
cultured human adipocytes were analyzed using qPCR (TaqMan
probe method). Mature adipocytes were cultured in the presence of 1
mmol/l DEX. Expression of miR-26b was identified to be
significantly altered by DEX stimulation. As demonstrated in
Fig. 4, miR-26b expression
markedly decreased after 4–8 h of exposure. Following this, the
levels of miR-26b expression were moderately increased. However,
the expression of miR-26b remained decreased in comparison with the
untreated cells.
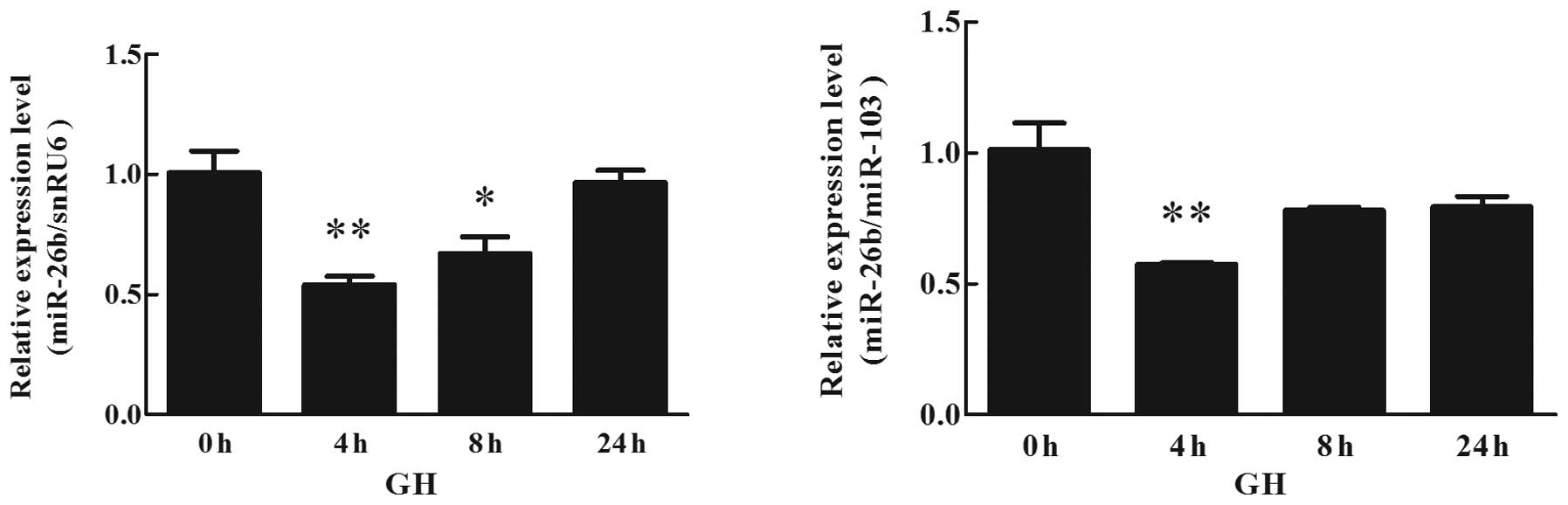
Effects of GH on miR-26b expression in
human adipocytes
The effects of 100 nmol/l GH on miR-26b expression
in cultured human adipocytes were analyzed using qPCR (TaqMan probe
method). Differentiation of human preadipocytes was induced and
adipocyte cultures were prepared for use in the experiments. The
miR-26b expression in human adipocytes treated with 100 nmol/l GH
for different durations (4, 8 and 24 h) was detected. The
expression of miR-26b was significantly downregulated in a
time-dependent manner at 4 h following the initiation of GH
stimulation. As demonstrated in Fig.
5, miR-26b expression markedly decreased after 4 h of exposure.
Following this, the levels of miR-26b expression were marginally
increased. However, the expression of miR-26b remained relatively
lower than that in the untreated cells.
Discussion
Due to increased morbidity and mortality, obesity
and obesity-associated diseases have become a major public health
problem worldwide. Currently, the prevalence of obesity and
obesity-associated diseases among children and adolescents, which
is a vulnerable age for the development of obesity, is also
increasing at an alarming rate (17). Although childhood and adolescent
obesity is correlated with a number of additional problems,
including hyperinsulinemia, poor glucose tolerance and a raised
risk of type 2 diabetes, hypertension, sleep apnea, social
exclusion and depression, the greatest health problems will emerge
as the present childhood obesity epidemic progresses to the next
generation of adults (18).
Therefore, prevention of childhood and adolescent obesity is a
priority. In particular, the population of adolescents who are
already obese, urgently require strategies to prevent their
progression into adulthood obesity.
Obesity is caused by an excessive accumulation of
adipose tissue. Adipose tissue is composed of functional units
termed adipocytes, is involved in the regulation of energy and
glucose homeostasis, and appears to function as an endocrine organ
(19,20). Therefore, assessing its
associations with energy-source materials and hormones is important
for combating obesity and its complications. However, the molecular
mechanisms underlying the effects of energy-source materials or
hormones on obesity-related insulin sensitivity have not yet been
fully clarified.
miRNAs are a novel class of small, non-coding RNA
molecules that function as negative gene regulators (8,21).
miRNA expression levels vary spatially and temporally in
vivo and alter in response to internal and external signals.
miRNA dysregulation is often associated with disease states,
including obesity and insulin resistance. For example, Kloting
et al (14) isolated miRNA
from different fat depots of overweight and obese individuals in
2009 and Zhao et al (22)
profiled 220 miRNAs in pancreatic islets, adipose tissue and liver
isolated from diabetes-resistant (B6) and diabetes-susceptible
(BTBR) mice. miR-26b is an intronic miRNA located in the intron of
the CTDSP1 gene, which is able to regulate its host
transcript (23). Emerging
evidence has indicated that miR-26b is associated with the
development of obesity. Several studies have demonstrated this,
including in abdominal subcutaneous adipose tissue where miR-26b
was closely correlated with the number of infiltrating macrophages
(14) and was upregulated during
adipogenesis in mice (15). In our
previous study, it was also demonstrated that miR-26b was
differentially expressed during differentiation of human
preadipocytes and was affected by a number of adipokines, including
tumor necrosis factor-α (TNF-α), leptin and resistin (16). This evidence suggests that miR-26b
is closely associated with the development of obesity. Thus, it was
hypothesized that energy-source materials or hormones may
participate in regulating the development of obesity via
interacting with miR-26b.
Increased circulating concentrations of fatty acids
and glucose are prominent features of type 2 diabetes and are also
involved in the development of obesity and insulin resistance. FFA
accumulation triggers several components of the insulin resistance
syndrome and increases the risk of diabetes (24). Furthermore, plasma FFA
concentrations are usually elevated in obese individuals (25). By contrast, obesity-induced glucose
intolerance reflects a failure to mount one or more compensatory
response through insulin-secreting β cells to systemic insulin
resistance (26). Therefore, FFAs
and glucose are important in obesity and insulin resistance,
however, the details of the mechanisms underlying their effects
remain elusive. In the present study, it was observed that FFAs and
glucose inhibit miR-26b expression in human adipocytes,
respectively, and that the effect of high glucose levels on miR-26b
was more potent, in comparison with low glucose levels. Due to the
fact that elevated circulating concentrations of fatty acids and
glucose are associated with insulin resistance, miR-26b may be
involved in regulating the development of obesity and insulin
resistance via increasing the insulin sensitivity of human
adipocytes.
Glucocorticoids and GH have important roles in
lipolysis and are able to increase plasma glucose levels. Numerous
studies have indicated that high glucocorticoids in obese adipose
tissue promotes the uptake and storage of fatty acids by increasing
lipoprotein lipase levels (27),
lipogenesis and lipid storage (28,29).
Furthermore, GH has a pronounced lipolytic effect, particularly in
abdominal fat (30). In the
present study, it was identified that DEX and GH decrease the
expression of miR-26b in human adipocytes. However, DEX and GH have
potent effects for only a relatively short time, and with time
their actions become weaker. Therefore it was observed that DEX and
GH participate in the development of obesity and insulin resistance
via controlling miR-26b expression in human adipocytes. However,
the time-dependent decrease of their effects suggests the
involvement of other mechanisms.
In conclusion, the present study revealed that the
expression of miR-26b is affected by FFAs, glucose, DEX and GH in
human adipocytes. These obesity-associated factors are closely
correlated with the development of obesity and insulin resistance,
and it is therefore possible that miR-26b is involved in
obesity-related insulin resistance. Thus, we hypothesize that
miR-26b is involved in the progression and development of obesity
and insulin resistance. To determine whether these effects occur
independently further investigation is required.
Acknowledgements
This study was supported by grants from the National
Key Basic Research Program of China (grant no. 2013CB530604), the
National Natural Science Foundation of China (grant no. 81170797),
the Natural Science Foundation of Jiangsu Province China (grant no.
BK2011107), the Program for Innovative Research Teams of Jiangsu
Province (grant no. LJ201108) and Nanjing Technological Development
Program (grant no. 201104013).
References
|
1
|
Basham P, Luik J, Jeffery RW, et al: Is
the obesity epidemic exaggerated? Yes BMJ. 336:2442008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haslam DW and James WP: Obesity. Lancet.
366:1197–1209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gregoire FM, Smas CM and Sul HS:
Understanding adipocyte differentiation. Physiol Rev. 78:783–809.
1998.PubMed/NCBI
|
|
4
|
Kassi E, Pervanidou P, Kaltsas G and
Chrousos G: Metabolic syndrome: definitions and controversies. BMC
Med. 9:482011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lubrano C, Saponara M, Barbaro G, et al:
Relationships between body fat distribution, epicardial fat and
obstructive sleep apnea in obese patients with and without
metabolic syndrome. PloS One. 7:e470592012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee MJ and Fried SK: Glucocorticoids
antagonize tumor necrosis factor-α-stimulated lipolysis and
resistance to the antilipolytic effect of insulin in human
adipocytes. Am J Physiol Endocrinol Metab. 303:E1126–E1133.
2012.
|
|
7
|
Morita J, Hakuno F, Hizuka N, Takahashi S
and Takano K: Growth hormone (GH) or insulin-like growth factor
(IGF)-I represses 11beta-hydroxysteroid dehydrogenase type 1 (HSD1)
mRNA expression in 3T3-L1 cells and its activity in their
homogenates. Endocr J. 56:561–570. 2009. View Article : Google Scholar
|
|
8
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esau C, Kang X, Peralta E, et al:
MicroRNA-143 regulates adipocyte differentiation. J Biol Chem.
279:52361–52365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Q, Li YC, Wang J, et al: miR-17-92
cluster accelerates adipocyte differentiation by negatively
regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci USA.
105:2889–2894. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arora H, Qureshi R, Park AK and Park WY:
Coordinated regulation of ATF2 by miR-26b in γ-irradiated lung
cancer cells. PloS One. 6:e238022011.PubMed/NCBI
|
|
12
|
Li J, Kong X, Zhang J, Luo Q, Li X and
Fang L: MiRNA-26b inhibits proliferation by targeting PTGS2 in
breast cancer. Cancer Cell Int. 13:72013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han M, Yang Z, Sayed D, et al: GATA4
expression is primarily regulated via a miR-26b-dependent
post-transcriptional mechanism during cardiac hypertrophy.
Cardiovasc Res. 93:645–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klöting N, Berthold S, Kovacs P, et al:
MicroRNA expression in human omental and subcutaneous adipose
tissue. PloS One. 4:e46992009.PubMed/NCBI
|
|
15
|
Kajimoto K, Naraba H and Iwai N: MicroRNA
and 3T3-L1 pre-adipocyte differentiation. RNA. 12:1626–1632. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu G, Ji C, Shi C, et al: Modulation of
hsa-miR-26b levels following adipokine stimulation. Mol Biol Rep.
40:3577–3582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ebbeling CB, Pawlak DB and Ludwig DS:
Childhood obesity: public-health crisis, common sense cure. Lancet.
360:473–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lobstein T, Baur L and Uauy R; IASO
International Obesity TaskForce. Obesity in children and young
people: a crisis in public health. Obes Rev. 5(Suppl 1): 4–104.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosen ED and Spiegelman BM: Adipocytes as
regulators of energy balance and glucose homeostasis. Nature.
444:847–853. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galic S, Oakhill JS and Steinberg GR:
Adipose tissue as an endocrine organ. Mol Cell Endocrinol.
316:129–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao E, Keller MP, Rabaglia ME, et al:
Obesity and genetics regulate microRNAs in islets, liver, and
adipose of diabetic mice. Mamm Genome. 20:476–485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dill H, Linder B, Fehr A and Fischer U:
Intronic miR-26b controls neuronal differentiation by repressing
its host transcript, ctdsp2. Genes Dev. 26:25–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bergman RN and Ader M: Free fatty acids
and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol
Metab. 11:351–356. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boden G: Role of fatty acids in the
pathogenesis of insulin resistance and NIDDM. Diabetes. 46:3–10.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kahn SE, Hull RL and Utzschneider KM:
Mechanisms linking obesity to insulin resistance and type 2
diabetes. Nature. 444:840–846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fried SK, Russell CD, Grauso NL, et al:
Lipoprotein lipase regulation by insulin and glucocorticoid in
subcutaneous and omental adipose tissues of obese women and men. J
Clin Invest. 92:2191–2198. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee MJ, Gong DW, Burkey BF and Fried SK:
Pathways regulated by glucocorticoids in omental and subcutaneous
human adipose tissues: a microarray study. Am J Physiol Endocrinol
Metab. 300:E571–E580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu CY, Mayba O, Lee JV, et al: Genome-wide
analysis of glucocorticoid receptor binding regions in adipocytes
reveal gene network involved in triglyceride homeostasis. PloS One.
5:e151882010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gravhølt CH, Schmitz O, Simonsen L, Bülow
J, Christiansen JS and Møller N: Effects of a physiological GH
pulse on interstitial glycerol in abdominal and femoral adipose
tissue. Am J Physiol. 277:E848–E854. 1999.PubMed/NCBI
|